Study on Hyaluronic Acid and Its Derivatives
Hyaluronic acid (HA), also known as vitreous acid, is a kind of linear macromolecular acidic mucopolysaccharide widely distributed in human body and animal body.In 1934, Professor Meyer of Columbia University isolated hyaluronic acid from the vitreous body of cow's eye, and then Kendell et al. extracted hyaluronic acid from the fermentation broth in 1937. After years of research, people have a clear understanding of the structure, properties and functions of hyaluronic acid, and it has been applied in many fields such as beauty, health care products, clinical and pharmaceutical products.
1 Distribution of hyaluronic acid
Hyaluronic acid is widely distributed in nature, in the body, more than 50% of hyaluronic acid exists in the skin, lungs and intestines. In addition, it is also found in the interstitial tissues such as synovial fluid, cartilage, umbilical cord and blood vessel wall. In early studies, the main source of hyaluronic acid was the umbilical cord. Currently, hyaluronic acid can be extracted from animal tissues, such as the corns of chickens, the vitreous humor of the eye, brain cartilage, joint fluids, or fermented by bacteria, such as Streptococcus, Pseudomonas aeruginosa, etc. [1]. The fermentation method of hyaluronic acid production is gradually replacing the tissue extraction method because of its low cost, abundant raw materials, easy to produce on a large scale, and high molecular quality of the hyaluronic acid obtained.
In recent years, the latest research on hyaluronic acid at home and abroad has focused on the optimisation of the fermentation process as well as the derivatisation and degradation of hyaluronic acid. However, the fermentation technology in China is not mature, so tissue extraction still has an irreplaceable role. At the same time, people are also trying to find hyaluronic acid from other organisms. China's marine resources are large in quantity, cheap and easy to obtain, and fish eyes are the waste of the fishery development process, if discarded, not only waste of resources, but also easy to form the eutrophication of the water body, endangering the ecological environment. However, the extraction of hyaluronic acid from fish eyes as raw material can not only achieve the effect of waste utilisation and comprehensive development, but also reduce the cost and meet the economic demand.
2 Structure and properties of hyaluronic acid
2.1 Structure of hyaluronic acid
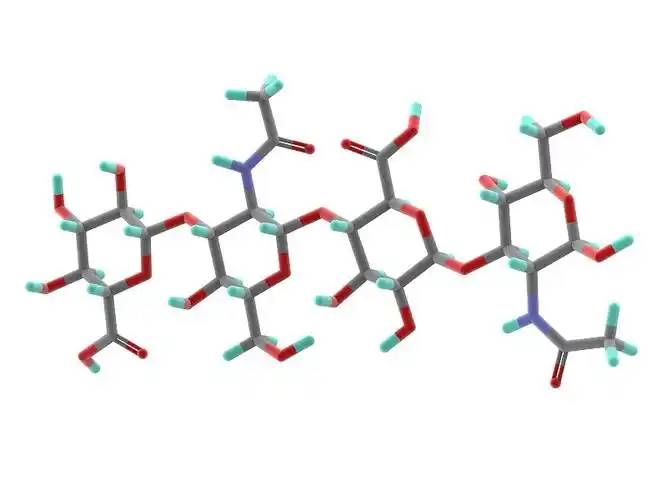
Hyaluronic acid is the only non-sulfur mucopolysaccharide known so far, it is a linear straight chain polysaccharide polymer formed by repeating arrangement of disaccharide units, D-glucuronic acid and N-acetylglucosamine are connected by β-1,3 glycosidic bond in each disaccharide unit, and the disaccharide units are connected by β-1,4 glycosidic bond. The molecule consists of two monosaccharides in a 1:1 molar ratio [2]. The structure of hyaluronic acid from different organisational sources is the same, but the length of the sugar chain and the molecular mass are different, the relative molecular mass is generally 105-107, and the number of disaccharide units is 300-1,100 pairs [3].
2.2 Properties of hyaluronic acid
Hyaluronic acid is generally a white amorphous solid, colourless and odourless, with strong hygroscopicity, very soluble in water, insoluble in organic solvents. Hyaluronic acid forms a rigid spiral column of 200nm in space, and the inner side of the column is strongly hydrophilic due to hydroxyl groups; at the same time, due to the continuous directional arrangement of hydroxyl groups, it forms a highly hydrophobic region on the molecular chain of hyaluronic acid[4]. At the same time, due to the continuous orientation of the hydroxyl groups, highly hydrophobic regions are formed in the molecular chain of hyaluronic acid [4]. Hyaluronic acid has a very strong water-absorbing capacity, good osmotic pressure and viscoelasticity in aqueous solution, and its affinity for adsorbed water is about 1,000 times its own mass, so it is recognised as a natural moisturising factor [5-6].
3 Preparation of hyaluronic acid
Hyaluronic acid can be prepared by tissue extraction or fermentation. Fermentation is not limited by the source of hyaluronic acid, has high yield and low cost, and is easy to form large-scale industrial production without the risk of contamination by pathogenic viruses of animal origin, so it has gradually become a hot research topic. However, the fermentation method is limited by high equipment requirements, high investment in the early stage, large volume of fermentation broth and large amount of bacteria and their metabolites, and so on, and the processing volume and complexity of hyaluronic acid isolation are higher than that of the tissue extraction method.
3.1 Tissue extraction
3.1.1 Terrestrial raw materials
The most commonly used raw material for tissue extraction is chicken crowns, which is usually crushed directly and then treated with acetone or ethanol, and then extracted with sterile water directly or heated.Dong Young Kang[7] cut frozen chicken crowns into pieces and repeated acetone precipitation several times, and after drying, they obtained 80g of chicken crowns dried powder, and 500mg of hyaluronic acid was extracted, with a final yield of 0.6%. Wang Jian et al[8] used domestic crude trypsin enzyme to extract chicken crowns after grinding, and filtered with 120 mesh filter cloth and diatomaceous earth at 60 ℃, meanwhile, the effect of secondary enzyme digestion on the purity and molecular mass of hyaluronic acid was also investigated, and the final yield of hyaluronic acid from chicken crowns was 0.4%~0.6%.

The vitreous body of the eye is another major source of hyaluronic acid, and in the early stage, the eyeballs of terrestrial organisms such as cows and sheep were mainly used as the main raw material. Guo Yutao et al[9] used the vitreous body of bovine eyes as raw material, peeled off the outer skin, removed the lens, and obtained the vitreous body fluid. After a series of separation and purification process, the final hyaluronic acid recovery rate of 79.5%.
3.1.2 Marine biological resources
Hyaluronic acid extracted from terrestrial organisms often contains some pathogenic bacteria, which brings safety problems to the products. Therefore, the current research hotspot is to use relatively safe aquatic organisms as raw materials, of which the most widely used is fish eye. Fish-eye is a waste product in the process of fishery development because it is abundant, cheap and easy to obtain, and if it is discarded, it is not only a waste of resources, but also prone to cause eutrophication of the water body, which is harmful to the ecological environment. Therefore, the extraction of hyaluronic acid from fish eyes as a raw material can not only achieve the effect of waste utilisation and comprehensive development, but also reduce the cost of hyaluronic acid extraction [10]. Qin Qian'an et al. [11] thawed the squid eye and stripped out the vitreous body, put it into acetone degreasing for 24h, dried, crushed, and then extracted with 0.2mol /L sodium chloride solution. After neutral protease digestion, the final hyaluronic acid yield reached 85.7% and the protein removal rate reached 91.1%.
Yao Meiqin et al.[12] investigated the effect of protein removal by Sevage method, isoelectric point precipitation (IEP) and trichloroacetic acid (TCA) after obtaining the crude extract of hyaluronic acid from squid eyes, and the protein content of IEP method contained the least amount of protein, and the protein content of the final product was 3.06%, with the total yield of hyaluronic acid of 2.96%. Amagai et al.[13] obtained high purity hyaluronic acid by repeated CPC precipitation-dissolution and alcohol precipitation with 95% ethanol solution containing 10% potassium acetate.Muradoa et al.[14] obtained hyaluronic acid with molecular mass of 2,000 kDa and purity of 99.4% by using ultrafiltration and dialysis of the hyaluronic acid extract from swordfish. Lu Jiafang [15] used DEAE-Sephadex A-25 column chromatography to purify hyaluronic acid from squid eyes, and eluted with distilled water and 0.95mol/L NaCl solution step by step to obtain two hyaluronic acid fractions, which accounted for 5.22% and 82.37% of the sample volume, respectively.

Besides fish eyes, other aquatic organisms are also rich in hyaluronic acid. Sun Zhihua et al.[16] studied the extraction of hyaluronic acid from the mucus of loach, and the results showed that the extract contained hexanedioic acid and aminocaproic acid, and the infrared spectroscopy analysis showed that the extract was in complete agreement with the scanning pattern of hyaluronic acid standard. Nicola et al[17] obtained hyaluronic acid for the first time from the mollusc bivalve purple mussel, and the purity of hyaluronic acid reached 97% after degreasing, enzyme digestion and anion-exchange resin, etc. Giji[18] extracted hyaluronic acid with a molecular mass of 1,365 kDa from the liver of stingray, and the analytical results showed that it was of high purity and good antioxidant activity. In recent years, with the increasing market demand for hyaluronic acid, the production of hyaluronic acid from aquatic organisms has gradually become an important issue in the rational development of marine resources.
In addition to cockles, fish eyes and loaches, hyaluronic acid has also been extracted from pig skin, forest frog skin and egg shell membrane [19-20].
3.2 Preparation of modified hyaluronic acid
The hyaluronic acid obtained by both tissue extraction and microbial fermentation has the disadvantages of poor stability, sensitivity to hyaluronidase and free radicals, easy degradation, short retention time in the body, and lack of mechanical strength in aqueous system, which greatly limits the application, so it is necessary to modify it to improve its mechanical strength and anti-degradation properties [21].
3.2.1 Cross-linking hyaluronic acid
The cross-linking of hyaluronic acid refers to the intermolecular cross-linking reaction between hyaluronic acid and cross-linking agent with relevant functional groups, or the intramolecular cross-linking reaction with cross-linking agent as catalyst, to obtain the molecular mesh structure with different cross-linking degree, which results in the growth of molecular chain of hyaluronic acid, the increase of average molecular mass, the enhancement of viscous-elasticity, the relative weakening of water solubility, and the enhancement of mechanical strength [22-23]. Commonly used cross-linking methods include hydrazide cross-linking, disulfide cross-linking, polyethylene glycol cross-linking, aldehyde cross-linking and carbodiimide cross-linking.
( 1) Hydrazide cross-linking: Hydrazide compounds can be used as cross-linking agents to modify flowable gels into brittle and mechanically hard gels, and the most commonly used cross-linking agent is adipic dihydrazide (ADH), which is used to produce stable HA-ADH derivatives of hyaluronic acid in the presence of a large amount of adipic dihydrazide. Xu et al. [24] prepared HA-ADH gel films by chemically modifying hyaluronic acid molecules using ADH as a cross-linking agent. The crosslinked film was obviously dissolved in the buffer, and the solubility was lower than that before crosslinking, and the stability was improved.
(2) Carbodiimide cross-linking: Carbodiimide (EDC) can react with the carboxyl group of hyaluronic acid in acidic solution to form N-acyl urea compounds, and then add with different carbodiimides to form cross-linking derivatives with good stability, high rigidity, high biodensity, and high hyaluronic acid enzyme degradation resistance [25]. Lai et al.[26] investigated the biocompatibility of EDC-crosslinked hyaluronic acid gel in the anterior chamber of the mouse eye, and the results showed that compared with glutaraldehyde-crosslinked membranes, these gel membranes were more biocompatible with the eye and had a higher tensile resistance.

(3) Sulfone cross-linking: The rapid cross-linking of divinyl sulfone (DVS) with the hydroxyl group of hyaluronic acid at room temperature resulted in gels with different properties. The cross-linking degree of the gel can be changed by controlling the concentration of hyaluronic acid, molecular mass, HA/DVS value and pH of the reaction medium. Wang Yanguo et al[27] obtained DVS-HA gels by cross-linking DVS at room temperature, and used ethanol precipitation to remove the residual DVS, and finally made cross-linked hyaluronic acid dry powder.
(4) Photocrosslinking: Photocrosslinking has the advantages of fast reaction, good reproducibility and non-toxic solvent, which is very suitable for the preparation of hyaluronic acid hydrogel. Luo Chunhong et al.[28] used glycidyl methacrylate (GMA) to chemically modify hyaluronic acid, and then crosslinked it to form hydrogel under radiation. The results showed that by increasing the degree of GMA substitution of hyaluronic acid, the cross-linking density of hydrogel could be increased, which led to smaller pore sizes, improved mechanical properties, and slower degradation rate of the gel. In the further study, Luo Chunhong constructed a self-reinforced double cross-linked hyaluronic acid hydrogel. Firstly, hyaluronic acid microspheres with different cross-linking densities were prepared by reversed-phase microemulsion polymerisation (primary cross-linking), and then modified with glycidyl methacrylate (GMA) to introduce reactive double bonds, and then the GMA-modified hyaluronic acid molecular chain was used as the base phase and the modified microspheres as the reinforcing phase, and then cross-linking was performed twice under ultraviolet radiation, resulting in a self-reinforced double-cross-linking hyaluronic acid hydrogel with double-cross-linking structure. This kind of hydrogel improves the mechanical strength of hyaluronic acid and prolongs the sustained release time of proteins[29] .
2.2.2 Non-crosslinked hyaluronic acid
( 1) Esterification: Esterification of hyaluronic acid includes hydroxyl and carboxyl modification, i.e., the hydroxyl group in the structure of hyaluronic acid undergoes esterification with acids or anhydrides, or the carboxyl group reacts with alcohols, phenols, epoxides, or halogenated hydrocarbons to form esterified derivatives. Vazquez et al[30] permeated sodium hyaluronate into acid in a cation exchange resin, added tetrabutylammonium hydroxide to neutrality, freeze-dried the hyaluronic acid, dissolved it in anhydrous dimethylsulfoxide (DMSO), and added p-chloromethylstyrene to obtain the ester compound HA-VB. This compound can be further cross-linked under the action of ultraviolet light.
(2) Graft modification: The grafting reaction of hyaluronic acid involves the grafting of small molecules or polymers onto the main chain of hyaluronic acid. Oldinski et al[31-32] prepared a biomaterial for bone tissue repair by graft copolymerisation of hyaluronic acid with high density polyethylene (HDPE). Palumbo et al. [33] prepared low molecular mass tetrabutylammonium salt of hyaluronic acid (HA-TBA), and then reacted it with NHS-activated polylactic acid (PLA-NHS) in dimethylsulfoxide to obtain the graft copolymer HA-PLA.
(3) Hydrophobic modification: Hyaluronic acid is highly hydrophilic, often exists in the form of sodium salt, and is insoluble in most organic solvents, so it is difficult to modify or combine it with many hydrophobic substances.Pravata et al.[34] modified sodium hyaluronate with cetylammonium bromide (CTA-Br) to obtain hydrophobic CTA-HA, and then grafted poly(lactic acid) (COL-OLA), which was chloride terminated, to NHS activated polylactic acid (PLA-NHS) in dimethylsulfoxide. (Then COL-OLA was grafted onto CTA-HA in dimethylsulfoxide to obtain the degradable derivative CTA-HAOLA, which can be further self-assembled in aqueous solution to form a hydrogel.
4 Application of hyaluronic acid
It is well known that hyaluronic acid has been widely used in cosmetics, ophthalmology and joint surgery due to its unique physicochemical properties. It is worth noting that the effect of hyaluronic acid is closely related to its molecular mass, which varies according to the purpose of use. High molecular mass hyaluronic acid has a good moisturising and lubricating effect and is mostly used in ophthalmology or joint surgery; medium molecular mass hyaluronic acid has a good slow-release effect and is often used in cosmetics and post-surgical anti-adhesion; and small molecular mass hyaluronic acid has anti-tumour, immunomodulatory, and angiogenesis-promoting effects [35].
4.1 Applications of high molecular mass hyaluronic acid (HMWHA)
4.1.1 Treatment of joint diseases
Hyaluronic acid is the main component of articular cartilage and synovial fluid. In osteoarthritis, rheumatoid arthritis, and other infectious and non-infectious arthritis, the concentration and molecular mass of hyaluronic acid in the synovial fluid are reduced, and the cartilage is degraded and destroyed, which leads to physiological dysfunction of the joints [36]. Therefore, in the treatment of joint diseases, hyaluronic acid can be supplemented to restore the lubrication function of synovial fluid and promote joint repair, and the effect of high molecular mass hyaluronic acid is better than that of low molecular mass hyaluronic acid.
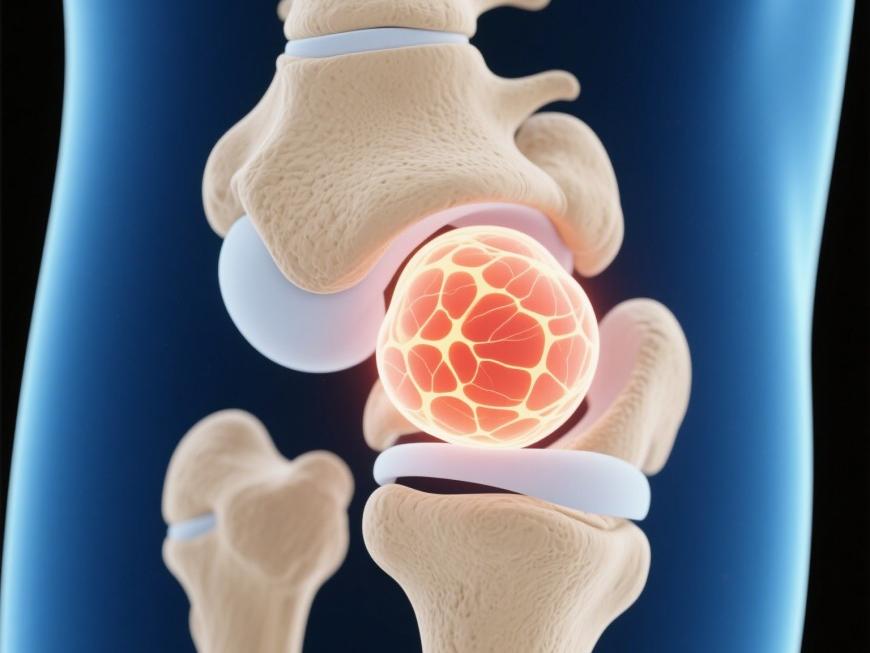
According to Ji[37] , regular intra-articular injections of 1% exogenous high molecular mass hyaluronic acid can not only increase the content of hyaluronic acid in the intra-articular cavity, but also act as a synovial fluid to protect articular cartilage from wear and tear and slow down the degeneration of articular cartilage. Fu Lifeng [38] compared the efficacy of Synvisc (欣维可) and Hyalgan (海尔根) with relative molecular mass of 6 × 106-7 × 106 on rabbit knee osteoarthritis, and the results showed that the degree of damage to the knee cartilage in the Synvisc group was lower than that in the Hyalgan group. The results showed that the degree of cartilage damage in the knee joint of the Synvisc group was lower than that of the Hyalgan group, and the protective effect of Synvisc was stronger and the therapeutic effect was better.
4.1.2 Ophthalmic applications
The reticular structure of hyaluronic acid varies according to its molecular mass. Compared with hyaluronic acid of low molecular mass, hyaluronic acid of high molecular mass forms a more complete reticular structure, so its viscoelasticity is higher, hydrophilicity and lubrication are better, and it can stabilise the tear film, prevent the cornea from drying out, reduce the friction of ocular tissues, and alleviate the dry eye syndrome. It can stabilise the tear film, prevent corneal dryness, reduce the friction of ocular tissues and alleviate dry eye. When it is combined with fibronectin, it can promote the connection and extension of corneal epithelial cells and accelerate the healing of corneal wounds [39].
In addition, high molecular mass hyaluronic acid can prevent postoperative adhesions [40-41], and has the effect of slow release of drugs [42].
4.2 Application of low molecular mass hyaluronic acid (LMWHA)
Currently, hyaluronic acid and its derivatives as a drug delivery system is a hot research topic, which is based on the fact that hyaluronic acid can bind to some specific receptors on the cell surface, so its use as a drug carrier can improve the targeting of drugs, and at the same time prolong the duration of action of the drug in vivo, improve bioavailability, and enhance the therapeutic efficacy. Compared with high molecular mass hyaluronic acid, low molecular mass hyaluronic acid has the properties of low viscosity, anti-tumour and activation of immune cells, so it is often used as a drug carrier.
4.2.1 Hyaluronic acid nanoparticles
Choi et al [43] used hyaluronic acid to produce nanoparticles. After systemic administration to mice carrying tumours, the hyaluronic acid nanoparticles could circulate in the blood for 2 days and selectively accumulate at the tumour site. In addition, hyaluronic acid nanoparticles can be modified with hydrophobicity, or made into novel copolymers and graft derivatives to change the particle size and drug loading capacity and improve the targeting ability. It was shown in [44] that after systemic administration of hyaluronic acid nanoparticles, they usually accumulate in the liver first, but polyethylene glycolated hyaluronic acid nanoparticles can effectively reduce this phenomenon, and at the same time, their circulation time in the blood was significantly increased, and their accumulation effect in the tumour site was 1.6 times more than that of the unmodified hyaluronic acid nanoparticles.
4.2.2 Hyaluronic acid modified lipid carriers
Liposome is a widely used carrier in drug delivery system, which has slow release, targeting and biocompatibility. If attached with glycoconjugates such as hyaluronic acid, they can reach the target more effectively, but the attached hyaluronic acid must be low molecular mass hyaluronic acid and its oligosaccharides, because high molecular mass hyaluronic acid has high viscosity, which affects the rheological properties of the drug [45].
Yang Xiaoyan [46] prepared paclitaxel nanolipid carriers (PTX-NLC), and then adsorbed hyaluronic acid with relative molecular mass of 300,000 and 1,000,000,000 on the surface of PTX-NLC by charge adsorption to obtain the active targeting hyaluronic acid-modified paclitaxel nanolipid carriers (HA-NLC), respectively. The results showed that the use of relatively low molecular mass hyaluronic acid could produce a more stable carrier. The in vivo pharmacodynamic and pharmacokinetic studies showed that HA-NLC had better tumour-suppressing effect in vivo than paclitaxel injection Taisu ®, and prolonged the circulation time of the drug in vivo and reduced the cardiac and renal toxicity. At the same time, the total targeting efficiency of HA-NLC in the tumour was increased by about 1.4-fold, and the active targeting of tumour was obvious. Zhang Wenqiang[47] firstly degraded macromolecular hyaluronic acid and obtained hyaluronic acid with relative molecular mass of 150,000~200,000, and then prepared hyaluronic acid liposome by reversed-phase evaporation method and investigated its permeability, which provided theoretical basis for hyaluronic acid to be used in cosmetics with liposome as a carrier.
4.2.3 Coupling of hyaluronic acid and drugs
The carboxyl group, ammonia group and reducing end of hyaluronic acid can be amidated and esterified and coupled with antitumor drugs to form a drug coupling body, which can prolong the retention time of the former drug in the body and enhance the water solubility of the drug and the targeting of the tumour. Xin Dingtui [48] designed a new type of anticancer drug paclitaxel precursor system using low molecular mass hyaluronic acid as the carrier. Leucine, phenylalanine and valine were used as linker arms to bind with the drug molecules, and then linked with hyaluronic acid with a molecular mass of 9,800 Da, which resulted in a large increase in the molecular mass of the original drug, and thus the water solubility was affected, and paclitaxel's solubility increased, with good cell-killing effect and IC50 value lower than that of the original drug. Galer et al[49] used a hyaluronic acid-paclitaxel coupling in a mouse tumour model of squamous cell carcinoma of the neck (SCCHN), which effectively inhibited tumour growth and increased the survival rate of the mice compared with that of pure paclitaxel injection. Ding Baoyue et al.[50] used hyaluronic acid (MW = 150 000) to modify doxorubicin (DOX) and polyamide-amine bonding compounds to form a drug-carrying dendritic polymer nanocarrier drug delivery system, which could significantly increase the intracellular uptake of the drug compared with doxorubicin solution, and at the same time promote the entry of doxorubicin into the nucleus of the target cells, which could further improve the therapeutic efficacy.

4.2.4 Hyaluronic acid nanogels
Nanogels are usually hydrogel particles composed of chemically or physically crosslinked polymer networks, which can be used as a new type of drug carriers due to their high loading capacity and stability. Jieying Ding [51] investigated the effect of the molecular mass of hyaluronic acid on the sulfhydrylated hyaluronic acid in the preparation of sulfhydrylated hyaluronic acid-poly(vinyl alcohol) multilayered hydrogel film carriers. The results showed that the total sulfhydryl groups and disulfide bonds attached to the hyaluronic acid chain decreased with the increase of molecular mass, which could be attributed to the fact that the higher molecular weight of hyaluronic acid and the longer molecular chain made it more difficult for the free sulfhydryl groups to form disulfide bonds.Duceppe et al.[52] used chitosan with ultra-low molecular mass to make a new type of nanogel with hyaluronic acid. Duceppe et al. [52] used ultra-low molecular mass chitosan and hyaluronic acid to make a new type of nanogel. When chitosan and hyaluronic acid were mixed in a 4:1 ratio with a molecular mass of 5 kDa and 64 kDa respectively, a gel with an average size of 146 nm was obtained. Further studies showed that the transfection rate of DNA-encapsulated chitosan-hyaluronic acid gel could be increased from 0.7% to 25% under the same condition.
4.2.5 Hyaluronic acid microspheres
Li Dan et al[53] prepared sodium hyaluronate microspheres by emulsification-cross-linking method, which reduced the drug release rate, prolonged the drug release time and improved the bioavailability through the insoluble skeleton of microspheres. Liang Henglun et al.[54] concluded that hyaluronic acid as a single drug carrier has the following shortcomings: low molecular mass hyaluronic acid is easily retained and metabolised by the liver, and it is difficult to reach the target tissues; high molecular mass hyaluronic acid does not have active targeting because of the loss of receptor-mediated cytotoxicity. Therefore, Liang Henglun et al. [54] used low molecular weight sodium hyaluronate coupled with chitosan to prepare a kind of hyaluronic acid-chitosan coupled microspheres (DTX-HACTNPs) with an average particle size of 228 nm, hoping to retain the active targeting property of drug hyaluronic acid and overcome the other shortcomings, and the MTT assay demonstrated that hyaluronic acid coupled microspheres could reduce non-selective cytotoxicity and maintain the active targeting property of the drug through the active targeting property.
MTT assay showed that the hyaluronic acid-coupled drug microspheres could reduce the non-selective cytotoxicity and maintain the antitumour activity of the drug through active targeting. Similarly, Zhou Panghu et al[55] showed that hyaluronic acid-chitosan microspheres could significantly inhibit the activity of inducible nitric oxide synthase in osteoarthritic chondrocytes in vitro, avoiding the production of excessive NO, thus inhibiting the destruction of articular cartilage and protecting chondrocytes.
4.2.6 Hyaluronic acid nanoemulsion
Nanoemulsions are good carriers for transdermal drug delivery because of their small particle size, high transdermal permeability and high drug-carrying capacity. Gao Yuanyuan et al.[56] used hyaluronic acid with a molecular mass of 10-110 K as a carrier, and prepared a 10,11- methylenedioxycamptothecin (MD-CPT) encapsulated hyaluronic acid nanocarrier (HA-GMS) by microemulsion method, which had a significantly higher transdermal efficiency and improved drug efficacy compared with MD-CPT ethanol solution. Kong et al.[57] prepared O/W/S nanoemulsions by modifying hyaluronic acid, in which dichloromethane was the oil phase, HA-GMA was the aqueous phase, and Tween-80 and Spectra-20 were used as surfactants. The nano-emulsions had low protein dispersion, uniform distribution, and the smallest particle size was 39.7 nm, which was a good carrier for lipophilic drugs.

4.2.7 Other applications
Zhang Jinxiang et al.[58] found that small-molecule hyaluronic acid degraded by HMW- HA could activate the main immune cells in the liver, the blight cells, and promote the secretion of pro-inflammatory factors, which triggered the inflammatory response, while the high molecular mass of hyaluronic acid did not have this function. Low molecular mass hyaluronic acid can also act as an endogenous danger signalling molecule to enhance the humoral immune response to inactivated HAV antigens, and can therefore also be used as an immune adjuvant [59].
5 Hyaluronic acid market
With regard to pharmaceutical hyaluronic acid, the number of people suffering from diseases such as osteoarthritis of the knee has increased by 4 million from 2000 to 2010, which has led to a rapid growth in the demand for hyaluronic acid as a viscoelastic supplement. In Canada, the orthopaedic market spent $13 million in 2012 alone. In Japan, the market for hyaluronic acid for knee treatment is valued at more than $5 million, and there is growing demand for new treatment options. Due to the accelerated aging of the global population and the increasing research on hyaluronic acid in medicine, the use of hyaluronic acid as a non-steroidal anti-inflammatory drug, etc., will also help expand the market for hyaluronic acid in medicine [60].
6 Conclusion
With the improvement of living standards, health is becoming more and more important to people, and the development potential of hyaluronic acid market in China is increasing. China has a long coastline and rich marine resources, but a large amount of waste is generated in the production and processing process every year, which is not only a waste of resources, but also a great pressure on the environment. The use of cheap and easily available marine resources to extract hyaluronic acid not only reduces the production cost, but also reduces the impact of processing waste on the environment, and opens the way for the development of high value-added products.
Reference
[1]Eun Ju Oh ,Kitae Park ,Ki Su Kim ,et al.Target specific and long-acting delivery of protein,peptide,and nucleotide therapeutics using hyaluronic acid derivatives. Journal of Controlled Release,2010,141 :2-12.
[2]Cui Y,Duan Q,Li Y H.The research progress in hyaluronic acid.Chang chun University of Scienceand Technology,2011,34 (3) : 101-106.
[3]Meng F T,Tan C Y,Liu F J,et al.The characteristics,preparation and application of hyaluronic acid.Fine and Specialty Chemicals, 2012,20(5) : 17-22.
[4]Song L,Wang T F.Current research of hyaluronic acid.Journal of Shan Dong Polytechnic University,2012,26(2) : 15-18.
[5]Pan H M.Current research of hyaluronic acid.Si Chuan Food and Fermentation,2003,1 (39) : 5-9.
[6]Liu H,Liu A J.Products saint in cosmetics———hyaluronic acid.Chemistry Teaching,2012,11 :72-75.
[7 ]Dong Young Kang.Extraction of hyaluronic acid from rooster comb and characterization using flow field-flow fractionation ( FlFFF ) coupled with multiangle light scattering ( MALS) .Journal of Separation Science,2010,33 ( 22 ) : 3530- 3536.
[8]Wang J,Xie Z,Wang Q.The optimization of the industrial production conditions of cockscomb hyaluranic acid.Chinese Journal of Biochemical Pharmaceutics,2011,32(6) :472-475.
[9 ]Guo Y T,Chen B,Jiang Y R , et al.A new method of purificati on of hyaluronic acid from bovine vitreous body.Fine Chemicals, 2008,25 (3) : 246-259.
[10]José Antonio Vázquez ,Isabel Rodríguez-Amado ,María Ignacia Montemayor.Chondroitin sulfate,hyaluronic acid and chitin / chitosan production using marine waste sources : characteristics, applications and eco-friendly processes : a review.Marine Drugs, 2013,11 :747-774.
[11]Qin Q A,Zhou X M,Li J R. Study on enzymatic hydrolysis of hyaluronic acids primary extract from squid eyes.Science and Technology of Food Industry,2009,30( 1) : 214-216.
[12]Yao M Q,Chu J J,Chen X E.Study on the extraction technology of hyaluronic acid from squid eyes.Food Science and Technology, 2009,34(3) : 241-244.
[13]Amagai I ,Tashiro Y ,Ogawa H.Improvement of the extraction procedure for hyaluronan from fish eyeball and the molecular characterization.Fisheries Science,2009,75 (3) : 805-810.
[14]Muradoa M A,Montemayora M I,Cabo M L,et al.Optimization of extraction and purification process of hyaluronic acid from fish eyeball.Food and Bioproducts Processing,2012,90 ( C3 ) ,491- 498.
[15]Lu J F.Studies on the Extraction,Degradation and Bioactive of Hyaluronic Acid from Squid Eyes.Ningbo : Ningbo University, 2010.
[16]Sun Z H,Wang J J.Study on the preparation and physicochemical properties of hyaluronicacid in mucilage of loach.Pharmaceutical Biotechnology,2001,8 ( 1) :42-44.[17]Nicola Volpi,Francesca Maccari.Purification and characterization of hyaluronic acid from the mollusc bivalve Mytilus galloprovincialis.Biochimie,2003,85 :619-625.
[18]Giji Sadhasivam.Isolation and characterization of hyaluronic acid from the liver of marine stingray Aetobatus narinari.International Journal of Biological Macromolecules,2013,54 : 84-89.
[19]Nakano T,Ikawa N,Ozimek L.Extraction of glycosaminoglycans from chicken eggshell.Poultry Science,2001,80 :681-684.
[20]Katarína Vulganová , Eva ürgeová . Extraction of hyaluronic acid from eggshell membranes.Current Opinion in Biotechnology, 2013,24( 1) : S106.
[21]Wu L J,Luan T,Zhang F.Progress on the biomedical modification and functionalization of hyaluronic acid.Natural Product Research and Development,2012,24 : 1690-1695,1679.
[22]Xu J,Ai L,Bai H Y,et al.Research progress of hyaluronic acid modified.Polymer Bulletin,2011,2 :78-84.
[23]Wang J Q.Modification Functionalization of Hyaluronic Acid.Wuxi: Jiangnan University,2013.
[24]Xu X,Lu M Q,Ye W X,et al.Synthesis and characterization of cross-linking hyaluronic acid film by ADH.Guangdong Chemical, 2012,39(2) :47-48.
[25]Yang H,Xiong W Y,Ying G Q,et al.Development of preparation and application of crosslinked hyaluronan.Chemical Industry and Engineering Progress,2006,25 ( 12) : 1410-1414.
[26]Lai J Y.Ocular biocompatibility of carbodiimide cross linked hyaluronic acid hydrogels for cell sheet delivery carriers.Journal of Biomaterials Science,2010,21 : 359-376.
[27]Wang Y G,Fu J G,Guo L B.Study on the preparation of cross- linked sodium hyaluronate gel.Technology and Development of Chemical Industry,2011,40(9) : 8-9.
[28]Luo C H,Zhao J H.Preparation and properties of light crosslinking hyaluronicacid hydrogels. Polymer Materials Science and Engineering,2011,27 (7) : 163-166.
[29]Luo C H.Fabrication and Properties of Self-reinforced Hyaluronan Hydrogel with a Doublecrosslinked Network.Jinan : Jinan University,2013.
[30]Vazquez C P,Boudou T,Dulong V,et al.Variation of polyelectrolyte film stiffness by photocross-linking : a new way to control cell adhesion.Langmuir,2009,25 (6) : 3556-3563.
[31]Oldinski R A.Synthesis and characterization of a hyaluronan- polyethylene copolymer for biomedical applications.J Biomed Mater Res,Part B : Appl Biomater,2010,94 : 441-446.
[32 ]Oldinski R A ,et al.Dynamic mechanical analysis and biomineralization of hyaluronan-polyet-hylene copolymers for potential use in osteochondral defect repair.Acta Biomater,2010, 7 : 1184-1191.
[33]Palumbo F S,Pitarresi G,Mandracchia D ,et al.New graft copolymers of hyaluronic acid and polylactic acid : synthesis and characterization.Carbohyd Polym,2006,66(3) : 379-385.
[34]Pravata L,Braud C,Boustta M,et al.New amphiphilic lactic acid oligomer-hyaluronan conjugates : synthesis and physicochemical characterization.Biomacromolecules,2007,9( 1) : 340-348.
[35]Hang Y S,Pan Y M,Xu J.Research on the function and application of hyaluronic acid with different molecular weights.Chinese Journal of Dialysis and Artifical Organs,2010,21 (4) : 22- 25.
[36]Ling P X,He Y L,Zhang Q.The effect of hyaluronic acid in treatment of osteoarthritis.Food and Drug,2010,21 (4) : 22-25.
[37]Ji X M.Sodium Hyaluronate Injection for the Repair of Articular Cartilage in Traumatic Arthritis of Rabit Knees : An Experimental Study.Shijiazhuang : Hebei Medical University,2011.
[38]Fu L F.Observe and analysis the efficacy in the treatment of rabbit knee osteoarthritis with different molecular weight hyaluronic acid.Zhejiang Medical Journal,2008,30(4) : 340-341.
[39]Ling P X,Zhang T M,Li Q.The progress of application and research of hyaluronic acid in the ophthalmology drugs.Chinese Remedies and Clinics,2004,4(9) : 697-699.
[40]Hu H Z,Sun J G,Pu G M,et al.The clinical effect observation of hyaluronic acid in the prevention of abdominal cavity and pelvic operation postoperative adhesion.China Modern Doctor,2013,10
(51) : 147-149.
[41]Lou D B.Protecting flexor tendon from adhering by sodium hyaluronate products with different molecular weight.Chinese Journal of Modern Applied Pharmacy,2002,19(4) : 334-336.
[42]Li Q.Study on the Action Mechanism of Sodium Hyaluronate as a Mediadepartment of Ophthalmology Drugs.Jinan : Shandong University,2006.
[43]Choi K Y,Chung H,Min K H,et al.Self-assembled hyaluronic acid nanoparticles for active tumor targeting.Biomaterials,2010, 31 ( 1) : 106-114.
[44]Ki Young Choi ,Kyung Hyun Min ,Hong Yeol Yoon ,et al.
PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo.Biomaterials,2011,32 : 1880-1889.
[45]Lou Q,Qian H,Wang F S.Research progress of hyaluronan in malignant tumor targeting therapy.Chinese Journal of Biochemical Pharmaceutics,2008,29(5) : 359-361.
[46]Yang X Y.The Studies on Hyaluronic Acid Coated Paclitaxel Loaded Nanostructured Lipid Carriers. Jinan : Shandong University,2012.
[47]Zhang W Q.Study on the Preparation of LMWHA—Containing Liposome and Percutaneous in vitro.Guangzhou : South China University of Technology,2010.
[48]Xin D C.Study on Self-Assembling Hyaluronic Acid 一 Paclitaxel Drug Delivery System.Changsha : Hunan University,2010.
[49]Galer C E,Sano D,Ghosh S C,et al.Hyaluronic acid-paclitaxel conjugate inhibits growth of human squamous cell carcinomas of the head and neck via a hyaluronic acid-mediated mechanism.Oral Oncol,2011,47 ( 11) : 1039-1047.
[50]Ding B Y,Lv Z L,Zhu Q F ,et al.Hyaluronic acid-modified doxorubicin PAMAM nanoparticles to overcome cancer multi-drug resistance.Chinese Pharmaceutical Journal,2013,48 (22) : 1933- 1937.
[51]Ding J Y.Thiolated Hyaluronic Acid / Polyvinyl Alcohol Hydrogel Multilayer Membrane Carrier for Protein Drugs for Oral Administration.Shanghai : Fudan University,2010.
[52]Duceppe N,Tabrizian M.Factors influencing the transfection efficiency of ultra low molecular weight chitosan /hyaluronic acid nanoparticles.Biomaterials,2009,30( 13) : 2625-2631.
[53]Li D,Chu Y.Preparation technology of sodium hyaluronate-loaded microspheres.China Pharmacy,2014,25 ( 1) : 51-53.
[54]Liang H L,Li J,Tong J,et al.Targeting effect of hyaluronic acid coupled chitosan nanoparticles on non-small cell lung cancer.Journal of Clinical Rehabilitative Tissue Engineering Research, 2011,15 (21) : 3847-3850.
[55]Zhou P H,Ma B L,Qiu B,et al.Effects of hyaluronic acid and chitosan microspheres on inducible oxide synthase activity and expression in osteoarthritis chondrocytes. Medical Journal of Wuhan University,2013,34(5) : 682-690.
[56]Gao Y Y,Kong M,Chen X J,et al.The research about the roal of hyaluronic acid nano micro-emulsion loading MD-CPT transdermal drug delivery in the study of scar tissue.Progress in Biochemistry and Biophysics,2014,41 (2) : 202-208.
[57]Kong M,Chen X G,Park H J.Design and investigation of nanoemulsified carrier based on am-phiphile-modified hyaluronic acid.Carbohydr Polym,2011,83 (2) :462-469.
[58]Zhang J X,Wang H,Xiao H,et al.Low molecular weight hyaluronic acid fragments activate Kupffer cells through toll receptor 4 signaling.Science China,2008,38 ( 11) : 1041-1047.
[59]Shu X M,Hu N Z,Lan Y.Study on low molecular weight hyaluronic acid enhance humoral immune response induced by HAV antigen.Chinese Journal of Cellular and Molecular Immunology,2008,24(2) : 183-185.
[60]Long Liu,Yanfeng Liu,Jianghua Li,et al.Microbial production of hyaluronic acid : current state ,challenges ,and perspectives.Microbial Cell Factories,2011,10 :99.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese