Study on Hyaluronic Acid Regulates Melanin Metabolism
Abnormal melanin synthesis and metabolism is the main mechanism for the development of melasma and other pigmented diseases, and it is also one of the important features of photoaging skin[1-2] . With the economic development and the improvement of people's living standard, there is an increasing demand for healthy and youthful skin, and how to better regulate melanin metabolism is of great clinical significance. Hyaluronic acid has a wide range of biomedical applications due to its good tolerance and non-immunogenicity. In recent years, it has been used as a filler in aesthetic medicine, playing an important role in facial rejuvenation and improvement of skin imperfections[3] . Currently, little research has been reported on the relationship between hyaluronic acid and melanin metabolism. Whether hyaluronic acid can regulate melanin metabolism deserves in-depth study. In this paper, we will review the roles of hyaluronic acid and cells involved in melanin biometabolism in recent years, so as to clarify the correlation between hyaluronic acid and melanin biometabolism, and to provide information for further research.
1 Biological characteristics of hyaluronic acid
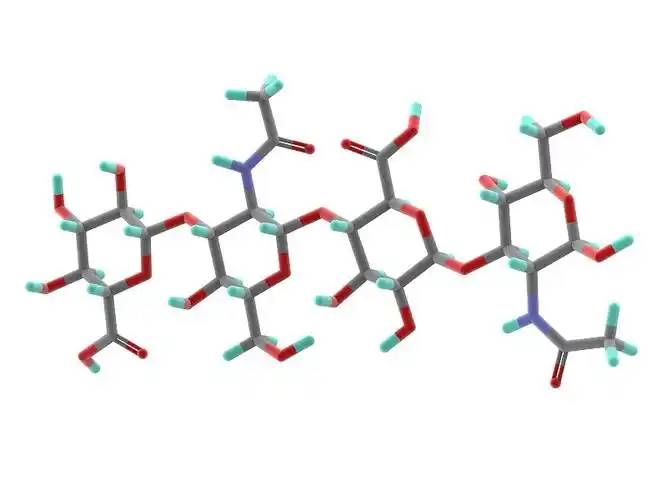
Hyaluronic acid is a non-sulfated glycosaminoglycan composed of repeating disaccharide units of D-glucuronic acid and N-acetylglucosamine. It has a wide range of molecular weights, and its concentration and distribution in the human body vary according to tissue type, age, and disease status[4] . Hyaluronic acid acts as a lubricant and shock absorber in the skin and joints due to its viscosity, elasticity and other rheological properties[5] ; it also participates in the regulation of cellular functions by activating intracellular signaling pathways through binding to hyaluronic acid receptors (e.g., CD44) on the cell surface. The size of the hyaluronic acid molecule is a major determinant of the activation of receptor-mediated signalling pathways, and only hyaluronic acid chains of a specific mass range are able to activate the corresponding receptor-mediated cell signalling, which is regulated by the balance between hyaluronic acid biosynthesis and degradation [4,6].
2 Melanin biometabolism is regulated by a variety of factors.
Melanin is synthesised in melanocytes and transported to keratinocytes, and a variety of intra- and extracellular factors are involved in the regulation of melanin biometabolism, either positively or negatively, by influencing melanocyte proliferation and viability, dendrite formation, melanin synthesis, and the transport of melanosomes [7-8].
Inflammation and oxidative stress affect melanin synthesis: in post-inflammatory hyperpigmentation, melanocytes respond to inflammation by increasing cell proliferation and activity, and melanin production increases and is transferred to neighbouring keratinocytes through the dendrites, leading to hyperpigmentation[9] ; UV radiation causes lipid peroxidation of cell membranes, which leads to an increase in the level of reactive oxygen species (ROS) in the melanocytes, which may stimulate the production of hyperpigmentation in melanocytes[10] . This may stimulate melanocytes to produce excessive amounts of melanin[10] ; in the pathogenesis of vitiligo, it is widely accepted that ROS lead to molecular and organelle dysfunction, triggering further immune responses and ultimately leading to melanocyte death[11] .
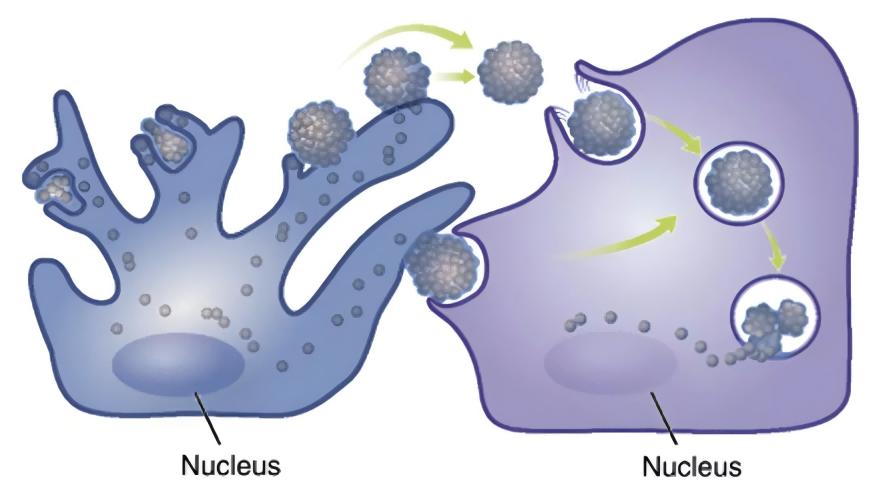
Neighbouring cells to melanocytes, such as keratinocytes, fibroblasts and immune cells, are involved in the metabolic regulation of melanin through the secretion of paracrine factors[8,12-13] . Pigmentary skin diseases are often accompanied by abnormal expression of cytokines derived from multiple melanocyte-adjacent cells: in the epidermis of hyperpigmented areas of sunspots, the expression of stem cell factor (SCF) and endothelin-1 derived from keratinocytes is up-regulated[14] ; in the hyperpigmented skin lesions of melasma, the expression level of secretory frizzled-associated protein 2 (sFRP2) derived from fibroblasts is significantly up-regulated[15] . (sFRP2), a fibroblast-derived secretory frizzled-related protein, is significantly up-regulated[15] . Traditional paracrine factors include keratinocyte-derived α-MSH, basic fibroblast growth factor (FGF-2), fibroblast-derived keratinocyte growth factor (KGF), and the secreted protein Dickkopf-1 (DKK1), among others[13,16-18] . Various inflammatory mediators such as histamine, prostaglandin E2, IL-6, IL-17 and tumour necrosis factor (TNF) are also involved in the regulation of melanogenesis[19-20] . In recent years, more and more factors have been found to be involved in the paracrine function of melanocyte neighbouring cells[21-23].
3 Possible mechanisms of hyaluronan regulation of melanin biometabolism
Lim et al[24] designed a self-controlled study using hyaluronic acid to fill the tear troughs. 4 weeks after topical injection of hyaluronic acid, a decrease in localised melanin content as measured by a skin melanometer was observed compared to the control side, suggesting the potential of hyaluronic acid to influence melanin biometabolism.
3.1 Direct effect of hyaluronic acid on melanocytes
It has been found that 0.2-5 g/L hyaluronic acid can promote the proliferation of melanocytes and increase the tyrosinase activity of melanocytes, thus promoting the synthesis of melanin; 10 g/L hyaluronic acid can inhibit the proliferation of melanocytes, decrease the tyrosinase activity, and inhibit the synthesis of melanin [25].
Hyaluronic acid has significant anti-inflammatory and antioxidant effects[26-27] : some studies have suggested that low molecular weight hyaluronic acid induces inflammation, while high molecular weight hyaluronic acid has an anti-inflammatory effect.26 Takabe et al.[28] found that UV treatment of melanocytes resulted in the inhibition of hyaluronic acid synthesis and the expression of the receptor CD44, and the inhibition of IL6, IL8, CXCL1, CXL1, and CXL1 receptors in melanocytes, IL6, IL8, CXCL1 and CXCL10 release in melanocytes; UV light and hyaluronan hydrolase co-treatment of melanocytes increased the release of the above inflammatory factors; exogenous addition of hyaluronic acid tablets did not significantly alter the production of inflammatory factors; the above results suggested that the unhydrolysed hyaluronic acid, which was present in the periphery of the melanocytes, could play a protective role in the inflammatory response of melanocytes, but the study did not focus on the role of the hydrolysis in this process in the melanocytic inflammation. The results suggest that hyaluronic acid, which is present around melanocytes and is not hydrolysed, could play a protective role in the inflammatory response of melanocytes, but the study did not focus on whether melanin synthesis was affected in this process. In the skin, hyaluronic acid acts as an antioxidant and free radical inhibitor[29] .
In our previous study, we observed that hyaluronic acid intervention down-regulated ROS levels in normal and aged fibroblasts, and Campo et al.[30] found that the addition of hyaluronic acid to the oxidative system induced by Fe2+ and ascorbate in fibroblasts inhibited lipid peroxidation through chelation, suppressed ROS production, reduced DNA fragmentation and protein oxidation, and limited cellular growth through the addition of hyaluronic acid. The addition of hyaluronic acid to the ascorbate-induced ROS-generating oxidative system can inhibit lipid peroxidation through chelation, inhibit ROS production, reduce DNA fragmentation and protein oxidation, and limit cell death, thus exerting antioxidant effects. It is not known whether hyaluronic acid can directly exert an antioxidant effect on melanocytes in the current study, and further investigation is needed.
3.2 Involvement of hyaluronic acid in the regulation of paracrine function of melanocyte neighbouring cells
In previous studies, hyaluronic acid has attracted much attention because of its role in inflammation and wound healing[26,32] . Recently, it has been found that hyaluronic acid can regulate the expression and secretion of growth factors and interleukins by acting on keratinocytes, fibroblasts and immune cells in the skin.
3.2.1 Effect of hyaluronic acid on paracrine action of keratinocytes
In the keratinocytes of aging mice, the binding of hyaluronic acid fragments (no more than 27 kDa) to its receptor CD44 promotes RhoA-ROK signalling, while the interaction between high molecular hyaluronic acid (700 ~ 1 000 kDa) and CD44 stimulates the activation of Rac-PKNγ, which affects the synthesis and secretion of keratinocytes [33]. UVB irradiation induces the degradation of hyaluronic acid into smaller fragments and selectively activates different cellular signalling mediated by CD44 in keratinocytes[34] .
In vitro studies have shown that 1% of high molecular hyaluronic acid (700-1,700 kDa) can increase the secretion of α-MSH from keratinocytes into the supernatant[35] . Hyaluronic acid oligosaccharides inhibited the release of IL-8 and TNF-α from LL-37-treated keratinocytes[36] . Hu et al.[37] found that very low molecular hyaluronic acid (0.8 kDa) and high molecular hyaluronic acid (1,200 kDa) inhibited the secretion of IL-6, IL-8, and IL-1 β by human epidermal keratinocytes after UVB irradiation, and the process involves the effect of hyaluronic acid on the secretion of TLRS and IL-1β in human epidermal keratinocytes[38] . This process involves the blockage of TLR4 activation by hyaluronic acid.
3.2.2 Effect of hyaluronic acid on fibroblast paracrine action
David-Raoudi et al.[38] investigated the effects of natural hyaluronic acid, 12- and 880-saccharide hyaluronic acid fragments on the proliferation of human fibroblasts and the expression of matrix-related genes, and found that all three types of hyaluronic acid could promote the adhesion and proliferation of cells, and increase the expression of matrix metalloproteinase-1 and matrix metalloproteinase-3, among which 12-saccharide hyaluronic acid could enhance the expression of type I collagen and TGFF-3 in fibroblasts. In particular, 12-saccharide hyaluronic acid enhances the expression of fibroblast type I collagen and TGF-β1.
Ciccone et al.39 reported that FGF-2 expression by fibroblasts was up-regulated when fibroblasts were exposed to 1 mg/mL of soluble hyaluronic acid, whereas the same concentration of a mixture of reticulated and free low-molecular-weight hyaluronic acid did not result in such an alteration.Asparuhova et al.[40] found that hyaluronic acid at 2,500 kDa (4 mg/mL) significantly increased the expression of human oral fibroblasts. Asparuhova et al.[40] found that 2,500 kDa of hyaluronic acid (4 mg/mL) significantly increased the expression of FGF-2, EGF2, IL-1 α, IL-1 β, and TNF in fibroblasts of human oral origin, and Quan et al.[41] showed an increase in the number of fibroblasts in the dermis, elongation of their morphology, and an increase in CCN2 in the dermis by subcutaneous injection of hyaluronic acid.
Radrezza et al.[42] applied quantitative proteomics to study the changes in protein profiles of normal human dermal fibroblasts with different concentrations of low molecular weight hyaluronic acid in the range of 20-50 kDa (0.125%, 0.25%, and 0.50%) and found that treatment with low molecular weight hyaluronic acid promoted the growth and proliferation of the cells, and increased the biosynthesis of proteoglycans, and the treatment of low molecular weight hyaluronic acid promoted cell growth and proliferation, and increased protein glycan biosynthesis at the highest concentration (0.50%). At the highest concentration (0.50%), inflammatory and immune responses were activated and the secretion of interleukins (IL-12, IL-1, IL-2, IL-4, etc.) and TNF-α was increased.
Lorén et al.[43] found that a 4.3-kDa hyaluronic acid fragment promoted the release of IL-6 and IL-8 from dermal fibroblasts via the CD44 receptor, but Olsson et al.[44] found that no increase in the release of IL-1 β, IL-6, IL-8, IL-10, IL-12, or TNF-α was detected after hyaluronic acid stimulation of fibroblasts from synovial membranes. IL-1β, IL-6, IL-8, IL-10, IL-12 or TNF-α were not detected after hyaluronic acid stimulation.
3. 2. 3 Effect of hyaluronic acid on paracrine effects of immune cells
Inflammatory factors secreted by immune cells also play an important role in the regulation of melanin synthesis. It was found that hyaluronic acid could inhibit the secretion of NO, IL-6 and TNF-α in LPS-induced RAW 264.7 cells; when used in helper T cells, low molecular weight hyaluronic acid mainly inhibited the secretion of IFN-γ, whereas high molecular weight hyaluronic acid inhibited the level of IL-4 [45].
3. 2. 4 Hyaluronic acid regulates the function of melanocytes by regulating the paracrine action of neighbouring cells
On the basis of the previous theory, we designed a fibroblast-melanocyte co-culture system, and after photo-aging of fibroblasts from human skin, we found that the intervention of hyaluronic acid at 1 mg/mL on photo-aged fibroblasts inhibited melanin synthesis in melanocytes in the co-culture system, but did not have any significant effect on the melanin synthesis in the normal fibroblast-melanocyte system, which preliminarily proved that under certain conditions, hyaluronic acid inhibits the synthesis of melanin in fibroblasts and melanocytes. Under certain conditions, hyaluronic acid can regulate the function of melanocytes by regulating the paracrine action of fibroblasts.
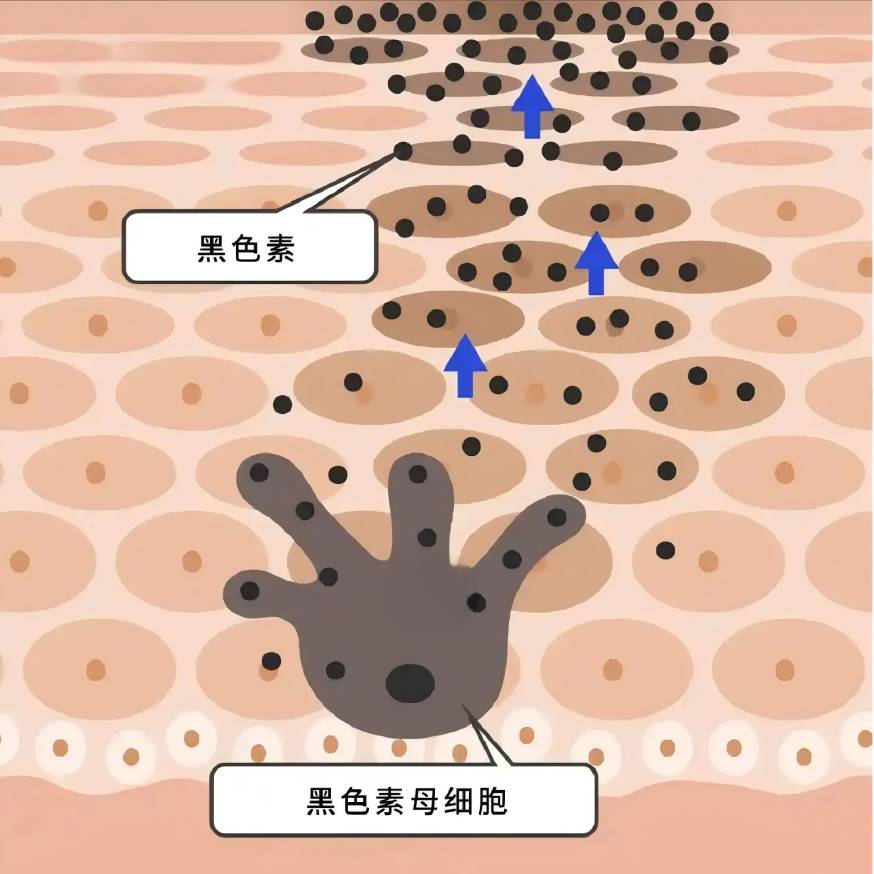
Zhou et al[46] found that UVB irradiation of keratinocytes induced the production of IL-18 and IFN-γ in a dose- and time-dependent manner, and IL-18 significantly increased the melanin content of melanocytes co-cultured with keratinocytes, while IFN-γ showed a significant inhibitory effect. D'agostino et al [47] found that a mixture of hyaluronic acid and chondroitin promoted the increase of melanin synthesis in keratinocyte-melanocyte co-cultures, but chondroitin or hyaluronic acid alone did not have a significant effect on melanin content. This may suggest that hyaluronic acid needs to be in a certain form or under a certain condition in order to play its role in regulating melanin synthesis.
4 Summary and Prospects
Although hyaluronic acid has been used for a long time and in a wide range of applications, its effects are not well understood because they are affected by a variety of factors such as molecular weight, cross-linking degree, concentration, receptor type, target cell type, etc. In the field of dermatology, hyaluronic acid has been used for a long time and in a wide range of applications. In the field of dermatology, few studies have been reported on the effects of hyaluronic acid on melanin biometabolism. The results of the existing clinical and basic studies suggest that hyaluronic acid may be related to melanin metabolism, especially the metabolic changes of melanin under external stimuli (e.g., UV irradiation), which is worthy of in-depth study. A deeper understanding of the functions of hyaluronic acid will help us to better select the right type of hyaluronic acid and realise its functions in aesthetic medicine and the treatment of pigmented diseases.
Reference
[1] Passeron T,Picardo M.Melasma,a photoaging disorder[J].Pig- ment Cell Melanoma Res,2018,31 (4) :461-465.
[2] Han A,Chien AL,Kang S.Photoaging[J].Dermatol Clin,2014, 32(3):291-299.
[3] Bukhari SNA,RoswandI NL,Waqas M,et al.Hyaluronic acid,a promising skin rejuvenating biomedicine:A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricos- metic effects[J].Int J Biol Macromol,2018,120 ( Pt B ):1682 - 1695.
[4]KobayashiT,ChanmeeT,Itano N.Hyaluronan:Metabolism and function[J].Biomolecules,2020,10( 11):1525.
[5]Cowman MK,Lee HG,Schwertfeger KL,et al.The content and size of hyaluronan in biological fluids and tissues[J].Front Immunol, 2015,6 :261.
[6]Weigel PH.Planning,evaluating and vetting receptor signaling stud-ies to assess hyaluronan size-dependence and specificity[J].Glyco- biology,2017,27 (9) :796-799.
[7] D'mello SA,Finlay GJ,Baguley BC,et al.Signaling pathways in melanogenesis[J].Int J Mol Sci,2016,17 (7):1144.
[8]Pillaiyar T,Manickam M,Jung SH.Recent development of signaling pathways inhibitors of melanogenesis[J].Cell Signal,2017,40:99 - 115.
[9] Silpa-archa N,Kohli I,Chaowattanapanit S,et al.Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology,patho- genesis,clinical presentation,and noninvasive assessment technique [J].J Am Acad Dermatol,2017,77 (4):591-605.
[10] Sies H,Stahl W.Nutritional protection against skin damage from sunlight[J].Annu Rev Nutr,2004,24:173-200.
[11]Xuan Y,Yang Y,Xiang L,et al.The role of oxidative stress in the pathogenesis of vitiligo:A culprit for melanocyte death[J].Oxid Med Cell Longev,2022,2022:8498472.
[12]Decean H,Perde-Schrepler M,Tatomir C,et al.Modulation of the pro-inflammatory cytokines and matrix metalloproteinases produc- tion in co-cultivated human keratinocytes and melanocytes[J]. Arch Dermatol Res,2013,305 (8) :705-714.
[13]Yuan XH,Jin ZH.Paracrine regulation of melanogenesis[J].Br J Dermatol,2018,178 (3):632-639.
[14]Bastonini E,Kovacs D,Picardo M.Skin pigmentation and pigmen- tary disorders:Focus on epidermal / dermal cross-talk[J].Ann Der- matol,2016,28 (3):279-289.
[15] Kim M,Han JH,Kim JH,et al.Secreted frizzled-related protein 2 ( sFRP2) Functions as a melanogenic stimulator ; the role of sFRP2 in UV-induced hyperpigmentary disorders[J].J Invest Dermatol, 2016,136( 1):236-244.
[16]Jiang L,Huang J,Lu J,et al.Ganoderma lucidum polysaccharide reduces melanogenesis by inhibiting the paracrine effects of kerati- nocytes and fibroblasts via IL-6 / STAT3 / FGF2 pathway[J].J Cell Physiol,2019,234( 12):22799-22808.
[17] Kovacs D,Cardinali G,Aspite N,et al.Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo[J]. Br J Dermatol,2010,163 (5):1020 -1027.
[18] Serre C,Busuttil V,Botto JM.Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation[J].Int J Cosmet Sci, 2018,40(4):328-347.
[19]Fu C,Chen J,Lu J,et al.Roles of inflammation factors in melano- genesis ( Review) [J].Mol Med Rep,2020,21 ( 3 ):1421 - 1430.
[20]Yoshida M,Takahashi Y,Inoue S.Histamine induces melanogene- sis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes[J].J Invest Dermatol, 2000,114(2):334-342.
[21]Yoon JE,Kim Y,Kwon S,et al.Senescent fibroblasts drive ageing pigmentation:A potential therapeutic target for senile lentigo[J]. Theranostics,2018,8 ( 17) :4620-4632.
[22] Kim Y,Kang B,Kim JC,et al.Senescent fibroblast-derived GDF15 induces skin pigmentation[J].J Invest Dermatol,2020, 140( 12):2478-2486.
[23]Xu Z,Chen L,Jiang M,et al.CCN1 / Cyr61 stimulates melanogen- esis through Integrin alpha6beta1,p38 MAPK,and ERK1 /2 signaling pathways in human epidermal melanocytes[J].J Invest Der- matol,2018,138 (8):1825 -1833.
[24]Lim HK,Suh DH,Lee SJ,et al.Rejuvenation effects of hyaluronic acid injection on nasojugal groove:prospective randomized split face clinical controlled study[J].J Cosmet Laser Ther,2014,16 ( 1):32-36.
[25] HONG Wei-Song, QIAN Guo-pei, XU Ai-E. Effects of sodium vitrate on the biological activity of melanocytes[J]. Effect of sodium vitrate on the biological activity of melanocytes[J]. Chinese Journal of Dermatology,2011,44(7) :491-493.
[26]Muto J,Sayama K,Gallo RL,et al.Emerging evidence for the es- sential role of hyaluronan in cutaneous biology[J].J Dermatol Sci, 2019,94( 1):190 -195.
[27]Litwiniuk M,Krejner A,Speyrer MS,et al.Hyaluronic acid in in- flammation and tissue regeneration[J].Wounds,2016,28 (3) :78 - 88.
[28]Takabe P,Karna R , Rauhala L,et al.Melanocyte hyaluronan coat fragmentation enhances the UVB-induced TLR-4 receptor signaling and expression of proinflammatory mediators IL6,IL8,CXCL1, and CXCL10 via NF-kappaB Activation[J].J Invest Dermatol, 2019,139(9):1993-2003.
[29] Marinho A,Nunes C,Reis S.Hyaluronic acid:A key ingredient in the therapy of inflammation [J]. Biomolecules, 2021, 11 ( 10):1518.
[30] Campo GM,Avenoso A,Campo S,et al.Reduction of DNA frag- mentation and hydroxyl radical production by hyaluronic acid and chondroitin-4-sulphate in iron plus ascorbate-induced oxidative stress in fibroblast cultures[J].Free Radic Res,2004,38 (6 ): 601-611.
[31] Cirillo N,Vicidomini A,Mccullough M,et al.A hyaluronic acid- based compound inhibits fibroblast senescence induced by oxidative stress in vitro and prevents oral mucositis in vivo[J].J Cell Physi- ol,2015,230(7):1421 -1429.
[32] Maytin EV.Hyaluronan:More than just a wrinkle filler[J].Glyco- biology,2016,26(6):553-559.
[33] Bourguignon LY,Bikle D.Selective hyaluronan-CD44 signaling promotes miRNA-21 expression and interacts with vitamin D func- tion during cutaneous squamous cell carcinomas progression follow- ing UV irradiation[J].Front Immunol,2015,6 :224.
[34] Bourguignon LY,Wong G,Xia W,et al.Selective matrix (hyalu- ronan) interaction with CD44 and RhoGTPase signaling promotes keratinocyte functions and overcomes age-related epidermal dys- function[J].J Dermatol Sci,2013,72( 1):32-44.
[35] Lagatta A,D' agostino A,Schiraldi C,et al.A biophysically-defined hyaluronic acid-based compound accelerates migration and stimulates the production of keratinocyte-derived neuromodulators [J].Cell Adh Migr,2019,13 ( 1):23-32.
[36] Lee SG,Yoon MS,Kim DH,et al.Hyaluronan Oligosaccharides Improve Rosacea-Like Phenotype through Anti-Inflammatory and Epidermal Barrier-Improving Effects[J].Ann Dermatol,2020,32 (3):189 -196.
[37]Hu L,Nomura S,Sato Y,et al.Anti-inflammatory effects of differ- ential molecular weight Hyaluronic acids on UVB-induced calpro- tectin-mediated keratinocyte inflammation[J / OL].J Dermatol Sci, 2022.[2022-06-10 ]. https: / /www. jdsjournal. com / article /S0923-1811 (22) 00158-X /fulltext.
[38] David-Raoudi M,Tranchepain F,Deschrevel B,et al.Differential effects of hyaluronan and its fragments on fibroblasts:relation to wound healing[J].Wound Repair Regen,2008,16 (2 ):274 - 287.
[39] Ciccone V,Zazzetta M,Morbidelli L.Comparison of the effect of two hyaluronic acid preparations on fibroblast and endothelial cell functions related to angiogenesis[J].Cells,2019,8 ( 12):1479.
[40]Asparuhova MB,Kiryak D,Eliezer M,et al.Activity of two hyalu- ronan preparations on primary human oral fibroblasts[J].J Perio- dontal Res,2019,54( 1):33-45.
[41] Quan T,Wang F,Shao Y,et al.Enhancing structural support of the dermal microenvironment activates fibroblasts , endothelial cells,and keratinocytes in aged human skin in vivo[J].J Invest Dermatol,2013,133 (3):658-667.
[42] Radrezza S,Baron G,Nukala SB,et al.Advanced quantitative proteomics to evaluate molecular effects of low-molecular-weight hyaluronic acid in human dermal fibroblasts[J].J Pharm Biomed Anal,2020,185:113199.
[43]Lorén CE,Dahl CP,Do L,et al.Low molecular mass myocardial hyaluronan in human hypertrophic cardiomyopathy [J].Cells, 2019,8 (2):97.
[44] Olsson M,Bremer L,Aulin C,et al.Fragmented hyaluronan has no alarmin function assessed in arthritis synovial fibroblast and chondrocyte cultures [J]. Innate Immun, 2018, 24 ( 2 ):131 -141.
[45]Zheng BW,Wang BY,Xiao WL,et al.Different molecular weight hyaluronic acid alleviates inflammation response in DNFB-induced mice atopic dermatitis and LPS-induced RAW 264.7 cells[J].Life Sci,2022,301:120591.
[46]Zhou J,Ling J,Wang Y,et al.Cross-talk between interferon-gam- ma and interleukin-18 in melanogenesis[J].J Photochem Photobiol B,2016,163:133 -143.
[47] D'agostino A,La Gatta A,Stellavato A,et al.Potential of biofer- mentative unsulfated chondroitin and hyaluronic acid in dermal re- pair[J].Int J Mol Sci,2022,23 (3):1686.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese