What Is Hyaluronic Acid?
Hyaluronic acid (HA) is a high-molecular-weight linear macromolecular acidic mucopolysaccharide composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine [1]. Hyaluronic acid was first isolated from the vitreous humour of cattle in 1934, and it was discovered that hyaluronic acid is also widely found in the interstitial matrix of connective tissue in animals and humans. Among these, the vitreous humour of the eye, skin, umbilical cord, cartilage and synovial fluid of joints have high levels of hyaluronic acid. Hyaluronic acid from different sources has basically the same structure, but hyaluronic acid from different sources has different molecular weights[2]. As a multifunctional matrix in the body, hyaluronic acid has important physiological functions such as regulating cell proliferation, differentiation, migration, lubricating joints, protecting cartilage, promoting wound healing, resisting oxidation, and anti-aging.
Hyaluronic acid has a strong water-retaining effect, and its moisturising effect is higher than that of other moisturising substances found in nature. It is known as an ideal natural moisturising factor and has been widely used in clinical medicine and cosmetics production. With the approval of hyaluronic acid as a new raw material for food this year, the application fields of hyaluronic acid are constantly expanding. At the same time, consumers' health awareness is constantly improving, and the demand for hyaluronic acid raw materials is constantly expanding. Industrial preparation of high-quality hyaluronic acid is essential. This article provides an overview of the physiological functions, preparation, separation and purification, and application fields of natural hyaluronic acid, with the aim of providing a reference for the development and utilisation of hyaluronic acid.
1 Distribution and physiological functions of hyaluronic acid in the body
1.1 Distribution of hyaluronic acid in the body
Natural hyaluronic acid is widely distributed in various tissues of higher animals, although the amount varies. It is mainly distributed in the cell matrix and lubricating fluid, including human umbilical cord, synovial fluid, skin, thoracic lymphatic fluid, vitreous humour, and rooster comb. The rooster comb is currently the animal tissue with the highest hyaluronic acid content. The hyaluronic acid content of various organisms is shown in Table 1 [3]. Hyaluronic acid is widely distributed in various tissues of the human body. The distribution of hyaluronic acid in the tissues of different organisms is basically the same, with the main difference being in molecular weight. The molecular weight of hyaluronic acid in normal biological tissues is approximately 1000–8000 kDa. Different molecular weights stimulate different receptors or pathways in three-dimensional structures, exerting different effects [4].
1.2 Physiological functions of hyaluronic acid
1.2.1 Lubricates joints and protects cartilage
Hyaluronic acid is widely distributed in the intercellular matrix and cell matrix. It is the main component of synovial fluid in the joints and is distributed on the surfaces of cartilage and ligaments. Hyaluronic acid has good viscoelasticity. When walking, the synovial fluid is viscous to reduce joint friction. When performing high-impact actions such as running, the synovial fluid is elastic to buffer the stress on the joints. When the joint is under load, the synovial fluid changes from a fluid to an elastic body to protect the articular cartilage [5]. There is a lot of evidence to suggest that osteoarthritis in elderly patients is caused by oxidative stress. Osteoarthritis is the wear and tear of articular cartilage. When attacked by reactive oxygen species, the long-chain hyaluronic acid is broken down into hyaluronic acid fragments, weakening the overall structure of the cartilage [6].
1.2.2 Promotes wound healing
The wound healing process can be divided into four stages: hemostasis, inflammation, proliferation and maturation. When an injury occurs, the amount of hyaluronic acid in the wound increases. Due to its large molecular weight, hyaluronic acid is used as an early temporary structure [7]. During the inflammation stage, damaged cells begin to secrete exudates containing salts, water and proteins [8]. This stage is characterised by redness and heat at the injury site, pain and dysfunction [9]. Hyaluronic acid binds to the CD44 receptor on the surface of leukocytes and endothelial cells, causing fewer leukocytes to migrate to the inflammation site and reducing the degree of wound swelling [10]. The CD44 receptor plays an important role in the inflammatory response, in which high molecular weight hyaluronic acid stimulates the anti-inflammatory response and low molecular weight hyaluronic acid induces the inflammatory response. In the proliferation phase, the wound is rebuilt with new collagen tissue, the extracellular matrix is secreted, and the wound begins to shrink under the action of myofibroblasts [11]. In the maturation phase, the unorganized collagen forms cross-links, reducing scarring and enhancing the elasticity of the skin in the wound area.
1.2.3 Regulating cell proliferation, migration and differentiation
Hyaluronic acid is an important regulatory factor affecting the processes of cell proliferation, migration and differentiation. The presence of hyaluronic acid helps to hydrate local tissues, weaken the fixation of cells to the extracellular matrix, and promote cell separation, migration and even division. The hyaluronic acid receptors on the cell surface can also be linked to some kinases related to cell movement [12].
During the early stages of mitosis, hyaluronic acid levels increase, and levels drop sharply after mitosis enters the G1 phase (the period between the completion of the previous mitosis and the beginning of the synthesis phase). High levels of hyaluronic acid cause the release of growth factors, and by forming an extra-cellular membrane, it affects cell-cell interactions and accelerates cell proliferation [13]. However, it has not yet been observed that hyaluronic acid directly promotes mitotic activity. This signalling and regulatory effect of hyaluronic acid is related to its molecular weight. Different molecular weights trigger different signalling pathways. Low molecular weight hyaluronic acid induces cell proliferation. In addition, low molecular weight hyaluronic acid can enhance the expression of pro-inflammatory factors, while high molecular weight hyaluronic acid has the opposite effect [14].
1.2.4 Angiogenic effect
It has been reported that low molecular weight hyaluronic acid can stimulate the expression of signal molecules, stimulate the proliferation and migration of vascular endothelial cells, and high molecular weight hyaluronic acid can inhibit endothelial cell proliferation and migration, thus having an anti-angiogenic effect [15]. However, most of the evidence supporting the effect of hyaluronic acid on cell growth has been produced using tumour xenografts. Some data show that injecting low molecular weight hyaluronic acid can inhibit tumour growth [16], which conflicts with the above concept and indicates that there may be more complex pathways and interactions that require further research.
1.2.5 Antioxidant activity
Studies have found that hyaluronic acid can eliminate free radicals and has a certain degree of antioxidant activity. High molecular weight hyaluronic acid can protect cells from the effects of reactive oxygen species, which, in excess, can damage proteins, lipids and DNA. Some of the antioxidant properties of hyaluronic acid include its ability to reduce ultraviolet-induced apoptosis and acid-induced DNA damage [17]. Feng Ning et al. [18] studied the serum superoxide dismutase activity after oral administration of hyaluronic acid and found that hyaluronic acid has an in vivo antioxidant effect. Yu Haihui et al. [19] found that the mucus hyaluronic acid of Andrias davidianus has a certain in vitro antioxidant activity and can scavenge DPPH.,.OH, ABTS+.and reduce Fe3+. Some scholars speculate that the antioxidant properties of hyaluronic acid are due to the hydroxyl functional groups in the structure of hyaluronic acid, which can absorb reactive oxygen species [14].
1.2.6 Anti-aging effect
Studies have found that the amount of hyaluronic acid in the human body decreases with age. Compared to the age of 20, the amount of hyaluronic acid decreases by 75% at the age of 60. The older the person, the lower the amount of hyaluronic acid in the body. The amount of hyaluronic acid in the body also varies among people of the same age. People with a high amount of hyaluronic acid in the body look younger, while people with symptoms of aging have significantly lower amounts of hyaluronic acid in the body [20]. A decrease in the amount of hyaluronic acid in the skin reduces the space filled by the intercellular gel-like matrix, causing the cells to be arranged closely together. Collagen loses water and hardens, making the skin rough and losing its elasticity. Studies have found that hyaluronic acid can heal skin damage caused by ultraviolet radiation, and high concentrations of hyaluronic acid can affect collagen expression [21].

In summary, the physiological functions of hyaluronic acid are closely related to its molecular weight. Hyaluronic acids with different molecular weights play different roles in physiological functions such as wound healing, regulation of cell proliferation, migration, differentiation, angiogenesis and antioxidant activity. Low molecular weight hyaluronic acid induces inflammatory responses, induces cell proliferation, stimulates the proliferation and migration of vascular endothelial cells, and high molecular weight hyaluronic acid has better antioxidant activity than low molecular weight hyaluronic acid. This difference in physiological function leads to differences in its ultimate application in products.
2 Structure and properties of hyaluronic acid
2.1 Structure of hyaluronic acid
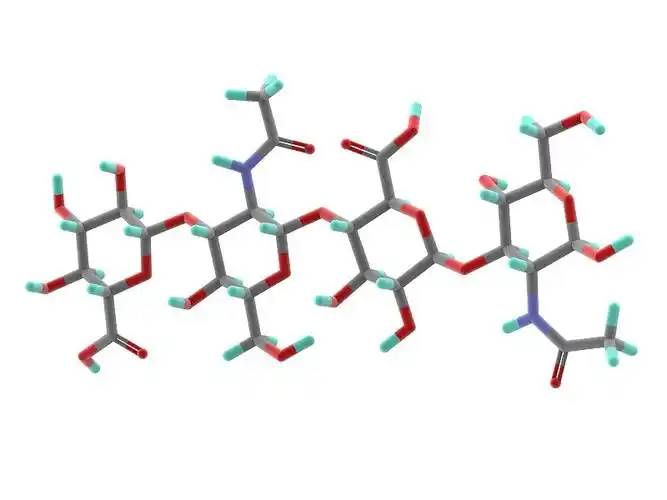
Hyaluronic acid is a high molecular weight acidic mucopolysaccharide composed of alternating glucose units linked by β-1,3-glycosidic bonds and N-acetylglucosamine units linked by β-1,4-glycosidic bonds. The primary structure of hyaluronic acid is shown in Figure 1 [22]. Hyaluronic acid, as the only currently discovered non-sulfur-containing glycosaminoglycan, differs from common glycosaminoglycans in that it is synthesized via cell membrane surface membrane proteins rather than by the cell's Golgi apparatus [23].
2.2 Physical and chemical properties of hyaluronic acid
Hyaluronic acid is a white amorphous solid with the common properties of acidic mucopolysaccharides. It is soluble in water but insoluble in organic solvents such as ethanol [24]. Hyaluronic acid aqueous solutions have specific rheological properties, with good viscoelasticity. Low concentrations or small molecular weight hyaluronic acid exist as monomers, with little change in viscosity. High molecular weight and high concentration hyaluronic acid has good viscoelasticity[25], and exhibits non-Newtonian fluid characteristics, making it very suitable for simulating synovial fluid. The viscoelasticity of synovial fluid is related to the concentration of hyaluronic acid[13].
A reasonable change in the molecular weight and solution concentration of hyaluronic acid can obtain better viscoelasticity. Due to the presence of hydrogen bonds between the monosaccharides in the hyaluronic acid molecule chain, hyaluronic acid at low concentrations can also form a unique honeycomb network structure, allowing hyaluronic acid to adsorb about 1000 times its own moisture, which has strong moisturising properties[26]. Hyaluronic acid with different molecular weights has different physical and chemical properties. High molecular weight hyaluronic acid has higher viscosity, while the random curled structure of long-chain hyaluronic acid is more stable, and short chains are more likely to expand [27]. The method and biological pathway by which cells differentiate between high molecular weight and low molecular weight hyaluronic acid are still unknown.
3 Preparation and purification of hyaluronic acid
3.1 Sources of hyaluronic acid
3.1.1 Animal tissue sources
Animal tissue sources can be divided into terrestrial sources and marine sources. Currently, hyaluronic acid is mainly extracted from terrestrial animal tissues such as the rooster comb, human umbilical cord, egg shell membrane, and pig skin. The rooster comb is widely used for hyaluronic acid extraction because it is an animal tissue with a high hyaluronic acid content. Due to the limited supply of terrestrial animal tissue, large-scale production is not possible. Researchers are constantly trying to extract hyaluronic acid from other animal tissues or other sources of raw materials. Marine biological resources such as animal residues, waste, and by-products have always received widespread attention due to their long-term economic and environmental benefits.
They have significant potential as a source of substances such as hyaluronic acid [28]. Researchers have extracted hyaluronic acid from biological tissues such as the ocular vitreous of marine organisms such as the eyes of cuttlefish, squid, tuna, frog skin, fish mucus, and the aqueous humour of freshwater mussels [19, 25, 29]. Yi et al. [29] first extracted hyaluronic acid from the ocular vitreous of tuna, with a final extraction rate of 0.013%. and Haihui Yu et al. [19] extracted it from the surface mucus of the Chinese giant salamander. When the amount of added trypsin was 1.5%, the yield of hyaluronic acid was 1.7041 mg/g. The structure of the extracted hyaluronic acid was the same as the standard product. Compared with the tissues of land animals such as the rooster comb and umbilical cord, the extraction rate was low, but it can be used as a stable source of hyaluronic acid extraction.
3.1.2 Microbial fermentation source pathway
Hyaluronic acid is widely distributed in the cell envelope of some bacteria, protecting the cells from oxygen damage. Previous research on hyaluronic acid in bacteria was mainly aimed at exploring the composition and function of the envelope. Shiseido in Japan was the first to apply the fermentation method to the industrial production of hyaluronic acid. The synthesis of hyaluronic acid in the cell is complex and continuous. Glucose is converted to gluco-6-phosphate by glucokinase, and then to the precursors uridine diphosphate N-acetylglucosamine and uridine diphosphate glucuronic acid by various enzymes such as isomerase and glucuronic acid phosphatase and other enzymes to produce the precursor substances uridine diphosphate-N-acetyl-glucosamine and uridine diphosphate-glucuronic acid, which are alternately added to the hyaluronic acid molecule chain under the action of hyaluronic acid synthase [30].
Streptococcus zooepidemicus from group C is the main source of hyaluronic acid [31]. Due to its pathogenicity and endotoxins in wild-type strains, it has become common practice in actual production to modify wild-type strains and produce hyaluronic acid through non-pathogenic strains [32]. The main means of strain treatment are genetic engineering, mutagenesis breeding and protoplast breeding. JIN et al. [33] improved the hyaluronic acid synthesis pathway of Bacillus subtilis by integrating the leech-derived hyaluronidase LHyal gene, regulating the expression of LHyal by sequence optimization and N-terminal fusion His tag strategy, and obtaining a high-yield strain that accumulates hyaluronic acid to 19.38 g/L after 100 h of fermentation in a 3 L fermenter. Wei Chaobao et al. [34] selected Streptococcus zooepidemicus, which has a short production cycle and high strength, for construction on this basis, and obtained a high-yield strain that can alleviate the problem of dissolved oxygen during fermentation. At present, the synthesis of hyaluronic acid has been achieved through the heterologous expression of hyaluronic acid synthase in different hosts such as Bacillus subtilis [35], Lactobacillus [36] and Bacillus glutamicum [37].
3.2 Preparation of hyaluronic acid
3.2.1 Preparation of hyaluronic acid from animal tissue sources
The production of hyaluronic acid from animal tissue sources often involves tissue extraction. The complete process includes pretreatment, extraction, separation and purification, drying, etc. The processing technology is relatively mature, the extraction method is simple, and most of the extracted hyaluronic acid is of high molecular weight [38], with high viscosity and good moisturising properties. It is mainly used in the pharmaceutical and cosmetics industries. The main extraction methods are salt extraction and enzyme extraction. The addition of inorganic salts and enzymes can break the complexation of hyaluronic acid and proteins in animal tissue. In addition, enzymes can hydrolyse impurities such as proteins and nucleic acids, which is beneficial for the extraction of hyaluronic acid [39].
KALKANDELEN et al. [40] successfully extracted hyaluronic acid from the comb of a chicken by defatting the tissue homogenate with acetone and extracting it multiple times with a sodium acetate solution. However, the tissue extraction method is complicated and the extraction rate is low. Enzyme extraction has become a research hotspot due to its high efficiency. Currently, the commonly used enzymes for extraction include neutral proteases, pepsin, trypsin, papain, etc. Ürgeová et al. [41] compared the results of extracting hyaluronic acid from eggshell membranes using pepsin, trypsin and papain. The results showed that trypsin was more effective than the other two enzymes. At a pH of 8, 37 °C and a trypsin dosage of 50 U/g for enzymolysis of eggshell membranes, the hyaluronic acid extraction rate was 44.82 mg/g eggshell membrane. In order to obtain a better extraction effect, enzyme mixtures or ultrasound are often used in experiments to assist extraction. Chen Shengjun et al. [42] used ultrasound (200 W, 30 kHz) to assist trypsin and complex protease to extract from tilapia eyes. After optimisation, the hyaluronic acid yield was 11.44%, which is about 5% higher than that obtained by simple enzymatic hydrolysis.
3.2.2 Preparation of microbial hyaluronic acid
The microbial fermentation process mainly includes the following steps: seed culture, fermentation, separation and purification, and drying. At present, research on improving the extraction efficiency of microbial fermentation mainly focuses on cultivating excellent strains, selecting suitable culture media, and optimizing fermentation conditions. There have been many studies on obtaining high yields of hyaluronic acid by controlling the conditions of the culture medium and fermentation process. Compared to the preparation of hyaluronic acid by tissue extraction, one advantage of the microbial fermentation method is that the molecular weight of hyaluronic acid can be controlled during the fermentation process. This is also the main content of current research on the fermentation process of hyaluronic acid. The regulation of hyaluronic acid molecular weight is affected by hyaluronic acid synthase and the relative strength of its binding to the substrate, the concentration ratio of hyaluronic acid precursor substances to hyaluronic acid synthase concentration [43]. Fructose-6-phosphate produced from carbon sources can be used to synthesise lactic acid, inhibit bacterial growth and hyaluronic acid synthesis. It is possible to inhibit other pathways that compete with hyaluronic acid for carbon sources (such as glycolytic pathways), so that more carbon sources can be used for hyaluronic acid synthesis, thereby increasing hyaluronic acid production and molecular weight [44].
The balance of metabolic fluxes can affect the molecular weight of hyaluronic acid [45]. Research has been carried out on fermentation conditions that affect hyaluronic acid production and molecular weight, such as temperature, aeration, pH, stirring speed, etc. Certain research has been conducted on fermentation conditions that affect the yield and molecular weight of hyaluronic acid, such as Liu Jinlong et al. [46] who studied the effect of fermentation conditions on the molecular weight of hyaluronic acid synthesized by Streptococcus equi subsp. zooecium. Batch culture fermentation mode is more conducive to the production of high molecular weight hyaluronic acid than glucose feeding culture mode. Within the range of 0–45% dissolved oxygen concentration, the relative molecular weight increased by 109.4% with increasing dissolved oxygen levels. Low temperatures are conducive to hyaluronic acid synthesis, and the yield and molecular weight of hyaluronic acid are relatively high at low temperatures. At 33 °C, the yield and molecular weight of hyaluronic acid are 4.41 g/L and 2.54×106, respectively. pH has a different effect on the yield and molecular weight of hyaluronic acid. The highest yield of hyaluronic acid (3.72 g/L) was obtained at pH 7, and the lowest yield (3.01 g/L) was obtained at pH 8. However, the highest molecular weight (2.38×106) was obtained at pH 8, indicating that high-quality hyaluronic acid production can be achieved by controlling the fermentation process conditions during the production process.
Animal tissue extraction and microbial fermentation are the two most common methods for producing hyaluronic acid. Tissue extraction is used to extract hyaluronic acid from animal tissue. This method was often used in the early days, but the extraction process is complicated, the yield of hyaluronic acid is low, and there are restrictions on the source of raw materials. With the progress of science and technology, fermentation has become the mainstream method for industrial production of hyaluronic acid due to its advantages of low cost, high yield, and ease of large-scale production. With the continuous improvement of the preparation method, people's demand for hyaluronic acid production has gradually shifted from high yield to high quality. Current research focuses on producing hyaluronic acid with specific molecular weight through genetic engineering, mutagenesis and other methods to meet the needs of hyaluronic acid in different applications. Establishing an efficient and safe preparation method of hyaluronic acid to produce hyaluronic acid with specific molecular weight that meets various application scenarios will become a research hotspot.
3.3 Separation and purification of hyaluronic acid
Regardless of whether the tissue extraction method or the fermentation method is used, the crude hyaluronic acid extracted contains some proteins, nucleic acids and other impurities, which need to be separated and purified to obtain pure hyaluronic acid. According to the principle of separation and purification, it can be roughly divided into three methods: precipitation, filtration and adsorption.
3.3.1 Precipitation
The main precipitation methods are quaternary ammonium salt precipitation and organic solvent precipitation. The principle of the quaternary ammonium salt purification method is that the quaternary ammonium salt and hyaluronic acid have different charges in an aqueous solution. The two form a complex and precipitate out in a low salt solution, but dissociate and dissolve in a high salt solution, thereby achieving the purpose of removing impurities that do not complex with hyaluronic acid. Commonly used quaternary ammonium salts include cetylpyridinium bromide (CPB), cetyltrimethylammonium bromide (CTAB), cetylpyridinium chloride (CPC) and other long-chain quaternary ammonium salts [47]. This method of purification yields high-purity hyaluronic acid with good results, and can remove impurities that do not complex with quaternary ammonium salts. The organic solvent precipitation method mainly affects the dielectric constant of the medium to cause intra- and intermolecular aggregation, thereby achieving the purpose of removing proteins [48].
Compared with restricted reagents such as chloroform and acetone, ethanol is more widely used due to its safety and low cost. Song Lei et al. [49] optimised the factors affecting the purity of hyaluronic acid after ethanol extraction by combining plate and frame filtration to obtain a high purity hyaluronic acid with a content of 93.71%. CAVALCANTI et al. [50] investigated the effect of the ratio of ethanol to fermentation broth on the dielectric constant and the effect of pH on the purification of hyaluronic acid. At a pH of 4 and an ethanol fermentation liquid ratio of 2:1, the purity of the hyaluronic acid was 55%, the recovery rate was 85%, and the precipitation of the organic solvent was used for the initial purification of the hyaluronic acid with good results.
3.3.2 Filtration
The principle of filtration is to retain particles on a porous membrane based on particle size. Compared to organic solvent precipitation, filtration does not involve the consumption of organic solvents, is simple to implement, and can be industrialised. However, the protein removal effect of filtration alone is not good, and pore blockage will occur as purification progresses, limiting its application in the purification of hyaluronic acid. Tangential filtration or the use of filter aids can greatly reduce pore blockage [51]. GÖZKE et al.[52] proposed an electrofiltration technique combining membrane filtration and electrophoresis. The electric field has a strong promoting effect on the filtration of hyaluronic acid. Compared with conventional filtration, the concentration factor based on the sample osmotic mass is increased by nearly 4 times in the same experimental time. Moreover, this filtration method will not negatively affect the molecular structure and average molecular weight of hyaluronic acid, providing new possibilities for the downstream purification process of hyaluronic acid.

3.3.3 Adsorption
Adsorption is a purification method for hyaluronic acid that is based on the selective retention of compounds on the surface of a porous solid. Commonly used adsorbents include activated carbon, resins, and silica gel. Activated carbon is an ideal material for separating and purifying hyaluronic acid because it has strong adsorption of proteins and nucleic acids and weak adsorption of high-molecular-weight neutral polysaccharides. Wei Linna et al. [53] used ethanol precipitation combined with activated carbon adsorption in the process of extracting hyaluronic acid from plateau zokor tissues. The recovery rate of the extracted hyaluronic acid can reach 72.73%. CAVALCANTI et al. [50] found that the structure of hyaluronic acid at different pH values has an important effect on the precipitation performance. At pH 4, the recovery rate of hyaluronic acid was 85%, and at pH 7, the recovery rate of hyaluronic acid was 70%. During the use of activated carbon, adjusting the pH to an appropriate value can increase the recovery rate of hyaluronic acid.
Electrophoresis is a widely used method for separating proteins, and its separation efficiency is affected by the gel. Compared with other operations, it has a lower purification efficiency for hyaluronic acid. Ion exchange chromatography is also one of the widely used methods for purifying biological macromolecules. This method is gentle and does not cause changes in molecular structure, but it is relatively expensive. It is necessary to select suitable exchange resins and exchange conditions, and the operation is complex. It is mainly used in the production of medical grade hyaluronic acid. Ni Hangsheng et al. [54] used a strong acid cation exchange resin in tandem with a strong base anion exchange resin modified with a histidine group. The impurity proteins in the crude hyaluronic acid were purified by exchange adsorption with the strongly acidic cation exchanger in an acidic solution, and eluted with sodium chloride solution. The protein content of the obtained high-quality hyaluronic acid is less than 0.075%, the average molecular weight is greater than 9.41×105, and the yield of purified weight is 58%~61%.
Separation and purification is an essential step in the preparation of high-purity, high-quality hyaluronic acid. At present, there is relatively little research on the effect of various purification operations on the purity of hyaluronic acid during purification. CAVALCANTI et al. [51] expressed the degree of purification as a percentage of hyaluronic acid or protein in the solution, and summarized the change in the purity of hyaluronic acid during the purification process.
The hyaluronic acid fermentation broth derived from Streptococcus zooepidemicus first underwent an isopropanol precipitation operation, with a protein content of 14.1%; a silica gel adsorption operation, with a protein content of 4.5%; and a charcoal filter module combining filtration and adsorption, with a protein content of only 0.6%. Finally, the protein content reached 0.06% after dialysis filtration. Each separation and purification method has its own advantages and disadvantages. In actual industrial production, a reasonable combination of several separation and purification methods is often used to achieve the maximum effect, depending on the source of the raw materials and the different requirements of the end products.
4 Application of hyaluronic acid
4.1 Application in the food sector
Hyaluronic acid is widely used in the Japanese food market. In addition to health foods, it is also widely used in ordinary foods such as beverages, soft candies, and jams. In the US food market, hyaluronic acid is mainly used as a dietary supplement [55]. At present, the main products containing hyaluronic acid in China are health foods, and the main effect is to improve skin moisture. Cha Shenghua et al. [56] developed a kind of bird's nest can with sodium hyaluronate as the main raw material, which can effectively improve skin moisture without other adverse reactions. The main types on the market are capsules, oral administration, and powdered drinks. After hyaluronic acid is absorbed through oral digestion, the precursor of hyaluronic acid synthesis in the body increases, which increases the content of hyaluronic acid in the body and concentrates it in the skin tissue, thereby enhancing the skin's water retention capacity, softening the stratum corneum, further improving skin elasticity and reducing wrinkles[57].

4.2 Application in cosmetics and daily necessities
Hyaluronic acid is found in large quantities in the human body and other living tissues. It has extremely strong moisturising properties and is mainly used in cosmetics as a moisturising agent, thickener and emulsifier [58−59]. At present, almost all types of cosmetic formulations on the market contain hyaluronic acid. Hyaluronic acid can easily form a hydrated film on the skin to enhance the lubrication of the skin, promote the absorption of active substances by the skin, and to a certain extent, the formation of the film can isolate bacteria, which is beneficial to anti-inflammatory and repair of the skin and delay skin aging [60]. Hyaluronic acid is a component that exists in skin tissue itself, which is safer. In addition, as hyaluronic acid has an anti-inflammatory and restorative effect in the mouth, it can be added to toothpaste to provide a certain degree of moisturising and efficacy[61]. The application of hyaluronic acid in daily necessities is constantly expanding and deepening.

4.3 Medical applications
Hyaluronic acid is an important component of synovial fluid in the joints and plays an important physiological role in joint protection. Abnormal synthesis or metabolism of hyaluronic acid in the joints can lead to joint diseases. At this time, exogenous hyaluronic acid can be injected to supplement the synovial fluid and improve the physiological function of the joints[62]. Due to its unique physical and chemical properties and biocompatibility, hyaluronic acid is widely used in ophthalmic surgeries related to the retina and cataracts.
Hyaluronic acid is used as a filler in medical aesthetics to inject under the skin to eliminate facial wrinkles and scars and give the face a plump appearance[63]. Hyaluronic acid spray can be used to repair the patient's face after laser surgery, effectively restoring skin barrier damage[64]. Hyaluronic acid derivatives are also widely used in ophthalmic preparations. For example, sodium hyaluronate can replace the role of tear mucin and is used to treat dry eye disease and relieve dry eye symptoms [65]. Studies have found that the body's hyaluronic acid content will increase during the occurrence of many diseases. Therefore, clinically, the level of hyaluronic acid in the serum can be used to reflect changes in various diseases, which is of great significance for auxiliary diagnosis.
Hyaluronic acid is widely used in food, cosmetics, daily necessities and medicine. Its application in functional skin care products, ophthalmology and orthopedics is relatively mature. There is still huge potential for its application in the food industry. Oral hyaluronic acid is milder than external application and injection, and can stimulate vitality from the inside out. In January 2021, the National Health Commission approved the addition of hyaluronic acid as a new raw material for food to be added to ordinary foods. This indicates that the application of hyaluronic acid in the food sector will see large-scale growth. In addition, there are many modification sites on the hyaluronic acid molecule, and modification of its active groups, such as cross-linking, esterification, and grafting, gives it better physicochemical properties and resistance to enzymatic hydrolysis [66], allowing hyaluronic acid to be used in more complex environments. With technological progress, the application of hyaluronic acid in various fields will become more and more in-depth.
5 Conclusions and outlook
Hyaluronic acid has important physical and chemical properties and physiological functions. It has a wide range of applications and a large market demand. Global sales of hyaluronic acid raw materials are showing an upward trend. At present, the main methods for industrial production of hyaluronic acid are animal tissue extraction and microbial fermentation. The microbial fermentation method has the advantages of low cost and easy mass production. With the continuous expansion of hyaluronic acid application scenarios and the growing market demand, establishing an efficient and safe hyaluronic acid extraction and purification process, modifying hyaluronic acid molecules to produce specific molecular weight hyaluronic acid that meets different application scenarios will become research hotspots.
Reference
[1]ZHANG K, JIAN J, ZHANG Z P. Research progress on the structure, properties, modi- fication and application of hyaluronic acid[J]. Polymer Bulletin, 2015,9:217−226.
[2] JEON O, SONG S J, LEE K, et al. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities[J]. Carbohydrate Polymers,2007, 70(3):251−257.
[3] KOGAN G, ŠOLTÉS L, STERN R, et al. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications[J]. Biotechnology Letters,2006,29(1):17−25.
[4] COWMAN M K, LEE HG, SCHWERTFEGERK L, et al. The content and size of hyaluronan in biological fluids and tissues[J]. Frontiers in Immunology,2015(6):261.
[5]HLAVÁCEK M. The role of synovial fluid filtration by cartil- age in lubrication of synovial joints-I. Mixture model of synovial fluid[J]. Journal of Biomechanics,1993,26(10):1145−1160.
[6] MAREK P, MAłGORZATA K, JACEK K, et al. The oxidat- ive stress in knee osteoarthritis patients an attempt of evaluation of possible compensatory effects occurring in the disease developme- nt [J]. Medicina,2019,55(5):150.
[7] VOIGT J, VICKIE R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized con- trolled trials[J]. Wound Repair and Regeneration,2012,20(3):317− 331.
[8] HOTAMISLIGILG S. Inflammation, metaflammation and im- munometabolic disorders[J]. Nature,2017,542(7640):177−185.
[9] KOJOUHAROV H V, TREJO I, CHEN B M. Modeling the ef- fects of inflammation in bone fracture healing[C]// American Insti- tute of Physics Conference Series. American Institute of Physics Conference Series, 2017.
[10] GRISHMA S P, ROHAN B, CHARLES D E. Numerical in- vestigation of leukocyte rolling, adhesion and bond formation on surface coated with varying p-selectin density[J]. Cell Press, 2019, 116(3): 18.
[11] LANDÉN N X, LI D Q, STÅHLE M. Transition from in- flammation to proliferation: A critical step during wound healing[J]. Cellular and Molecular Life Sciences: Cmls,2016,73(20):3861− 3885.
[12] JOHN CH W Y, ABATANGELO G. Functions of hyaluron- an in wound repair[J]. Wound Repair and Regeneration,1999,7(2): 79−89.
[13] HUI E, GIMENO K I, GUAN G, et al. Spatiotemporal con- trol of viscoelasticity in phototunable hyaluronic acid hydrogels[J]. Biomacromolecules,2019,20(11):4126−4134.
[14] DOVEDYTIS M, LIU Z J, BARTLETT S. Hyaluronic acid and its biomedical applications: A review[J]. Engineered Regenera-tion,2020,1:102−113.
[15] SLEVIN M, KRUPINSKI J, GAFFNEY J, et al Hyaluronan- mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways[J]. Matrix Biology: Journal of the International Society for Matrix Biology, 2007, 26(1): 58–68.
[16] ZHONG Y N, KATHARINA G, CHENG L, et al. Hyaluron- ic acid-shelled acid-activatable paclitaxel prodrug micelles effect- ively target and treat CD44-overexpressing human breast tumor xenografts in vivo[J]. Biomaterials,2016:84.250−261.
[17] MOSELEY R, LEAVER M, WALKER M, et al. Comparis- on of the antioxidant properties of HYAFF ® -11p75, AQUACEL ® and hyaluronan towards reactive oxygen species in vitro[J]. Bioma- terials,2002,23(10):2255−2264.
[18]FENG N, SHI Y L, GUO F X, et al. Study on the effect of oral hyaluronic acid on skin moisture improvement and in vivo antioxidant effect[J]. Food and Drugs,2016,18(6):386−390.
[19]YU H H, LI W, TONG C Q. Extraction of hyaluronic acid from body surface mucus of giant salamander and its antioxidant activity[J]. Agricul- tural Products Processing,2018,10:18−21.
[20]GUO X P, HE Y L, SUN M L, et al. Application of hyaluronic acid in health products[J]. Chinese Journal of Biochemical Pharmaceutics,2002, 23(1):49−51.
[21]WU B J, NI H L, ZHU M L, et al. Research progress and application of hyaluronic acid[J]. Chinese Journal of Aesthetic Plastic Surgery,2018,29(4): 252−254.
[22] LAURENT T C. Biochemistry of hyaluronan[J]. Acta Oto- laryngologica Supplementum,1987,442:7−24.
[23]CHEN J S, WANG J Q, YI Y, et al. Research progress of hyaluronic acid and its derivatives[J]. Chinese Journal of Bioengineering,2015,35(2): 111−118.
[24] WANG C F. The application status of hy- aluronic acid[J]. Chinese Journal of Medical Devices,2018,42(1): 74−76,78.
[25] SHA K. Research on the extraction techno- logy of hyaluronic acid from the skin of Chinese forest frog[D]. Changchun: Jilin Agricultural University, 2004.
[26] JIANG S, LIANG H. Hyaluronic acid-a highly sought after beauty tool[J]. Healthy World,2020(2):25−27.
[27] MARY K. C, SHIRO M. Experimental approaches to hyalur- onan structure [J]. Carbohydrate Research,2005,340(5):791−809.
[28] TRIVEDI N, BAGHEL R S, BOTHWELL J, et al. An integ- rated process for the extraction of fuel and chemicals from marine macroalgal biomass[J]. Scientific Reports,2016,6(1):30728.
[29] YI Y, XU J, MEI J F, et al. Study on the extraction process of hyaluronic acid from tuna eyes[J]. Journal of Zhejiang University of Technology, 2018,46(3):276−281.
[30] ARINOBU Y, ATAMAS S P, OTSUKA T, et al. Molecular cloning and characterization of a putative mouse hyaluronan syn- thase[J]. Biological Chemistry,1996,271(38):23400−23406.
[31]DONATELLA C, ILEANA D I, ELISABETTA C, et al. En- gineering S. equi subsp. zooepidemicus towards concurrenttion of hyaluronic acid and chondroitin biopolymers of biomedical interest[J]. AMB Express,2017,7(1):61.
[32] PAN N C, PEREIRA H C B, SILVA M L C, et al. Improve- ment production of hyaluronicacid by Streptococcus zooepidemicus in sugarcane molassesugarcane molasses[J]. Biotechnology and Ap- plied Chemistry,2017,182(1):276−293.
[33] JIN P, KANG Z, YUAN P H, et al. Production of specific- molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168[J]. Metabolic Engineering 2016, 35: 21–30.
[34]WEI C B, DU G C, CHEN J, et al. Construction of an engineered strain of fermenting hyaluronic acid oligosaccharide Streptococcus zooepidemicus[J]. Chinese Journal of Biological Engineering,2019, 35(5):805−815.
[35] ADAM W, WESTBROO K, XIANG R, et al. Metabolic en- gineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis[J]. Metabolic Engineering,2018,47:401−413.
[36] SUNGUROğLU C, SEZGIN D E, AYTAR Ç P, et al. Higher titer hyaluronic acid production in recombinant Lactococcus lactis[J].Preparative Biochemistry & Biotechnology,2018,48(8): 734−742.
[37] CHENG F, YU H, STEPHANOPOULO G, et al. Engineer- ing Corynebacterium glutamicum for high titer biosynthesis of hya- luronic acid[J]. Metabolic Engineering,2019,55:276−289.
[38] YAMADA T, KAWASAKI T. Microbial synthesis of hyalur- onan and chitin: New approaches[J]. Biosci Bioeng, 2005, 99(6): 521–528.
[39] SADHASIVAM G, MUTHUVEL A. Isolation and character- ization of hyaluronic acid from marine organisms[J]. Advances in Food and Nutrition Research,2014,72:61−77.
[40] KALKANDELEN C, SU S, SAATCIOGLU E, et al. Hyalur- onic acid production and analysis from rooster comb[C]// 2020 Med- ical Technologies Congress (TIPTEKNO). Antalya, 2020: 1−4.
[41]ÜRGEOVÁ E, VULGANOVÁ K. Comparison of enzymatic hydrolysis of polysaccharides from eggshells membranes[J]. Nova- Biotechnologica et Chimica,2016,15(2):133−141.
[42]CHEN S J, CHEN H, GAO R C, et al. Technology conditions for the extraction of hyaluronic acid from tilapia eyes by ultrasonic-as- sisted enzymatic hydrolysis[J]. Journal of Nuclear Agriculture, 2014,28(8):1446−1452.
[43]GAO J J, YANG S L. Research progress on the production of high mo- lecular weight hyaluronic acid by microbial fermentation[J]. Chinese Journal of Bioengineering,2017,37(5):118−125.
[44]DONG Z H. Research on mutation breeding and molecular weight controllable technology of hyaluronic acid production by fermentation[D]. Hangzhou: Zheji- ang University of Technology, 2017.
[45] KARAMI M, SHAHRAKY M K, RANJBAR M, et al. Pre- paration, purification, and characterization of low-molecular-weight hyaluronic acid[J]. Biotechnology Letters,2021,43(1):133−142.
[46] LIU J L, ZHAO G Q, Li Z M, et al. Effect of culture condition on the molecular weight of hyaluronic acid synthesized by Streptococcus equisimilis[J]. Journal of Food Science and Biotechnology,2015,34(2):209−214.
[47] AMAGAI I, TASHIRO Y, OGAWA H. Improvement of the extraction procedure for hyaluronan from fish eyeball ansd the mole- cular characterization[J]. Fisheries Science,2009,75(3):805−810.
[48] LI Y, SHI S, YANG X, et al. The deproteinization, antioxid- ant acticities and inhibitory effect on a-amylase of polysaccharides from corn silk[J]. Biochem Biotechnol, 2019, 15(2): 83–90.
[49]SONG L, MENG G Q, GUO Y F, et al. Study on the extraction and purifica- tion process of hyaluronic acid in fermentation broth[J]. Shandong Agricultural Sciences,2017,49(3):134−139.
[50] CAVALCANTI A D D, MELO B A G, OLIVEIRA R C, et al. Recovery and purity of high molar mass bio-hyaluronic acid via precipitation strategies modulated by pH and sodium chloride[J]. Biochem Biotechnol, 2019, 188: 527–539.
[51] CAVALCANTI A, MELO B, FERREIRA B, et al. Perform- ance of the main downstream operations on hyaluronic acid purifica- tion[J]. Process Biochemistry,2020,99:160−170.
[52] GÖZKE G, KIRSCHHÖFER F, PRECHTL C, et al. Electro- filtration improves dead-end filtration of hyaluronic acid and presents an alternative downstream processing step that overcomes technological challenges of conventional methods[J]. Engineering in Life Science,2017,17(9):970−975.
[53]WEI L N, WANG Y, WEI D B, et al. Extraction technology and molecular characterization of hyaluronic acid from plateau zokor tis- sues [J]. Biotechnology Bulletin,2017,33(3):151−161.
[54]NI H S, LI R, HE Y L, et al. Purification of hyaluronic acid by ion exchange chromatography[J]. Chinese Journal of Pharmaceuticals,2001,11:5−8.
[55]LIU S, WANG J Z. The charac- teristics of hyaluronic acid and its application in food[J]. Chemical Engineering Design Communications,2018,44(8):62.
[56] CHA S H, WANG J L, LIAN C C, et al. Development of hyaluronic acid collagen rock sugar bird's nest and its effect on improving skin moisture[J]. Food Industry,2020,41(2):129−134.
[57]JIANG Q Y, LING P X, CHENG Y N, et al. Distribution of oral hyaluronic acid in animals[J]. Chinese Journal of Biochemical Pharmaceutics,2008, 29(2):73−76.
[58]ZHU C, ZHU Y, WEI W, et al. Preparation and application of coumarin- modified hyaluronic acid granular emulsifier[J]. Journal of Func- tional Polymers,2016,29(4):388−396.
[59]FU S Y, LI J. Research on the progress of facial mask[J]. Chemical Management,2017,26:117−119.
[60]MENG L L, DU T, WANG X X, et al. Research progress in the application of hyaluronic acid in cosmetics[J]. Shandong Chemical Industry,2018, 47(18):52−54,56.
[61]XU H Y, WANG H Y, XIAO X H, et al. Application of hyaluronic acid in toothpaste[J].Oral Care Products Industry,2020,30(6):13−17.
[62] YANG W, CHEN Z H, YI Z Y, et al. The effect of intra-articular injection of hyaluronic acid and placebo in the treat- ment of early and midstage knee osteoarthritis: A meta-analysis based on randomized, double-blind, controlled, and clinical trials[J]. Chinese Tissue Engineering Research,2021,25(23):3760−3766.
[63]LIU L T, WU L, ZENG D L, et al. The application progress of hyaluronic acid for injec- tion[J]. Journal of Practical Dermatology,2020,13(6):352−355.
[64]MA Y, PENG P, ZHAO Q. Application effect and safety of hyaluronic acid spray in skin repair of patients after facial laser surgery[J]. Clinical Medical Research and Practice,2020, 5(36):175−177.
[65]YU H L. Clinical observation of 0.3% sodium hyaluronate combined with Pranoprofen in the treatment of dry eye[J]. Electronic Journal of Clinical Medicine,2019,6(60):71.
[66]HU L J, LIU F L, LI L C, et al. Synthesis of amphiphilic hyaluronic acid derivatives and their application in antitumor nano-drug delivery systems[J]. Progress in Pharmaceutical Sciences,2017,41(11):804−811.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese