What Is the Use of Hyaluronic Acid on Skin?
Hyaluronic acid is a common polysaccharide widely found in various tissues of the human body, including dermal tissue and connective tissue. Since its first isolation from bovine vitreous humour by Meyer et al. in 1934, various functions of hyaluronic acid have been discovered. Due to its hydrophilicity, viscoelasticity, biocompatibility, and lack of immunogenicity, hyaluronic acid has found applications in topical drug delivery systems, wound healing, barrier therapy for diseases, and the treatment of inflammatory conditions.
1 Physical and chemical properties
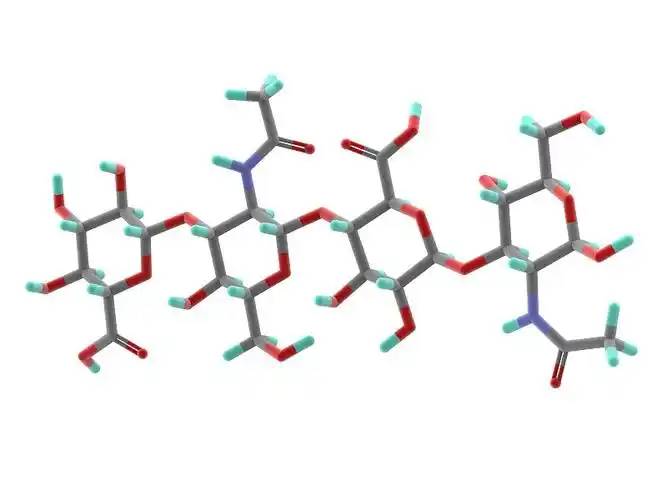
Hyaluronic acid is a linear polysaccharide polymer composed of D-glucuronic acid and N-acetylglucosamine, with the molecular formula (C₁₄H₂₁NO₁₁)_n. Based on its molecular weight, hyaluronic acid can be classified into high-, medium-, and low-molecular-weight types. Most of the properties of hyaluronic acid are related to its molecular weight. High-molecular-weight hyaluronic acid (HMW-hyaluronic acid) has a molecular weight range of 103–104 kDa, medium-molecular-weight hyaluronic acid has a molecular weight range of 200–103 kDa, and low-molecular-weight hyaluronic acid (LMW-hyaluronic acid) has a molecular weight range of 10–200 kDa. Through hyaluronidase, mechanical force, oxidative stress, and other regulatory processes of hyaluronic acid degradation and metabolism, high-molecular-weight hyaluronic acid can be degraded into hyaluronic acid polymers (or fragments) of different sizes. Low-molecular-weight hyaluronic acid can also be de novo synthesised by hyaluronic acid synthase during inflammatory processes [1]. In the human body, hyaluronic acid typically exists in a high-molecular-weight form with a size of approximately 104 kDa. It can bind to water equivalent to 1,000 times its own weight through hydrogen bonds [2] and achieve moisturising effects by reducing water evaporation and inducing hydration of the stratum corneum.
2 Biological Properties
2.1 Biocompatibility
Unlike collagen, which is highly allergenic, hyaluronic acid lacks antigenic epitopes [3], making it safer as a topical drug matrix. Another advantage of hyaluronic acid is that it can be rapidly degraded by hyaluronidase when adverse events occur [4]. Different molecular weights also influence the biological properties of hyaluronic acid. High-molecular-weight hyaluronic acid has higher viscosity, longer retention time, and better biocompatibility; low-molecular-weight hyaluronic acid exhibits the opposite characteristics [5].
2.2 Tissue Repair Effects
When inflammation occurs or trauma forms, topically applied high-molecular-weight hyaluronic acid binds with its receptor CD44 to form a hyaluronic acid-CD44 complex, which may trigger a series of reactions in fibroblasts, such as changes in the cellular cytoskeleton and regulation of the tissue healing process [6]. During trauma, hyaluronic acid promotes fibroblast migration to the wound site via CD44 expression on fibroblast cells, thereby facilitating tissue healing. However, the receptors of small-molecule hyaluronic acid differ from those of large-molecule hyaluronic acid, and thus this effect is not observed.
Small-molecule hyaluronic acid can promote tyrosine phosphorylation and activate phospholipase PLCγ1, thereby activating the protein kinase C (PKC) signal transduction pathway and the mitogen-activated protein kinase (MAPK) signal transduction system, promoting mitosis of vascular endothelial cells, promoting angiogenesis, and facilitating tissue repair [6].
Komorowicz et al. [7] reported that hyaluronic acid with molecular weights of 500 kDa and 1500 kDa caused fibrin to form lateral cross-links rather than branches, thereby altering the fibrin aggregation pattern and inhibiting fibrinolysis; furthermore, on the surface of fibrin clots, 500 kDa and 1500 kDa hyaluronic acid significantly inhibited tissue plasminogen activator (tPA)-catalysed plasminogen activation; therefore, in tissue injury and inflammation, hyaluronic acid can stabilise fibrin by altering its structure and solubility.
2.3 Regulatory effects on inflammation
In most studies, high-molecular-weight hyaluronic acid exhibits anti-inflammatory effects when applied topically, while lower-molecular-weight fragments exhibit pro-inflammatory effects. This is primarily due to different receptors on the cell surface for hyaluronic acid of different molecular weights, with high-molecular-weight hyaluronic acid primarily binding to CD44 and lower-molecular-weight hyaluronic acid primarily binding to toll-like receptors (TLR) [5].
High-molecular-weight hyaluronic acid binds to CD44 to form a hyaluronic acid-CD44 complex. The signal transduction cascade triggered by CD44 includes PI3K, PDK1, AKT, and the Ras phosphorylation cascade involving RAF1, MEK, and ERK1/2, thereby reducing inflammatory responses and inhibiting the production of reactive oxygen species (ROS). When inflammation occurs, the production of hyaluronic acid synthase increases, leading to enhanced de novo synthesis of small-molecule hyaluronic acid. Concurrently, under the influence of ROS or enzymatic actions such as hyaluronidase, more large-molecule hyaluronic acid is degraded into small-molecule hyaluronic acid. However, simultaneously, CD44 regulates inflammatory responses by downregulating TLR-4 expression. During this process, as large-molecule hyaluronic acid is degraded, ROS are also cleared, exerting an antioxidant effect. Additionally, hyaluronic acid regulates the expression of inflammatory cells, antigen-presenting cells, dendritic cells, and macrophages at the site of inflammation through CD44, exhibiting anti-inflammatory effects [8–11].
In the interaction between small-molecule hyaluronic acid and TLR, TLR-2 and TLR-4 activate NF-κB protein through Myeloid Differentiation Factor 88 (MyD88)-dependent and MyD88-independent pathways, exhibiting pro-inflammatory effects. In the MyD88-independent pathway, hyaluronic acid increases the expression of interferon-induced pro-inflammatory genes through type I interferon [9].
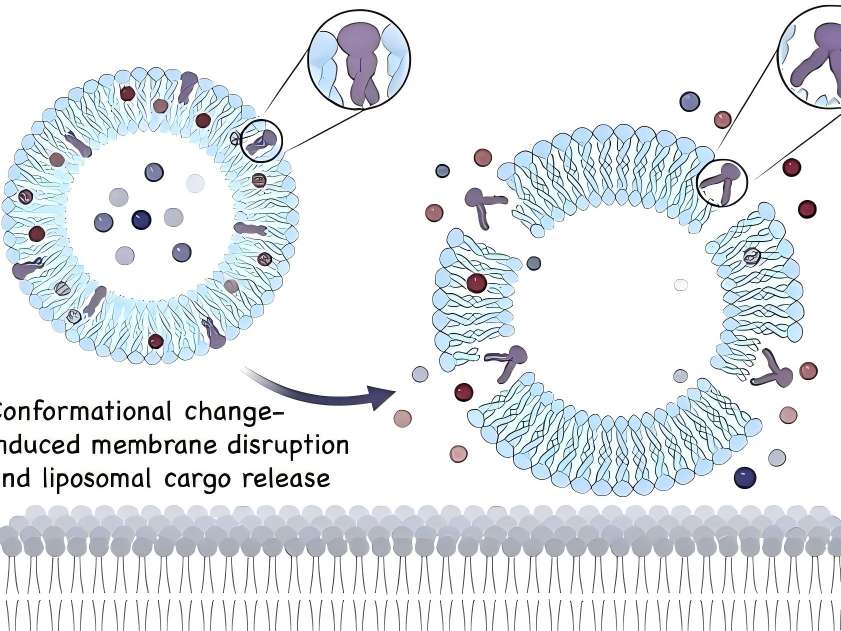
3 Clinical Applications
3.1 Topical Drug Delivery Systems
In topical drug formulations for skin diseases, liposomes containing hyaluronic acid are present on the skin surface. Hyaluronic acid can act as a mucosal adhesive agent when the drug takes effect or is absorbed. Additionally, as a topical drug formulation matrix, hyaluronic acid can prolong drug retention time [12].
Friedrich et al. [13] conducted experiments on regulating inflammatory responses associated with acute injury. The combination of high-molecular-weight hyaluronic acid gel with a monoclonal antibody that antagonises TNF-α influenced the bioavailability of the monoclonal antibody, prolonging the drug's action time at the inflammatory site, increasing drug residence time, and thereby promoting wound healing more effectively. Here, it not only acts as a mucosal adhesive to aid drug absorption but also alters the drug's residence time. In more drugs, such effects of hyaluronic acid may be further elucidated.
A 3% diclofenac gel with 2.5% high-molecular-weight hyaluronic acid as the matrix (trade name: Solaraze) is increasingly used in the treatment of actinic keratosis. In vitro Franz cell studies showed that compared with the buffer solution control group, after 7 days of treatment with hyaluronic acid formulations, a higher percentage of diclofenac remained in the epidermis (41% vs 25%) [14]. Clinical applications demonstrated good efficacy in clearing skin lesions, with the most common adverse reactions being contact dermatitis, skin dryness, rash, and epidermal peeling; no severe adverse reactions were reported [15].
Advances in nanotechnology have made the application of new technologies possible, such as hyaluronic acid-based nanocapsule polymers, which have shown promising efficacy as a novel topical drug delivery matrix in experimental studies. Compared with non-polymeric micelle solutions containing similar drug concentrations, in vitro skin penetration analysis showed that after 5 hours of local drug application, the drug concentration in the epidermis increased by 3 times, while the drug concentration in the dermis increased by 6 times. Additionally, hyaluronic acid polymer micelles enhanced the drug's bioactivity [16].
3.2 Wound healing
Given that hyaluronic acid promotes tissue repair, it can shorten wound healing time and reduce scar formation. Many clinical studies have reported such effects of hyaluronic acid. In the healing of 21 wounds caused by erbium laser, the wounds of subjects treated with oat Rhealba extract and high-molecular-weight hyaluronic acid formulations healed within 9 days, while the control group treated with panthenol and hydroxyproline formulations also healed within 9 days. while those treated with resveratrol copper healed in 12 days, and the untreated control group healed in 16 days. The hyaluronic acid group showed a significant reduction in healing time [17]. In a clinical trial involving 60 cases of local burns (average burn area approximately 3% of body surface area) treated with a high-molecular-weight hyaluronic acid zinc gel, wound size reduced to 50% of the original wound size by day 5, 93.3% of participants achieved complete epithelialisation by day 21, and 91.7% of participants experienced pain relief by day 10. No wound infections occurred during the wound healing process [18].
In a controlled experiment involving 30 cases of second-degree burn healing, olive oil and high-molecular-weight hyaluronic acid had similar effects on burn healing time, but olive oil was more effective in preventing scar formation [19]. In a randomised clinical trial comparing the topical application of high-molecular-weight hyaluronic acid and a neutral medium on 89 cases of leg venous ulcers, the results showed that by day 45, the ulcer healing area was significantly larger in the hyaluronic acid group compared to the control group (hyaluronic acid group: 73.4 ± 4.6% vs. control group: 46.9 ± 9.6%, P = 0.011), and the number of healed ulcers at 45 and 60 days was significantly higher than that in the control group (45 days: hyaluronic acid group 31.1% vs. control group 9.3%, P = 0.011) (60 days: hyaluronic acid group 37.8% vs. control group 16.3%, P = 0.024) [20].
3.3 Barrier therapy for the disease
Studies have shown that impaired epidermal barrier function plays a significant role in the development of atopic dermatitis and other allergic diseases, and dry skin is more prone to eczema. The addition of moisturisers containing hyaluronic acid can improve the integrity of the stratum corneum. Some studies have shown that barrier therapy can reduce the frequency and severity of skin disease flare-ups and decrease the need for topical corticosteroids or topical calcineurin inhibitors. Among these, hyaluronic acid may play a crucial role in the epidermis' natural response to trauma, including keratinocyte migration in atopic dermatitis and cell proliferation during wound healing and barrier repair [21].
Palmer et al. [22] conducted a randomised controlled trial to evaluate the efficacy and safety of MAS063D (a cream containing high-molecular-weight hyaluronic acid) in treating 30 patients with atopic dermatitis. After two weeks of treatment, MAS063D significantly reduced the itch severity index (EASI) in patients; Draelos et al. [23] conducted a controlled trial comparing a 1% high-molecular-weight hyaluronic acid-based pimecrolimus gel with a medicinal ceramide cream in the topical treatment of atopic dermatitis. At the start of the experiment, and at weeks 2 and 4, patients were assessed for erythema, crusting, lichenification, skin peeling, itching, stinging, and burning. At week 2, patients in the hyaluronic acid group showed significant improvement in eczematous lesions compared to the control group, but there was no significant difference in lesion recovery by week 4. Hyaluronic acid-based formulations are easier to apply and absorb than traditional drugs and have a milder odour, improving patient compliance. However, this study had a small sample size, no control group was established, and an appropriate assessment system was lacking, necessitating further experiments to support these results [24].
3.4 Inflammatory diseases
The anti-inflammatory regulatory mechanisms of hyaluronic acid have been demonstrated in some drug delivery media, skin barrier function repair, and wound healing. Additionally, topical hyaluronic acid has shown efficacy in treating facial seborrheic dermatitis. However, in in vitro experiments on inflammation regulation, small-molecule hyaluronic acid often exhibits pro-inflammatory effects (as described in Section 2.3), so the mechanism of topical hyaluronic acid in treating inflammatory diseases remains unclear. The main drugs used for facial seborrheic dermatitis are corticosteroids and antifungal agents, but their application is limited due to adverse reactions and drug sensitivity issues. A clinical trial by Schlesinger et al. [25] showed that topical application of 0.2% sodium hyaluronate improved symptoms such as erythema and itching in patients with facial seborrheic dermatitis. However, this clinical trial had a small sample size, and whether small-molecule hyaluronic acid regulates inflammation in a positive or negative manner remains controversial, so further clinical studies are needed to confirm its efficacy.
4 Conclusion
Topical hyaluronic acid is safe and effective in dermatology. With the maturation of bacterial fermentation methods for hyaluronic acid production, its biosafety has been further enhanced, and production costs have decreased compared to previous methods. This makes it more suitable for topical applications. With the continuous development of biotechnology, the emergence of new materials such as nano-particle polymer micelles may enable hyaluronic acid to exhibit better physicochemical properties for topical use in dermatology.
In many skin lesions, such as psoriasis, the normal hyaluronic acid reticular structure is partially missing in the spinous layer and granular layer, and hyaluronic acid is present in the stratum corneum of dyskeratosis. Whether correcting the abnormal distribution of hyaluronic acid in the epidermis of psoriasis patients can improve the symptoms of related skin diseases is worthy of further study. Studies have shown that with the increase of age, the expression of hyaluronic acid and CD44 receptor in the skin showed a downward trend. At this time, the role of exogenous hyaluronic acid in various aspects was weakened, so whether there were differences in the dosage and frequency of use at different ages is still worth discussing. Because hyaluronic acid exerts its antioxidant effect through its own degradation and ROS elimination mechanism, its application in anti-aging drugs is worth tracking.
Reference
[1] Liang J ,Jiang D ,Noble PW.Hyaluronan as a therapeutic target in human diseases[J].Adv Drug Deliv Rev ,2016, 97 : 186-203.
[2]Stern R,Asari AA,Sugahara KN.Hyaluronan fragments : an information-rich system[J].Eur J Cell Biol,2006,85 ( 8) : 699-715.
[3] Nowacki M ,Pietkun K ,Pokrywczyńska M ,et al.Filling effects,persistence ,and safety of dermal fillers formulated with stem cells in an animal model[J].Aesthet Surg J, 2014,34(8) : 1261-1269.
[4]DeLorenzi C.Transarterial degradation of hyaluronic acid fill- er by hyaluronidase[J].Dermatol Surg,2014,40( 8) : 832- 841.
[5]Zhao N,Wang X ,Qin L ,et al.Effect of molecular weight and concentration of hyaluronan on cell proliferation and os- teogenic differentiation in vitro[J].Biochem Biophys Res Commun,2015,465(3) : 569-574.
[6] Slevin M ,Kumar S ,Gaffney J.Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing respon- ses[J].J Biol Chem,2002,277(43) : 41046-41059.
[7] Komorowicz E ,Balázs N ,Varga Z ,et al.Hyaluronic acid decreases the mechanical stability,but increases the lytic re- sistance of fibrin matrices[J].Matrix Biol,2017,63 : 55-68.
[8]Petrey AC,de la Motte CA.Hyaluronan,a crucial regulator of inflammation[J].Front Immunol,2014,5 : 101.
[9]Naor D.Editorial : Interaction Between Hyaluronic Acid and Its Receptors ( CD44 ,RHAMM) Regulates the Activity of Inflammation and Cancer[J].Front Immunol,2016,7 : 39.
[10]Vigetti D ,Karousou E ,Viola M ,et al.Hyaluronan : bio- synthesis and signaling[J].Biochim Biophys Acta,2014, 1840(8) : 2452-2459.
[11] Kim Y ,Lee YS ,Hahn JH ,et al.Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdel- ta,Rac1 ,ROS ,and MAPK to exert anti-allergic effect [J].Mol Immunol,2008,45(9) : 2537-2547.
[12]Law CH,Li JM,Chou HC,et al.Hyaluronic acid-depend- ent protection in H9C2 cardiomyocytes : a cell model of heart ischemia-reperfusion injury and treatment[J].Toxi- cology,2013,303 : 54-71.
[13] Friedrich EE ,Washburn NR.Transport patterns of anti - TNF- α in burn wounds : Therapeutic implications of hyalu- ronic acid conjugation[J].Biomaterials,2017,114 : 10-22.
[14]Brown MB,Hanpanitcharoen M,Martin GP.An in vitro in- vestigation into the effect of glycosaminoglycans on the skin partitioning and deposition of NSAIDs[J].Int J Pharm, 2001,225( 1-2) : 113-121.
[15]Gupta AK,Paquet M,Villanueva E,et al.Interventions for actinic keratoses[J].Cochrane Database Syst Rev,2012, 12.
[16] mejkalová D,Muthn∮ T,Neporová K,et al.Hyaluronan polymeric micelles for topical drug delivery[J].Carbohydr Polym,2017,156 : 86-96.
[17]Sabadotto M,Theunis J,Black D,et al.In vivo assessment of the effect of a cream containing Avena Rhealba( ) extract and hyaluronic acid on the restoration of the skin barri- er in de-epidermised skin produced with an erbium-YAG laser[J].Eur J Dermatol,2014,24(5) : 583-588.
[18]Juhász I,Zoltán P,Erdei I.Treatment of partial thickness burns with Zn-hyaluronan : lessons of a clinical pilot study [J].Ann Burns Fire Disasters,2012,25(2) : 82-85.
[19]Campanati A,De Blasio S,Giuliano A,et al.Topical ozon- ated oil versus hyaluronic gel for the treatment of partial-to full-thickness second-degree burns : A prospective ,com- parative,single-blind,non-randomised,controlled clinical trial[J].Burns,2013,39(6) : 1178-1183.
[20]Humbert P,Mikosinki J,Benchikhi H,et al.Efficacy and safety of a gauze pad containing hyaluronic acid in treatment of leg ulcers of venous or mixed origin : a double-blind, randomised,controlled trial[J].Int Wound J ,2013,10 (2) : 159-166.
[21]Maytin EV,Chung HH,Seetharaman VM.Hyaluronan par- ticipates in the epidermal response to disruption of the per- meability barrier in vivo[J].Am J Pathol,2004,165 (4) : 1331-1341.[22]Palmer CN,Irvine AD,Terron-Kwiatkowski A,et al.Com- mon loss-of-function variants of the epidermal barrier pro- tein filaggrin are a major predisposing factor for atopic der- matitis[J].Nat Genet,2006,38(4) : 441-446.
[23]Draelos ZD.A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing e- mulsion cream in the treatment of mild-to-moderate atopic dermatitis[J].J Cosmet Dermatol,2011,10(3) : 185-188.
[24]Frankel A,Sohn A,Patel RV,et al.Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis[J].J Drugs Dermatol,2011,10(6) : 666-672.
[25]Schlesinger T,Rowland PC.Efficacy and safety of a low mo- lecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis final report[J].J Clin Aesthet Dermatol,2014,7(5) : 15-18.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese