Hyaluronic Acid Is Good for Skin Care Products
Hyaluronic acid (HA) is a polysaccharide composed of repeating units of N-acetyl-D-glucosamine and β-D-glucuronic acid linked by β-1,3-glycosidic bonds. The length and molecular weight of the hyaluronic acid molecule chain varies, The number of disaccharides is generally 300-11000 pairs, and the molecular weight ranges from 0.2 × 106 to 7.0 × 106. Hyaluronic acid is white, amorphous, odourless, and easily soluble in water, ether and other organic solvents. Its aqueous solution is acidic and negatively charged, and it moves towards the positive electrode during electrophoresis.
When the concentration is very high, hydrogen bonds form between the molecules, causing them to cross-link and exist in a network-like form. It has high viscoelasticity and osmotic pressure. In the 1930s, Mayer, a professor of ophthalmology at Columbia University in the United States, and others were the first to use an extraction method using animal tissue as a raw material to isolate hyaluronic acid from the vitreous body of a cow's eye. As one of the most widely distributed acidic mucopolysaccharides in the human body, hyaluronic acid is mainly found in the connective tissue matrix. and is one of the components of the skin. It has a good moisturising effect and is therefore widely used in cosmetics. It is known as the natural moisturising factor (NMF).
Hyaluronic acid binds to the CD44 receptor on cells and causes cell differentiation. When epidermal cells mutate into squamous cell carcinoma, the differentiation of epidermal cells also stops. Hyaluronic acid is a signalling substance that reflects cell physiology. It binds to receptors on the surface of epidermal cells to produce an irritating effect, which slows down differentiation towards apoptosis. Hyaluronic acid can remove reactive oxygen species generated by ultraviolet radiation in epidermal cells, thereby preventing skin damage. It is called an efficient scavenger of free radicals[1]. Free radicals cause lipid oxidation, and hyaluronic acid reacts with free radicals to decompose and then precipitate. Currently, the production of hyaluronic acid has been switched from extracting it from animal tissue to producing it using microbial fermentation, which has increased production and reduced costs[2].
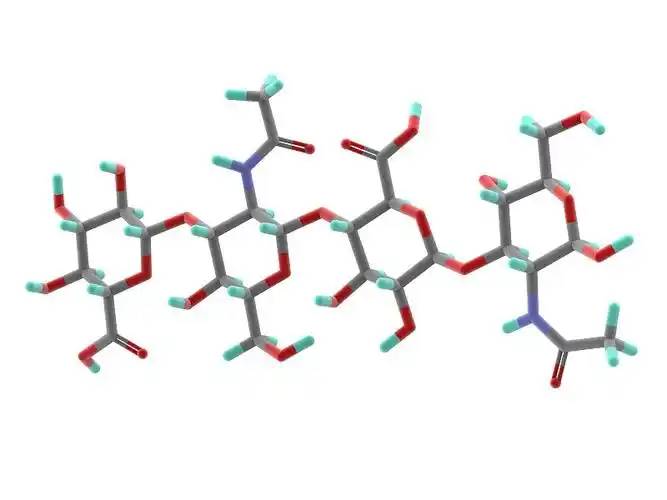
1 Application of hyaluronic acid in cosmetics
1.1 Moisturising effect
The main function of hyaluronic acid in cosmetics is to hydrate and moisturise, and its advantages are that its moisturising properties are less affected by the surrounding environment and it can retain moisture for a long time. Hyaluronic acid has the highest moisture absorption capacity at low humidity compared to glycerin, etc. [3], while its moisture absorption capacity at high humidity is the lowest. This property can be used to suitably moisturise the skin in different seasons and environments with different humidity levels. Hyaluronic acid is rarely used alone as a moisturising agent, but is often used in combination with other moisturising agents.
1.2 Emulsifier
Hyaluronic acid and phospholipids can form a stable emulsion when mixed in the oil and water phases without the addition of other emulsifiers. The emulsifier composed of hyaluronic acid and phospholipids is characterised by its emulsifying and moisturising properties, which are unmatched by other emulsifiers. A safe and effective emulsifier like hyaluronic acid can be used in the preparation of various cosmetics [4].
1.3 Thickening agent
Hyaluronic acid (molecular weight 3000kD) solution has a high viscosity[5]. Similarly, polyoxyethylene (molecular weight 100 ~ 5000kD) is one of the common thickening agents in cosmetics. The viscosity of the mixture of the two is greater than the sum of their respective viscosities. Therefore, hyaluronic acid-polyoxyethylene solution is a good thickening agent with excellent moisturizing properties. It can be used in creams, lotions, and other cosmetics, and is especially suitable for making various translucent viscoelastic gels, such as shaving gels, eye gels, sunscreens, etc.[6].
1.4 Fragrance fixative
Hyaluronic acid has the characteristic of surrounding substances with molecular sacs, so it can be used in fragrance products. Hyaluronic acid can be used as a fixative to bind the fragrance, reducing the rate of fragrance volatilisation and making the fragrance last longer. It is suitable for use in perfumes and other cosmetics. Hyaluronic acid has two other advantages: ① it reduces the adverse stimulation of fragrances on sensitive skin; ② it prevents certain fragrances from reacting with skin secretions to prevent the formation of unpleasant odours in creams, lotions, gels, etc.[7]

1.5 The nutritional effects of hyaluronic acid
Hyaluronic acid forms a film on the skin surface, which is beneficial for the adsorption of active substances by the stratum corneum. Hyaluronic acid can directly penetrate the dermis, promote skin tissue metabolism, and transport nutrients, thereby improving the physiological properties of the skin, thus achieving an effective supply of nutrients and producing a nourishing skin function [25]. For example, in facial masks and facial cleansers.
1.6 The antibacterial and anti-inflammatory effects of hyaluronic acid
Animals containing a small amount of hyaluronic acid can inhibit bacteria and play an anti-inflammatory role. Hyaluronic acid has the characteristic of forming a film, which can easily form a hydrated film to separate bacteria and play an anti-inflammatory effect. In addition, proteins and hyaluronic acid combine to form protein complexes in the skin matrix. This substance is abundant in the intercellular spaces and can form a gel that binds cells, ensuring the normal metabolism and water retention function of cell tissue, preventing harmful substances from invading cells, preventing the occurrence of various infections, and providing a certain degree of protection for the skin[8]. It has a wide range of applications in cosmetology and clinical medicine.
1.7 The restorative effect of hyaluronic acid
Hyaluronic acid can repair cell damage [9]. Hyaluronic acid levels increase at the wound site. During the different stages of wound healing, hyaluronic acid and fibrin play structural and regulatory roles together. Hyaluronic acid and the fibrin matrix promote or induce cell migration and regulate the functions of many cells involved in the inflammatory process. At the same time, Hyaluronic acid has excellent moisturising properties, maintains the relative stability of skin moisture, maintains an appropriate intracellular and intercellular water content, ensures a relative balance and free movement of various nutrients (including inorganic salts), and provides wound healing [10-11]. Studies have shown that when the skin has ulcers and minor burns, applying products containing hyaluronic acid can relieve pain, accelerate wound healing, and reduce the occurrence of scars.
1.8 Sun protection with hyaluronic acid
Exposure to UV light can lead to lipid peroxidation, damage cell membranes and cause pigmentation [12]. It can also limit the access of enzymes that catalyse lipid peroxidation to cell membranes, thereby reducing the entry and exit of free radicals outside the cell. Skin care products containing hyaluronic acid can induce cell differentiation. Combining sun protection with hyaluronic acid has a synergistic effect, as in the case of the Yilian Multi-Whitening Sunscreen [13].
1.9 The lubricating effect of hyaluronic acid
Hyaluronic acid is a polysaccharide with good film-forming properties [14-16]. When used on the skin, hyaluronic acid skincare products can be observed to have a good lubricating effect. When hyaluronic acid is used, a hydrated film forms on the skin surface, giving the skin a lubricated feeling. This effect cannot be compared with that of other skincare products.

1.10 The cosmetic effect of hyaluronic acid
Hyaluronic acid is a glycosaminoglycan that is not species-specific, is a component of the human body and does not cause an immune response. Lipid injections containing it have good compatibility and high safety, and generally do not cause allergic reactions, making hyaluronic acid an ideal filler material. Hyaluronic acid is injected into the desired area to fill it from the inside, restoring the skin's original radiance [17-19].
2. Application of hyaluronic acid derivatives in cosmetics
Hyaluronic acid has a short residence time in vivo and in vitro, which affects its efficacy. Because it has multiple functional groups on the surface, including carboxyl groups, it can be chemically modified to give it better rheological properties and resistance to enzymatic hydrolysis, increasing its prospects for development in the cosmetics field. Since hyaluronic acid has multiple sites that can be modified, such as carboxyl groups and hydroxyl groups, it is easy to transform it into amphoteric derivatives [20-23].
2.1 Sodium hyaluronate
Sodium hyaluronate (SH) is the sodium salt of hyaluronic acid. It is white and fibrous, has good moisturising properties, is easily soluble in water but insoluble in organic solvents such as ether [24-25]. SH aqueous solution is fluid and has good rheological properties. According to research, the human body contains more than 70% water, and it is very important to maintain a large amount of water in the body [26]. As people age, the amount of SH in the skin decreases, which leads to a decrease in skin moisture, rough skin, loss of elasticity, and the appearance of wrinkles [27]. Exogenous SH can effectively supplement the skin's endogenous SH, thereby preventing skin aging and having a beautifying effect. At present, SH is widely used in moisturizing products such as skin creams and lotions [28-29]. In addition, SH has also begun to be used in the medical cosmetology industry, and various beauty and health products have already appeared abroad[30], such as Hylaform, which can effectively eliminate frown lines, smile lines, and wrinkles around the eyes and nose as an injection, and can also increase the width of the nose and make the lips plump and bright[31], making people look younger and more beautiful. Injections containing SH are hypoallergenic[32] and easy to promote.
2.2 Acetylated hyaluronic acid
The hydroxyl groups of hyaluronic acid can undergo acylation reactions with anhydrides or acid chlorides to form ester compounds. Takashi[33] reported a method for synthesising acetylated hyaluronic acid (Ac-Hyal) using acetic anhydride as the acylation reagent. The physicochemical properties of this substance vary depending on the degree of substitution of the acetyl group. The higher the degree of substitution, the stronger the hydrophobicity of Ac-Hyal. Compared to hyaluronic acid, Ac-hyaluronic acid compounds exhibit superior moisturising and skin-softening properties when the degree of substitution is 3.0. As Ac-hyaluronic acid is soluble in organic solvents such as a 90% ethanol solution, it can be used as a superior moisturiser in both water-based and oil-based cosmetics [33-35].

2.3 Hyaluronate esters
Kana et al. [36-38] used acid hyaluronan or hyaluronan-TBA to react with trimethylsilyldiazomethane in the presence of dimethyl sulfoxide (DMSO) and methanol to form methyl hyaluronate. After methylation, hyaluronan's resistance to hyaluronidase degradation is enhanced. Methyl hyaluronate has different degrees of esterification, and its application fields are also different. It can be used in cosmetics, biomedicine, wound healing, various forms of tissue engineering scaffolds such as dressings, films, fibers and other broader fields. Lipoic acid (LA) is a biocompatible active molecule that can be rapidly degraded in the body. Picotti et al. [39] 40 - combined with LA to prepared a lipoic acid hyaluronic acid gel (Lipohyal). Lipohyal can be used in the cosmetics field for moisturising and anti-ageing, and also as an adjuvant to treat skin damage, such as burns, ulcers, wounds, dermatitis, radiation-induced skin hyperthermia, etc.
2.4 Hyaluronic acid hydrazide derivatives
After the adipic acid dihydrazide (ADH) compound is linked to hyaluronic acid, the remaining -NH2 can be linked to other carboxyl groups in the hyaluronic acid molecule, cross-linking the hyaluronic acid molecules within or between molecules. After the solvent evaporates, the soft, fluid gel can be modified into a hyaluronic acid hydrogel film with greater mechanical hardness, soluble and biodegradable hyaluronic acid hydrogel film with good biocompatibility. In addition, He Yanli et al. [41-43] also studied the biocompatibility of hyaluronic acid-ADH films and concluded that they have good resistance to enzymatic hydrolysis and biocompatibility.
Under the catalysis of acid, disulfide is refluxed into diester in ethanol, and diester is hydrolyzed into corresponding dihydrazine [44]. Shu et al. used hyaluronic acid and disulfide to cross-link and prepare a gel film, which showed great potential value in clinical applications such as wound healing and tissue repair after performance testing.

Zhang Wenqiang et al. [45-48] added hyaluronic acid to sodium tripolyphosphate (STMP) at a certain mass ratio, stirred well in a three-necked flask, allowed it to rapidly stir at pH 10 and 50 °C for 12 h, then subjected the resulting solution to two-stage dialysis with distilled water, purified it, and formed a film at room temperature. The water absorption capacity of the resulting film decreases, its water retention performance improves, and the film has better resistance to degradation. Both water retention performance and degradation resistance improve with increasing degree of cross-linking.

3 Prospects
At present, with the continuous development of the cosmetics industry, the pursuit of natural and safe cosmetic ingredients has become a future development trend. The continuous expansion of the application fields of hyaluronic acid and the development and application of its derivatives will inevitably become a research hotspot. In particular, China's cosmetics industry started late, and most raw materials need to be imported. The development of hyaluronic acid derivatives with new functions, high efficiency and non-toxicity has become one of the urgent problems to be solved in the development of China's cosmetics industry.
References
[1] Hu Woying. Research on drug-carrying system of hyaluronic acid nanoparticles[D]. Tianjin: Tianjin Medical University, 2007.
[2] HUANG Xiaozhong, GUAN Guoqiang. Progress of hyaluronic acid physiological function and its application [J]. Livestock and Feed Science,2015,36(01) :21-25.
[3] Ling Peixue. Hyaluronic acid[M]. Beijing : China Light Industry Press,2007.
[4] Zhuchao, Zhu Ye, Wei Wei et al. Preparation and application of coumarin-modified hyaluronic acid particle emulsifier[J]. Journal of Functional Polymers,2016,29(4) : 388-396.
[5] FU Si-Ya, LI Juan. An analysis of the research progress of facial mask[J]. Chemical Management,2017 (26) : 117 -119,121.
[6] YANG Su-Zhen, CHEN Gui-Rui. Characteristics of hyaluronic acid and its application in food[J]. Food Industry Science and Technology,2008,29(6) : 317-320.
[7] SONG Yongmin, DING Houqiang, GUO Xueping. Application of hyaluronic acid in food[J]. Food and Drugs,2014,16(04) :299-302.
[8] SUN Shangzhi, ZHANG Qingqing, ZHANG Hui. Functions and production of hyaluronic acid (HA) [J]. Journal of Changchun University of Traditional Chinese Medicine,2007,23 (3) : 88-89.
[9] CHEN Xiaoying, LU Xiangrong. Development of functional cosmetics[J]. Fujian Light Textile, 2006(3) : 1-5.
[10] SAN Lianhai, XIONG Xiong, GUO Hai. Preparation and application of hyaluronic acid[J]. Anhui Agricultural Science,2007,35 ( 11) : 3150-3151.
[11] CHEN Dejing, XU Weiliang, SU Wen, et al. Extraction of polysaccharides from the skin mucus of giant salamander (Andrias davidianus) and analysis of monosaccharide composition [J]. Natural Products Research and Development,2015 ( 10) : 1700 - 1705.
[12] LIU J-T. Hyaluronic acid and its application in cosmetics[J]. Guangzhou Chemical Industry, 2009,37(8) :71-73.
[13]ZHAO Li-Ying. Introduction to hyaluronic acid[J]. Toothpaste Industry,2006(1) :54-55.
[14]Kim E G ,Eom T K ,Kang S J. Severe visual loss and cerebral infarction after injection of hyaluronic acid gel[J].J Craniofac Surg,2014,25 :684-686.
[15]Jang J G,Hong K S,Choi E Y.A case of nonthrombotic pulmonary embolism after facial injection of hyaluronic acid in an illegal cosmetic procedure [J].Tuberc Respir Dis (Seoul) ,2014 ;77 :90-93.
[16]D.D.R.F,Biochemistry [M].Lippincott Williams & Wilkins,2014.
[17]Khutoryanskiy V V.Antimicrobial hydrogels based on autoclaved poly (vinyl alcohol ) and poly ( methyl vinyl etheraltmaleic anhydride ) mixtures for wound care applications[J].RSC Advances,2016,6 ( 60 ) : 55211-55219.
[18]Chen F ,Ni Y ,Liu B ,et al. Self - crosslinking and injectable hyaluronic acid -GD-functionalized pectin hydrogel for cartilage tissue engineering[J].Carbohydrate Polymers,2017,32( 1) : 166 -169.
[19]Cui N,Qian J,Liu T,et al.Hyaluronic acid hydrogel scaffolds with a triple degradation behavior for bone tissue engineering [J].Carbohydrate Polymers,2015,126 -128.
[20]Edwards K.New twist on an old favorite : gentian violet and methylene blue antibacterial foams[J].Advances in Wound Care,2016,5 ( 1) : 11 -15.
[21]Fiorica C,Palumbo F S,Pitarresi G,et al.Hyaluronic acid and beta cyclodextrins films for the release of corneal epithelial cells and dexamethasone[J].Carbohydrate Polymers,2017,9 (9) :7979-7979.
[22]González -Vázquez P,Larraneta E,McCrudden MTC,et al.Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis [J].Journal of Controlled Release,2017,265 : 30-40.
[23]Gustafson C T,Boakye -Agyeman F,Brinkman CL,et al. Controlled delivery of vancomycin via charged hydrogels[J]. Public Library Of Science,2015,11 ( 1) : 146-401.
[24]Hall C W,Mah T F.Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria[J]. FEMS Microbiology Reviews,2017,41 (3) : 276-278.
[25]Hemshekhar M,Thushara R M,Chandranayaka S,et al, Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine [J].International Journal of Biological Macromolecules,2016,86:917-919.
[26]Highley C B,Prestwich G D,Burdick J A.Recent advances in hyaluronic acid hydrogels for biomedical applications[J]. Current Opinion in Biotechnology,2016,40: 35-40.
[27 ]Jia Y ,Huo M ,Huang H , et al. Preparation and characterization of bacterial cellulose / hyaluronic acid composites. Proceedings of the Institution of Mechanical Engineers[J].Part N : Journal of Nanoengineering and Nanosystems,2015,229 :41-48.
[28]Jiao Y,Pang X,Zhai G.Advances in hyaluronic acid -based drug delivery systems[J].Current Drug Targets,2016,17 : 720-730.
[29]Korogiannaki M,Guidi G,Jones L,et al.Timolol maleate release from hyaluronic acid - containing model silicone hydrogel contact lens materials,2015,30 (3) : 361-366.
[30]Larraneta E ,Lutton REM ,Brady AJ,et al.Microwave- assisted preparation of hydrogel -Forming microneedle arrays for transdermal drug delivery applications[ J ]. Macromolecular Materials and Engineering,2015,300: 586 - 595.
[31]Lutton R E M,Larraneta E,Kearney M C,et al.A novel scalable manufacturing process for the production of hydrogelforming microneedle arrays[J].International Journal of Pharmaceutics,2015,494( 1) :417-429.
[32]McCoy C P,Irwin N J,Brady C,et al.An infection - responsive approach to reduce bacterial adhesion in urinary biomaterials[J].Molecular Pharmaceutics,2016,13 ( 8 ) : 2817-2822.
[33]Mero A,Campisi M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules[J].Polymers,2014,6 (2 ) : 346-369.
[34]Norsworthy A N ,Pearson M M. From catheter to kidney stone : the uropathogenic lifestyle of Proteus mirabilis[J]. Trends in Microbiology,2017,25 (4) : 304-315.
[35]Romanò C L,De Vecchi E,Bortolin M,et al.L Hyaluronic acid and its composites as a local Antimicrobial / Antiadhesive barrier[J]Journal of Bone and Joint Infection,2017,2( 1) : 1 - 2.
[36]Tong S Y C,Davis J S,Eichenberger E,et al.Staphylococcus aureus infections : Epidemiology ,pathophysiology ,clinical manifestations ,and management[J].Clinical Microbiology Reviews,2015,28(3) : 603-661.
[37]Tripodo G,Trapani A,Torre M L,et al.Hyaluronic acid and its derivatives in drug delivery and imaging : Recent advances and challenges[J].European Journal of Pharmaceutics and Biopharmaceutics,2015,97 (Pt B) :400-416.
[38]Kim J H,Choi J S,Yun J H,et al.Foreign body reaction to injectable hyaluronic Acid : late granuloma formation[J].Ann Dermatol,2015,74(9) :764-766.
[39]Haneke E,Adverse effects of fillers and their histopathology [J].Facial Plast Surg,2014,30(06) : 599-614.
[40]Elder D E,Elenitsas R , Rosenbach M,et al.Lever's histopathology of the skin [J]. Journal of Cutaneous Pathology,2014,41 (6) : 552-553.
[41]Koltowska K,Paterson S,Bower NI,et al.Mafba is a downstream transcriptional effector of Vegfc signaling essential for embryonic lymphangiogenesis in zebrafish[J].Genes Dev,2015,29( 15) : 1618 -1630.
[42]Wu Y C,Wang Y P,Chang J Y F,et al.Langerhans cells in lining epithelia of epidermoid cysts[J].J Dent Sci,2013,8 : 448-450.
[43]Chen H M,Wu Y C,Wei L Y,et al.Metastatic hepatocellular carcinoma of the anterior palatal gingiva[J].J Dent Sci, 2014,9 :202-204.
[44]Chang P E,Goh B B,Jing H N,et al,Clinical applications, limitations and future role of transient elastography in the management of liver disease[J ].World Journal of Gastrointestinal Pharmacology & Therapeutics,2016,7 ( 1 ) : 91 -106.
[45]Fernandez M,Trpo E,Degr D,et al.Transient elastography using Fibroscan is the most reliable noninvasive method for the diagnosis of advanced fibrosis and cirrhosis in alcoholic liver disease[J].European Journal of Gastroenterology & Hepatology,2015,27(9) : 1074 -1079.
[46]Parikh P,Ryan J D,Tsochatzis E.Fibrosis assessment in patients with chronic hepatitis B virus ( HBV ) infection [J].Ann Transl Med,2017,5 (3) :40-46.
[48]BeerKR , Julius H,Dunn M,et al.Remodeling of periorbital,temporal ,glabellar ,and crow's feet areas with hyaluronic acid and botulinum toxin [J].Journal of Cosmetic Dermatology,2014,13 (2) : 143 -150.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese