What Is Mannan Oligosaccharide?
Mannooligosaccharides, also known as mannan oligosaccharides and glucomannan oligosaccharides, are a class of antigenically active substances extracted from the cell walls of yeast cultures, which are widely found in the cell walls of many microorganisms, as well as in the seeds and tubers of plants, such as gelatinized lemongrass, konjac flour, and guar gum. It has been found that mannan-oligosaccharides have a variety of biological activities, which can enhance animal immunity, regulate glucose-lipid metabolism and maintain intestinal health, as well as have growth-promoting and antioxidant effects (Wang et al., 2018; Li Yuxin, 2015).
Manno-oligosaccharides are safe and non-toxic, with good physicochemical properties such as low heat and stability, and no adverse effects when used in combination with other additives, and they have been widely used as additives in food and animal feed in China and abroad (Zhao Xiaofeng, 2008). In this paper, we summarize the preparation methods, physiological functions and research progress of mannan oligosaccharide in livestock and poultry production, in order to provide reference for further exploration of its application in livestock and poultry production.

1 Physicochemical Properties of Mannan Oligosaccharides
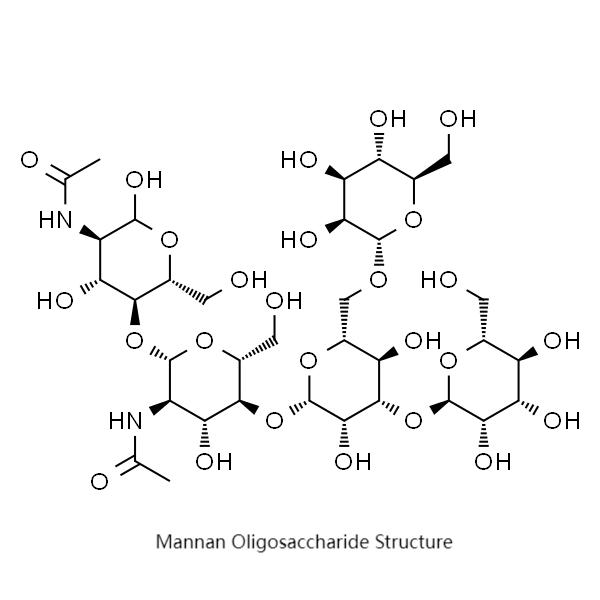
Manno-oligosaccharides are a class of oligosaccharides, consisting of several mannose molecules or mannose and glucose connected mainly by α-1,2 glycosidic bonds, α-1,3 glycosidic bonds, α-1,6 glycosidic bonds, β-1,4 glycosidic bonds, or β-1,3 glycosidic bonds (Ximei Yao, 2011).
The physical and chemical properties of mannan oligosaccharides from different sources are different, mannan oligosaccharides are soluble in water, insoluble in organic solvents, and coexistence of organic solvents can produce precipitation or crystallization. Manno-oligosaccharides have a certain viscosity, and the viscosity is inversely proportional to the temperature, in addition, when the pH is 1.5 ~ 3, the viscosity increases; when the pH is 3 ~ 9, the viscosity is more stable (Chen Xiaoying, 2017). Some manno-oligosaccharides with molecular weights ranging from 200 to 20 million have certain gelation effects (Yang et al., 2005), and the structural properties of manno-oligosaccharides are relatively stable. Manno-oligosaccharides are structurally stable and contain a large number of chemical bonds that cannot be hydrolyzed by amylase, and exist in the form of a mixture of polysaccharides (Y. Liang et al., 2013).
2 Production of Mannan Oligosaccharides
At present, the main methods for the preparation of Mannan Oligosaccharides are: (1) degradation, such as enzymatic degradation, oxidative acidification degradation, ultrasonic degradation and irradiation modification degradation, etc.; (2) synthesis, such as microwave solid state synthesis. Due to the high cost and technical difficulty of the synthesis method, most of the industrial production of Glyco-oligosaccharides adopt the degradation method.
2.1 Degradation
Polysaccharides are autohydrolized at 190 °C (Carvalheiro et al., 2004) and depolymerized by ionizing radiation such as c-rays, microwaves, and hydrothermal treatment (Devin et al., 2010; Singh et al., 2009). Weak acid (H2SO4) hydrolyzes galactomannans from Mimosa pudica seeds to manno-oligosaccharides (Joana et al., 1995); strong base (NaOH) hydrolyzes and depolymerizes mannans from the plant cell wall, and manno-oligosaccharides can be produced by further neutralization with strong acid (HCl) (Mia et al., 1995). The preparation of mannan oligosaccharides by degradation is divided into two steps: preparation of mannan and degradation of mannan. Li Ying et al. (2015) used hot water maceration to prepare mannan from wine yeast, and the optimal extraction conditions were 1:23 (g/mL), 124 ℃, 5 h, and 3 times, which resulted in a 14.27% yield of mannan. The degradation method of mannan varies depending on its source, Liu Zizheng (2016) found that the optimal reaction conditions for the preparation of mannan oligosaccharides by enzymatic hydrolysis (β-mannanase) were as follows: degradation temperature of 50 ℃, degradation pH of 5.5, reaction time of 2 h, enzyme substrate ratio of 150 U/g, and the target mannan oligosaccharides yield of 65.72% by using a one-way test and orthogonal test.
Chen Xiaoying (2017) determined the optimal process conditions for the preparation of manno-oligosaccharides from waste yeast by orthogonal test: papain enzyme concentration of 2.25‰, enzyme digestion time of 6 h, alkali temperature of 45 ℃, alkali digestion time of 5.5 h and liquid alkali concentration of 0.5 mol/L. The average yield of manno-oligosaccharides was 2.14% and the average content was 40.96% by parallel pilot test.
2.2 Synthesis Method
Using monosaccharides or disaccharides as substrates, microwave solid-state synthesis of oligosaccharides has fast reaction speed, high synthesis yield and no pollution. Li Xinming (2008) found that the optimal reaction conditions for microwave solid-state synthesis of mannose and glucose as reactants were as follows: microwave power of 1000 W, microwave processing time of 4 min, 15% additive of initiator, 3% additive of catalyst, and the synthesis yield was 86.50%. High performance liquid chromatography (HPLC) analysis of the initial glycooligosaccharides: monosaccharides accounted for 13.50%, disaccharides accounted for 3.82%, trisaccharides accounted for 7.56%, tetrasaccharides accounted for 6.84%, pentasaccharides accounted for 4.77%, hexasaccharides accounted for 5.54%, and heptasaccharides and above accounted for 57.97%.
3 The Main Biological Functions of Mannan Oligosaccharides
3.1 Antioxidant
Reactive Oxygen Clusters (ROS) as the most common free radicals in the organism, including superoxide ion (O2-), hydroxyl radical (-OH), and monoclinic oxygen, etc., and too many free radicals can be harmful to the organism. Intracellular free Fe2+ reacts with -OH to generate ROS (Xu Wenzhe, 2018), and Fe2+ deposition can lead to oxidative stress. Manno-oligosaccharides have the effect of scavenging free radicals (He Zhikun et al., 2013). Yang Xueshan et al. (2015) found that mannan oligosaccharides have good scavenging effects on -OH, O2-, diammonium salts (ABTS + - ), 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) free radicals, and chelated Fe2+ in in vitro assays.
Li XM (2008) found that mannan oligosaccharides enhanced the total antioxidant capacity (T-AOC) of mice by increasing the activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), Na+-K+-ATPase, and the content of glutathione (GSH) in animal experiments. Malondialdehyde (MDA) is a product of lipid peroxidation, which is cytotoxic, and the content of MDA can reflect the level of lipid peroxidation and oxidative stress (Quan et al., 2014). Guo Yungui et al. (2010) showed that mannan oligosaccharides could significantly reduce the MDA content in serum, liver, myocardium and muscle of Sanyo chickens. Therefore, the mechanism of action of mannan oligosaccharides may be through its own physicochemical properties and the reaction with free radicals in cells (Zhang Shuai, 2018), which can scavenge free radicals and play an antioxidant function; on the other hand, mannan oligosaccharides can activate antioxidant enzymes in the body, increase the activity of antioxidant enzymes and the content of antioxidant, and inhibit lipid peroxidation, so as to maintain the balance of the antioxidant in the organism.
3.2 Immunomodulation
Mannan Oligosaccharides are antigenically active substances with certain immunogenicity, which can induce an immune response (Li et al., 2017), and can be used as adjuvants of exogenous antigens to bind to the cell surface of certain toxins, viruses, and fungi (Kou et al., 2012) in order to enhance the immune response of the body to the antigens or to change the type of the immune response, and thus to enhance the cellular and humoral immunity of the animal organism. immunity of animal organisms. In addition to their coagulant and antigenic properties, mannan-oligosaccharides can react with protein receptors on the surface of immune cells, intervene in the signaling system on memory cells in lymph nodes and mucosal lamina propria to play an immunomodulatory role (Chen et al., 2005), and improve the function of natural anti-infective immunity through the activation of complement system (Xiong A-Ling, 2014).
3.2.1 Enhancement of Non-specific and Specific Immunity
On the one hand, mannan oligosaccharides can improve the non-specific immune function of the body by increasing the immune organ index and promoting the expression of genes related to natural immunity (Xiong A-Ling, 2014); on the other hand, mannan oligosaccharides can promote the proliferation of T and B lymphocytes, increase the CD4+/CD8+ ratio (Li Xin-Ming, 2008), and stimulate the key molecule of the Toll-like Receptor (TLR) signal pathway (Duan Ou-Dong, 2013), thus enhancing the body's specific immune function. 2013), which in turn improves specific immunity.
Studies have shown that mannan oligosaccharide can significantly increase the content of phagocytic index, erythrocyte count, thymic index and 50%CH50 in mice, and enhance the nonspecific immunity of animals (Shen Wenkang et al., 2015). Mannooligosaccharides can differentially up-regulate the expression of natural immunity-related genes AvBD9, TLR2, TLR4 and Cath-B1 in broiler tissues, and improve the natural immunity defense function of broilers by increasing the expression of TLRs in broiler tissues and up-regulating the expression of antimicrobial peptides such as p-antioxidant and Cathelicidins mediated by TLRs (Xiong A-Ling, 2014).
Li Xinming (2008) showed that the immunity indexes of tissues decreased significantly after aging modeling, but mannan oligosaccharide could significantly increase the indexes of liver, kidney, spleen and thymus, as well as the levels of immunoglobulin IgA, IgG and IgM in the serum of aging mice; under the stimulation of ConA, mannan oligosaccharide could significantly increase the proliferative index of thymus T-cells and the rate of transformation of splenic lymphocytes, thus enhancing the level of humoral and cellular immunity of the model mice.
Under the stimulation of ConA, mannan oligosaccharides significantly increased the proliferation index of thymus T cells and the conversion rate of spleen lymphocytes, which in turn enhanced the humoral and cellular immunity levels of mice in the model of aging. In addition, mannan oligosaccharide can activate the key molecules of TLR signaling pathway and improve the sensitivity of immune response in jejunal tissues of piglets, and on the other hand, it can improve the intestinal immunity of piglets by inhibiting the over-activation of the TLR signaling pathway in mesenteric lymph nodes (Duan Xudong, 2013).
3.2.2 Anti-inflammation
Inflammation is a defense response induced by injurious agents. In general, the inflammatory response is triggered by exogenous substances and products of tissue damage and is accompanied by the production of pro-inflammatory cytokines, recruitment and activation of immune cells, and the production of free radicals. Interleukins (IL) are immune factors, including IL-2, IL-4, IL-10, etc., which down-regulate inflammation, Interleukins (IL) are immune factors, including IL-2, IL-4, IL-10, etc., that downregulate inflammatory mediators and promote immune responses (Liang et al., 2012).
Che et al. (2013) found that mannan oligosaccharides increased serum IL-10 levels and the number of leukocytes and lymphocytes, and reduced the intensity of the inflammatory response. In a mouse model of acute colitis induced by sodium glucosulfate (DSS), mannan normalized the expression of intestinal mucin 2 and attenuated the local expression of pro-inflammatory cytokines IL-1α, IL-1β, IL-6 and monocyte chemotactic protein (MCP)-1, as well as the inflammatory vesicles of Toll-like receptor TLR4 and NLRP3. The protective effect of mannan oligosaccharides may be directly mediated through local macrophages, and in a lipopolysaccharide (LPS)-induced murine macrophage (RAW264.7) model, mannan oligosaccharides inhibit the production of IL-1α, IL-1β, IL-6 and granulocyte colony-stimulating factor (G-SCF) (Szilamer et al., 2016). It can be seen that mannan oligosaccharide can promote the production of immune factors by regulating the expression of non-immune and immune genes, thus exerting anti-inflammatory effects.
Mitogen-activated protein kinases (MAPKs) and nuclear transcription factor-κB (NF) are important downstream signaling pathways that regulate inflammatory responses (Kim et al., 2006). Excessive inflammatory mediators can activate NF-κB, causing it to detach from inhibitory protein-κB (IκB) and enter the nucleus to induce the expression of inflammatory mediator genes, thereby exacerbating the inflammatory response (Joel et al., 2002). In contrast, the MAPKs family proteins ERK, JNK and p38 are regulatory mediators targeting NF-κB when regulating the inflammatory response (Matthew et al., 2014).
According to Zhou et al. (2015), mannan oligosaccharide significantly reduced the binding of LPS to the cellular surface of mouse macrophage RAW 264.7 cells and the expression of LPS-induced TLR4 and cluster of differentiation (CD)14; significantly inhibited the LPS-induced stimulatory pathways of RAW 264.7 cellular NF-κBs and MAPKs, and mannan oligosaccharide inhibited the stimulatory pathways of NF-κBs and MAPKs by blocking the stimulation of NF-κBs and MAPKs. Mannooligosaccharide reduces LPS-induced inflammation by blocking the activation of NF-κB and MAPKs. In addition, mannan oligosaccharide exerts anti-inflammatory functions by inhibiting the LPS-induced increase in tumor necrosis factor (TNF)-α and interferon (IFN)-γ (Pourabedin et al., 2016).
3.3 Regulation of Microflora and Maintenance of Intestinal Health
Manno-oligosaccharides have been found to have good bacteriostatic effects in vitro (Bozkurt et al., 2016; Shine Long, 2016). Manno-oligosaccharides are effective in inhibiting the growth of pathogenic E. coli by lowering pH through lactic acid production by Lactobacillus (Hang Suqin, 2007). In the adhesion and inhibition of adhesion between Salmonella (or E. coli) and Caco-2 cells, mannan oligosaccharide has the effect of inhibiting the adhesion of Salmonella and E. coli, and there is a dose effect of mannan oligosaccharide on the inhibition of Salmonella at the concentration of 0.0005-0.005 mol/L. The inhibition of adhesion is as follows: α-glycoligosaccharides > β-mannan ≥ mannoglucosaccharides (GL) (GL) (Gao L. 2016; LL). 2016).
As the largest immune compartment in the body, the intestine has the function of preventing mucosal infections and regulating microbial colonization (Mehmet et al., 2010). Manno-oligosaccharides can increase the number of beneficial bacteria such as Bifidobacterium and Lactobacillus (Liu Zizheng, 2016), reduce the number of harmful bacteria such as Escherichia coli (Liu Weidong et al., 2011), and increase the diversity of intestinal microorganisms (Hang et al., 2012), which improves the composition of intestinal flora. Wang Hongshan et al. (2018) found that mannan oligosaccharides could selectively modulate some microorganisms and significantly increase the relative abundance of probiotics such as Eckermannia, Lactobacillus, and Bifidobacterium. In addition, mannan oligosaccharides can repair intestinal damage and maintain intestinal health.
Yuxin Li (2015) found that, in the E. coli poisoning test, mannan oligosaccharide reduced the mRNA expression of TLR4 and IL-1β in the intestinal mucosa without poisoning, and increased the number of inter-epithelial lymphocytes and cupped cells after E. coli poisoning, and that mannan oligosaccharide improved the local immune response by regulating the expression of genes of intestinal cytokines in the intestinal mucosa and altering the number of intestinal immune cells, thus maintaining the health of the intestine. This will help to maintain the health of the intestinal tract. Tight junctions are the main connection between the epithelial cells of the intestinal mucosa and play an important role in maintaining the integrity of the mechanical structure and normal function of the intestinal mucosal barrier (Ting Chen, 2016). The three most important tight junction proteins are ZO-1, Occludin and Claudins (Zhang et al., 2015). Wu Shi (2017) found that mannan oligosaccharides have certain repair functions on intestinal epithelial cells, compared with the LPS-injured group, mannan oligosaccharides caused a significant up-regulation of Caco-2 cell activity, a significant down-regulation of IL -6, TNF -α, IL -1β mRNA expression, a significant up-regulation of Claudin-1, ZO-1, and MUC-2 mRNA expression, and an up-regulation of ZO-1 protein expression This suggests that mannan can repair LPS-induced Caco-2 cell damage.
3.4 Regulation of Glycolipid Metabolism
Manno-oligosaccharides have been shown to regulate glucose and lipid metabolism. In a high-fat (HFD) mouse model, mannan oligosaccharide reduced liver and serum triglyceride (TG) levels, and significantly increased fecal TG and excreted fat content (Izumi et al., 2006). In a diabetic mouse model constructed by intraperitoneal injection of tetracycline, Qiyu Gao et al. (2012) found that mannan oligosaccharides significantly reduced TG, blood glucose, and cholesterol (CHO) levels in mice, and significantly increased high-density lipoprotein (HDL) cholesterol (HDL-C) levels, and that high doses of mannan oligosaccharides had a better effect on reducing glycolipids than low doses. Acetic acid, propionic acid and butyric acid are the main short-chain fatty acids in the intestine. Acetic, propionic and butyric acids control body weight by regulating energy intake and expenditure in the host (Dinesh et al., 2017) and help to reduce weight gain caused by high-fat diets (Den et al., 2015).
Wang Hongshan et al. (2018) found that mannan oligosaccharide can significantly increase the content of acetic acid, propionic acid and butyric acid in the cecum of mice on normal and high-fat diets, effectively slowing down the weight gain caused by high-fat diets, and improving the capacity of lipid metabolism of intestinal flora of mice. Silvia et al. (2015) found that mannan oligosaccharides can induce the body to preferentially retain long-chain polyunsaturated fatty acids (LC-PUFA) and reduce the level of fatty acids as β-oxidation substrates, thus altering the fatty acid composition of the liver and muscle, which is related to the reduction of the expression of the gene for desaturase in the liver. In addition, mannan oligosaccharides promote LC-PUFA accumulation and β-oxidation by affecting parameters related to intestinal-associated lymphoid tissues and regulating lipid metabolism in muscle and liver.
Leptin, a protein produced mainly by adipocytes, can regulate energy balance by suppressing starvation.Wang et al. (2018) found that the transcription level of leptin gene was significantly elevated in HFD mice, whereas the addition of mannan oligosaccharides resulted in a significant decrease in the transcription level of leptin gene, which inhibited body weight gain and adipose accumulation in HFD mice; furthermore, mannan oligosaccharides could reduce the transcription level of lipocalin gene to alleviate insulin resistance and glucose intolerance induced by HFD in HFD mice. In addition, mannan oligosaccharide can reduce insulin resistance and glucose intolerance induced by HFD mice by lowering the transcription level of the lipocalin gene.
4 Mannan Oligosaccharide in Livestock and Poultry Industry
4.1 Application in Pig Production
In swine production, mannan oligosaccharides have been shown to promote growth, improve feed remuneration, enhance animal immunity, increase antimicrobial potency, and improve meat quality (Porntrakulpipa et al., 2016; Su et al., 2016).Zhao et al. (2012) found that the addition of 0.1% mannan oligosaccharides not only significantly improved the growth performance of weaned piglets, but also significantly improved dry matter and nitrogen digestibility, and significantly reduced the diarrhea rate of piglets. The addition of 0.1% mannan oligosaccharide was found to significantly improve the growth performance of weaned piglets, and also significantly increase the digestibility of dry matter and nitrogen, and significantly reduce the rate of diarrhea. In addition, it was reported that mannan oligosaccharide did not improve the growth performance of animals (Hrvoje et al., 2016).

Neutralizing antibody is an antibody produced by the body stimulated by viral surface antigen with adsorption and penetration function.Porntrakulpipat et al. (2016) showed that 400 ppm of manno-oligosaccharides could effectively enhance PRRS-specific antibody but not neutralizing antibody, while 800 ppm of manno-oligosaccharides could significantly enhance neutralizing antibody. It can be seen that adding glycooligosaccharides to sow feed can help to strengthen the effect of PRRS vaccination in sows. In summary, the reports on the growth-promoting effect of mannan oligosaccharide tend to be positive, but its mechanism of action needs to be further investigated. In recent years, the research reports of mannan oligosaccharide in pigs at home and abroad are shown in Table 1.
Table 1 Major studies and applications of glycoligosaccharides in swine production
Manno-oligosaccharides Additions | Trial phase and duration | Test results References | ||
0.1% | Weaned piglets (21 d), test period 28 d | The growth-promoting effects were significant for ADG and ADFI Significantly higher apparent dry matter and nitrogen elimination rates; significantly lower diarrhea rates Zhao et al (2012) | ||
0.2% | Weaned piglets (21 d), test period 28 d | Significantly reduced the rate of piglet diarrhea and significantly increased the rate of diarrhea in piglets. ADG, and Ala ~ Gln and mannan oligosaccharides did not interact in Tee Ping et al. (2017) High growth performance | ||
0.2% | Weaned piglets (30 d), test period 28 d | Significantly higher ADG and lower G:F; Significantly higher ADG and lower G:F; Significantly higher ADG and lower G:F. Reduced serum levels of total protein, urea nitrogen, and total cholesterol Chun-zao Wu et al. 2011; Significantly improved antioxidant properties | ||
0.2% | Weaned piglets (28 d), test period 35 d | Significantly increased PHA activity in neutrophils sex; T lymphocytes CD4+ and CD8+ Valpoti Significantly higher ratio and improved immune performance | ||
0.1% | Piglets weighing about 8 kg, test period 28 d | Increased bifidobacteria counts. | , but promotes growth Su et al. (2016) | |
0.3% | Piglets weighing about 12 kg, test period 42 d | Significantly increased final body weight and ADG, and had a significant growth-promoting effect. 0.2% glyco-oligosaccharides can increase the birth weight of piglets and shorten the interval between the estrus of sows, and increase the number of sows in estrus. | Poeikhampha et al. (2011) | |
0.1%, 0.2%, 0.4% | Sows, trial period from gestation 85 d to weaning | For weaning weight of piglets and daily weight gain of piglets during lactation, 0.1%, 0.2% and 0.4% doses of manno-oligosaccharides significantly increased the IgG content in the whelping serum and colostrum of sows. | Li, Yuxin (2015) | |
0.04% | Sows, trial period from gestation 86 d to weaning | Significantly shortened the interval between weaning and oestrus in sows, and significantly increased the weaning weight of piglets and the daily gain of piglets during lactation. |
Duan et al. (2016) | |
4.2 Application in Poultry
In broiler production, mannan oligosaccharides can promote the expression of genes closely related to intestinal health, such as LUM, LYZ and APOA1, and thus regulate intestinal immune responses and protect animals from the toxicity of intestinal disease-causing bacteria to maintain intestinal health in broilers (Xiao et al., 2012). Wuwei et al. (2017) found that 50 and 75 mg/kg of mannan oligosaccharides could significantly increase the body weight and feed intake of broilers, in which 50 mg/kg of mannan oligosaccharides significantly improved feed remuneration; mannan oligosaccharides could also significantly increase CAT, SOD activity, GSH-Px activity and T-AOC activity, and the suitable amount of mannan oligosaccharides was 50 mg/kg.
Song Xinlei et al. (2018) found that mannan oligosaccharide can significantly increase the levels of IgA and IL-2 in the blood of broiler chickens, which can enhance the immunity of the body. In addition, mannan oligosaccharides have the effect of mitigating heat stress in broiler chickens (Sohail et al., 2010). In egg studies, Zaghini et al. (2005) showed that mannan oligosaccharide has the ability to adsorb and degrade aflatoxin B1 (AFB1), which can reduce the absorption of AFB1 in the gastrointestinal tract.

Bozkurt et al. (2016) found that the addition of mannose oligosaccharides to the diet of laying hens can significantly improve their egg production, egg weight and feed compensation, increase the antioxidant capacity, and reduce the number of pathogenic bacteria in the back part of the cecum. and reduced the number of pathogenic bacteria in the posterior segment of the cecum. In conclusion, adding appropriate amount of Glyco-oligosaccharides to poultry diets has many physiological functions such as improving growth performance, enhancing immunity and antioxidant capacity, but the mechanism of Glyco-oligosaccharides needs to be studied in depth. However, the mechanism of action of glyco-oligosaccharides needs to be studied in depth. The research reports on glyco-oligosaccharides in poultry in recent years are shown in Table 2.
Table 2 Major studies and applications of glycoligosaccharides in poultry production
Manno-oligosaccharides Additions | Poultry species and test duration Test results | bibliography | |
0.1% | In 1-day-old broilers, feed intake and ADG were significantly increased. Test period 42 d Fruit better than Chrysomycin | Jane Yunhua et al. (2016) | |
0.1% | Mannitoligosaccharides significantly increased meat flavor under heat stress conditions. Growth performance and GSH-px activity in the breast muscle of 1-day-old AA chicks. The test period was 42 d. Significantly reduced serum corticosterone concentration, MDA Concentration and 48 h drip loss |
Cheng et al. (2018) | |
0.1% | 36-week-old laying hens Test period 15 weeks | Significantly increased eggshell weight; Significantly decreased relative egg white weight of egg yolks; Significantly decreased egg white height and haf units | Bozkurt et al. (2012) |
0.1% | 82-week-old laying hens Test period 25 weeks | Significantly increased egg weight, egg production and improved feed cost; significantly increased liver antioxidant capacity and inhibited the growth of cecum pathogenic bacteria. | Bozkurt et al. (2016) |
0.05%, 0.1%, 0.15% |
55-week-old laying hens Test period 11 weeks | 0.1% and 0.15% mannan oligosaccharides significantly increased egg production rate and egg weight, and improved feed remuneration; addition of mannan oligosaccharides significantly reduced the number of Salmonella and increased the number of lactobacilli; mannan oligosaccharides increased the digestibility of DM and CP, and the digestibility was highest in the group with 0.05% mannan oligosaccharides. |
Jahanian et al. (2015) |
4.3 Application in Ruminants
Manno-oligosaccharides have been less studied in ruminants. Xiao Yu (2012) found that mannan oligosaccharides could significantly reduce the pH of goat rumen, significantly reduce serum MDA and ALT activity, significantly increase serum globulin and serum phosphorus content, significantly increase serum IgA at 21 d, and serum IgM at 7 and 14 d. Mannan oligosaccharides had the effects of improving fermentation parameters of goat rumen and enhancing the immune system. Xie Xinming et al. (2018) found that mannan oligosaccharide improved growth performance and immunity in Mongolian sheep.

Manno-oligosaccharides can increase calf ADG and feed compensation, increase serum immunoglobulin content and fecal bifidobacteria counts, and decrease fecal E. coli counts (Jin Yadong et al., 2016). Guo Tingting et al. (2017) reported that mannan-oligosaccharides significantly increased the total volatile acids and ammoniacal nitrogen content in the rumen of dairy cows, of which the acetic acid content in rumen fluid was significantly higher; the milk fat percentage was significantly higher, and the number of somatic cells in the milk was lower.Westland et al. (2017) found that mannan-oligosaccharides significantly increased colostrum weight of cows with the effect of improving growth performance.
5 Conclusion
As a new type of feed additive, mannan-oligosaccharides have been widely used in the feed industry. However, there are still some unsolved problems in the process of application, which constrain its popularization and application in aquaculture industry. In the future, we should increase the research on the mechanism of mannan oligosaccharides in the animal body, the way of adding mannan oligosaccharides in different stages of the animal and its appropriate amount, the effect of combining mannan oligosaccharides with other feed additives, and the interactions between mannan oligosaccharides and the intestinal flora of the animal, and so on. With the deepening of the research and the further clarification of the related mechanism of action, mannan-oligosaccharides will be more rationally and widely used, and its application value will be utilized to a greater extent.
Reference:
[1] Chen Ting . Study on the molecular regulatory mechanism of intestinal epithelial cell tight junction by Intestinal An Ⅱ formula based on MLCK-MLC signaling pathway: [Doctoral Dissertation][D]. Beijing: China Academy of Traditional Chinese Medicine, 2016.
[2] CHEN Xiaobing, HONG Biao, QIAO Yu. Beneficial effects, immunologic mechanism and application technology of mannan oligosaccharide[J]. China Animal Husbandry & Veterinary Medicine, 2005, 8: 6 ~ 8.
[3] Chen Xiaoying . Research on co-production of mannan oligosaccharide and glucan from waste yeast: [Master's thesis][D]. Hangzhou: Zhejiang University of Technology, 2017.
[4] Duan Xudong . Effects of dietary mannan-oligosaccharides on reproductive performance, immune function, offspring growth, immunity and intestinal microbiology in sows: [Master's thesis][D]. Ya'an: Sichuan Agricultural University, 2013.
[5] Gao Long . Study on the inhibition of Salmonella and Escherichia coli adhesion by mannan disaccharide: [Master's thesis] [D]. Beijing: Chinese Academy of Agricultural Sciences, 2016.
[6] GAO Qiyu, XU Guangcui, JIANG Yuanyuan . Effects of mannan oligosaccharides and chitosan on blood glucose and blood lipids in tetracycline-induced diabetic mice[J]. Sichuan Animal, 2012, 31(1): 129 ~ 131.
[7] GUO Tingting, HU Dandan, JIN Yadong, et al. Effects of mannan oligosaccharide on rumen fermentation and production performance of dairy cows in early lactation[J]. Feed Industry, 2017, 38(17): 56 ~ 60.
[8] GUO Yungui, YANG Bilin, SUN Jianhong, et al. Effects of konjac mannan oligosaccharide on antioxidant capacity of three yellow chickens[J]. Journal of Wuhan Institute of Biological Engineering, 2010, 3: 72-74.
[9] HANG Suqin . Effects of mannan oligosaccharide on intestinal microorganisms of weaned piglets: [Doctoral dissertation][D]. Nanjing: Nanjing Agricultural University, 2007.
[10] HE Zhikun , ZHAO Changhong , LI Mengting , et al. Drying and heating selenoacidification of konjac glucomannan oligosaccharide and antioxidant properties of its products [J]. Food Science, 2013, 34(5): 5 ~ 9.
[11] JIAN Yunhua, GAO Chunguo, JIANG Shouqun. Effects of mannan oligosaccharides on production performance and intestinal microflora of medium-fast yellow feather broilers[J]. Chinese Poultry, 2016, 38(11): 78 ~ 80.
[12] JIN Yadong, ZHANG Lili, CHEN Shaoshu. Effects of mannan oligosaccharides additive mode on growth performance, fecal flora and serum immunity indexes of lactating calves[J]. China Animal Husbandry & Veterinary Medicine , 2016, 43(11): 2922 ~ 2930.
[13] KOU Qing, LIANG Mijuan, TAO Liangliang, et al. Research and application of oligosaccharides [J]. Grain and Feed Industry, 2012, 5: 57 ~ 59.
[14] LI Guohui, WANG Jinrong, SU Lanli, et al. Research on the application of forage mannan oligosaccharides in animal production[J]. Feed Expo, 2017, 11: 20 ~ 23.
[15] LI Mei, LIU Wenli, ZHAO Guiying, et al. Effects of different oligosaccharides on immunity and productivity of piglets[J]. Anhui Agricultural Science, 2010, 38(28): 15655 ~ 15657.
[16] Li XM . Microwave solid-state synthesis of oligosaccharides and their antioxidant and immune activities in mice: [Doctoral dissertation] [[D]. Jiangnan University, 2008.
[17] LI Ying, YANG Ting, ZHU Xia, et al. Response surface method to optimize the process conditions of glucose extraction from grape wine with Saccharomyces cerevisiae mannan[J]. Food Industry Science and Technology, 2015, 36(16): 294 ~ 298.
[18] Li Yuxin . Effects of Beech yeast manno-oligosaccharides on productive and immunologic performance of pigs: [Doctoral dissertation] [D]. Beijing: China Agricultural University, 2015.
[19] LIANG Yong, LI Biao, DAI Jinjun . Progress in the application of mannan oligosaccharide in feed industry[J]. Feed Research, 2013, 1: 32 ~ 33+36.
[20] LIU Weidong, SU Fang, CHENG Pu . Effects of mannan oligosaccharides and probiotics on production performance and intestinal flora of broiler chicks[J]. Journal of Livestock Ecology, 2011, 32(1): 32 ~ 35.
[21] Liu Zizheng . Enzymatic preparation process and functional study of mannan oligosaccharides: [Master's thesis] [D]. Huazhong Agricultural University, 2016.
[22] SHEN Wenkang, ZHANG Mingjun, ZHAO Ningfang. Effect of mannan oligosaccharides on immunity of immunocompromised mice[J]. Hunan Animal Husbandry and Veterinary Medicine, 2015, 4: 13 ~ 15.
[23] SONG Xinlei, ZHU Lianqin, LIN Gang, et al. Effects of combined application of oligosaccharides and organic selenium on production performance and immunity of broiler chickens[J]. China Poultry, 2018, 40(8): 24 ~ 27.
[24] WANG Bin . Study on the effect of galactomannan oligosaccharide replacing antibiotics on pigs: [Master's thesis][D]. Changsha: Institute of Subtropical Agroecology, Chinese Academy of Sciences, 2006.
[25] WANG Hongshan, ZHANG Xiaojuan, LI Heng, et al. Beneficial effects of glyco-oligosaccharides in mice on high-fat diet[J]. Food and Fermentation Industry, 2018, 44(11): 63 ~ 68.
[26] NG Chun-zao, WANG Jian-hua. Effects of Lactobacillus and Oligosaccharides on growth and serum biochemical indexes of weaned piglets[J]. Jiangsu Agricultural Journal, 2011, 27(1): 94 ~ 99.
[27] Wu S . Synergistic repair effects of konjac mannan oligosaccharide and Bacillus subtilis on intestinal epithelial cell injury:[Master's thesis][D]. Huazhong Agricultural University, 2017.
[28] WU Wei, ZHENG Yunduo, JIA Linan, et al. Effects of glyco-oligosaccharides on early growth performance and related physicochemical indexes of broiler chicks[J]. Chinese Poultry, 2017, 39(01): 34 ~ 37.
[29] Xiao Y . Effects of functional oligosaccharides on rumen fermentation parameters and serum biochemical and immunological indices in goats: [Master's thesis][D]. Qingdao: Qingdao Agricultural University, 2012.
[30] Xie Mingxin, Wang Hairong, Yang Jinli, et al. Effects of yeast mannan oligosaccharides on growth performance, serum immunity and inflammation and antioxidant indices in Mongolian sheep [J]. Journal of Animal Nutrition , 2018, 30(1): 219 ~ 226.
[31] Xiong A-Ling . Effects of dietary mannan-oligosaccharides on growth performance and expression of natural immunity-related genes in broiler chickens: a study [Master's thesis][D]. Nanchang: Jiangxi Agricultural University, 2014.
[32] Xu Wenzhe . tBHQ and peroxide reductase 2 in ferrous ion-induced neurological injury: a study on the mechanism of action:[Doctoral dissertation][D]. Jinan: Shandong University, 2018.
[33] YANG Hua, WANG Zhenxi . Research Progress on the Application of Microecological Additives -- Glycoligosaccharides[J]. Jiangxi Journal of Animal Husbandry and Veterinary Medicine, 2005, 1: 22 ~ 23.
[34] YANG Xueshan, ZHU Xia, LI Ying, et al. Orthogonal experiments to optimize the extraction process of glucomannan from grape wine sludge yeast and its in vitro antioxidant effects [J]. Food Science, 2015, 36(18): 69 ~ 74.
[35] Yao Ximei. Preparation of konjac glucomannan oligosaccharides by continuous enzyme-membrane coupled reaction: [Master's thesis][D]. Beijing: Chinese Academy of Agricultural Sciences, 2011.
[36] Zhang Shuai . Protective effects and mechanism of konjac mannan oligosaccharide and Bacillus subtilis on LPS-induced intestinal oxidative damage: [Master's thesis] [D]. Wuhan: Huazhong Agricultural University, 2018.
[37] Zhao Xiaofeng . Graft modification and enzymatic preparation of galactomannan oligosaccharides from guar gum: [Master's thesis][D]. Nanning: Guangxi University, 2008.
[38] Bozkurt M, Bintas E, Kirkan S, et al. Comparative evaluation of dietary supplementation with mannan oligosaccharide and oregano essential oil in forced molted and fully fed laying hens between 82 and 106 weeks of age[J].Poultry Science, 2016, 95(11):2576.
[39] Bozkurt M, Kucukyilmaz K, Catli A U, et al. Performance, egg quality and immune response of laying hens fed diets supplemented with mannan- oligosaccharide or an essential oil mixture under mod - erate and hot environmental conditions [J].Poultry Science, 2012, 91(6):1379.
[40] Carvalheiro F, Esteves M P, Parajo J C, et al. Production of oligosaccharides by autohydrolysis of brewery 's spent grain [J].Biore- source Technology, 2004, 91(1):93 ~ 100.
[40] Che T M, Johnson R W, Kelley K W, et al. Mannan oligosac- charide improves immune responses and growth efficiency of nurs - ery pigs experimentally infected with porcine reproductive and res - piratory syndrome virus [J].Journal of Animal Science, 2013, 91(12): 5668 ~ 5679.
[42] Cheng Y F, Du M F, Xu Q, et al. Dietary mannan oligosaccha- ride improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress[J].Journal of Thermal Biol - ogy, 2018, 75.
[43] Den B G, Bleeker A, Gerding A, et al. Short -chain fatty acids protect against high -Fat diet-Induced obesity via a PPARγ-depen- dent switch from lipogenesis to fat oxidation [J].Diabetes, 2015, 64 (7): 398 ~ 408.
[44] Devin J R, George E I. Two-Stage Hydrothermal Processing of Wheat (Triticum aestivum) Bran for the Production of Feruloylated Arabinoxylooligosaccharides [J].Journal of Agricultural and Food Chemistry, 2010, 58(10): 6427 ~ 6432.
[45] Dinesh K D, Monica P, Renuka M, et al. Gut microbiota modu- lation and its relationship with obesity using prebiotic fibers and probiotics: a review [J ].Frontiers in Microbiology, 2017, 8: 563.
[46] Duan X D, Chen D W, Zheng P, et al. Effects of dietary man- nan oligosaccharide supplementation on performance and immune response of sows and their of fspring [J].Animal Feed Science and Technology, 2016, 218: 17 ~ 25.
[47] Hang S Q, Zhu W Y. Gut bacterial and Lactobacilli communi- ties of weaning piglets in response to mannan oligosaccharide and sugar beet pulp in vitro fermentation[J].Agricultural Sciences in Chi - na, 2012, 11(1): 122 ~ 133.
[48] Hrvoje V, Marko S, Svjetlana T, et al. Effect of mannan oligosac- charide supplementation on blood and intestinal immune cells, bacte- ria numbers and performance in weaned pigs [J].Acta Veterinaria Brno, 2016, 85(3): 267 ~ 276.
[49] Izumi T, Shigeyoshi F, Asako I, et al. Effects of mannooligosac- charides from coffee mannan on fat storage in mice fed a high fat di - et[J]. Health Science, 2006, 52(3): 333 ~ 337.
[50] Jahanian R, Ashnagar M. Effect of dietary supplementation of mannan -oligosaccharides on performance, blood metabolites, ileal nutrient digestibility, and gut microflora in Escherichia coli ~ chal- lenged laying hens [J].Poultry Science, 2015, 94(9):2165.
[51] Joana L M.S.G, Alain H, Carmen L.O.P, et al. Galactomannans from Brazilian seeds: characterization of the oligosaccharides pro- duced by mild acid hydrolysis [J].International Journal of Biological Macromolecules, 1995, 17(1):13.
[52] Joel L P, David B. Two pathways to NF-κB [J].Molecular Cell, 2002, 10(4):693 ~ 695.
[53] Kim H J, Lee H S, Chong Y H, et al. p38 mitogen -activated protein kinase up-regulates LPS-induced NF-κB activation in the development of lung injury and RAW 264.7 macrophages [J].Toxi- cology, 2006, 225(1): 36 ~ 47.
[54] Liang H E, Lee R R, Bando J K, et al. Divergent expression patterns of IL -4 and IL -13 define unique functions in allergic im- munity [J]. Immunology, 2012, 13(1):58 ~ 66.
[55] Matthew L T, James G C, Gareth D H, et al. Epithelial and stro- mal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll -like receptors TLR2, TLR1, and TLR6 [J].Endocrinology, 2014, 155(4):1453 ~ 1465.
[56] Mehmet L O, Hasan E S, Okur A A. Effects of mannanoligosac- charide and/or organic acid mixture on performance, blood parame- ters and intestinal microbiota of broiler chicks [J].Italian Journal of Animal Science, 2010, 8(4): 595 ~ 602.
[57] Mia Y, Michael J D, David B, et al. Characterization of oligosac- charides from an antigenic mannan of Saccharomyces cerevisiae [J]. Glycoconjugate Journal, 1998, 15(8): 815 ~ 822.
[58] Ping W Q, Qin H, You J, et al. Effects of alanyl-glutamine and mannan oligosaccharides on growth performance and diarrhea rate in weaned piglets [J]. Feed Industry, 2017.
[59] Poeikhampha T, Bunchasak C. Comparative effects of sodium gluconate, mannan oligosaccharide and potassium diformate on growth performances and small intestinal morphology of nursery pigs [J].Asian-Australasian Journal of Animal Sciences, 2011, 24(6): 844 ~ 850.
[60] Porntrakulpipat S, Tuangtananan Y, Schoosing S, et al. Mannan oligosaccharide supplementation during gestation can enhance porcine reproductive and respiratory syndrome virus (PRRS)-spe- cific antibody levels of sows and their piglets [J].Pakistan Veterinary Journal, 2016, 36(1) : 106 ~ 108.
[61] Pourabedin M, Chen Q, Yang M, et al. Mannan and Xylo - oligosaccharides modulate cecal microbiota and expression of inflam- matory related cytokines and reduce cecal Salmonella Enteritidis colo - nization in young chickens [J].Fems Microbiology Ecology, 2016, 93(1):fiw226.
[62] Prawitwong P, Takigami S, Phillips G O. Effects of irradiation on molar mass and properties of Konjac mannan [J].Food Hydrocolloids, 2007, 21(8): 1362 ~ 1367.
[63] Quan Q, Hao Q, Song T, et al. Evaluation of Antioxidant Ac- tivities of Ampelopsin and Its Protective Effect in Lipopolysaccha - ride-Induced Oxidative Stress Piglets [J].Plos One, 2014, 9 (9):e108314.
[64] Sang H M, Fotedar R, Filer K. Effects of dietary mannan oligosaccharide on the survival, growth, immunity and digestive en- zyme activity of freshwater crayfish, Cherax destructor Clark (1936) [J].Aquaculture Nutrition, 2015, 17(2): e629 ~ e635.
[65] Silivia T, Daniel M, Maria J C, et al. Effects of dietary concen- trated mannan oligosaccharides supplementation on growth, gut mu- cosal immune system and liver lipid metabolism of European sea bass (Dicentrarchus labrax) juveniles [J].Fish Shellfish Immunol, 2015, 42(2): 508 ~ 516.
[66] Singh V, Tiwari A. Hydrolytic fragmentation of seed gums under microwave irradiation [J].International Journal of Biological Macro - molecules. 2009, 44(2):186 ~ 189.
[67] Sohail M U, Ijaz A, Yousaf M U, et al. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccha - ride and Lactobacillus -based probiotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity[J]. Poultry Science, 2010, 89(9): 1934 ~ 1938.
[68] Su C W, Xiao F Y, Yi L I, et al. Effects of probiotics and man- nan oligosaccharides in the wheat diet on the productive perfor- mance and fecal microorganisms in nursing pigs[J].Heilongjiang Ani-mal Science and Veterinary Medicine, 2016.
[69] Szilamer F, Krisztian S, Zsuzsanna W, et al. Oligomannan prebi- otic attenuates immunological, clinical and behavioral symptoms in mouse model of inflammatory bowel disease [J].Scientific Reports, 2016, 6: 34132.
[70] Valpotia H, Zura Z I, Samardzija M, et al. Dietary supplementa- tion with mannan oligosaccharide and clinoptilolite modulates innate and adaptive immune parameters of weaned pigs [J].Polish Journal of Veterinary Sciences, 2018, 21(1): 83 ~ 93.
[71] Wang H, Zhang X, Wang X, et al. Mannan -oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice [J].Food Funct, 2018, 9: 3906 ~ 3915.
[72] Westland A, Martin R, White R, et al. Mannan oligosaccharide prepartum supplementation: effects on dairy cow colostrum quality and quantity[J]. Animal, 2017, 11(10):1 ~ 4.
[73] Xiao R, Mallonee D, Routt K, et al. Effects of yeast cell wall derived mannan oligosaccharides on jejunal gene expression in young broiler chickens [J ].Poultry Science, 2012, 91(7):1660.
[74] Zaghini A, Martelli G, Roncada P, et al. Mannanoligosaccharides and aflatoxin B1 in feed for laying hens: effects on egg quality, afla- toxins B1 and M1 residues in eggs, and aflatoxin B1 levels in liver[J]. Poultry Science, 2005, 84(6): 825 ~ 832.
[75] Zhang K, Hornef M W, Dupet A. The intestinal epithelium as guardian of gut barrier integrity [J].Cellular Microbiology, 2015, 17 (11): 1561 ~ 1569.
[76] Zhao P Y, Jung J H, Kim I H. Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood pro- file, and diarrhea score in weanling pigs[J].Journal of Animal Science, 2012, 90(3):833.
[77] Zhou R, Shi X Y, Gao Y, et al. Anti -inflammatory activity of guluronate oligosaccharides obtained by oxidative degradation from alginate in lipopolysaccharide -activated murine macrophage


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese