The 5 Production Methods of Glutathione
Glutathione production methods include extraction, chemical synthesis, biosynthesis, enzymes, and fermentation. Fermentation refers to the use of microbial metabolism to convert low-priced nutrients into glutathione. The microorganisms used in fermentation are easy to cultivate, the nutrients are inexpensive and easy to obtain, and the operation process is simple, so based on the above advantages, fermentation has become the most commonly used method to produce glutathione.
1. Solvent Extraction
The classic method of glutathione production is extraction, where the solvents can be acidic organic solvents, H2O, esters, enols and mixtures of these components in different proportions. Extraction is the original method of glutathione production, which utilizes the different solubility of glutathione in two different solvents to extract glutathione[1] . The extraction method is mainly used for the separation and extraction of plant and animal tissues, using the method of extraction and precipitation, due to the difficulty of obtaining raw materials and the extremely low content of intracellular glutathione in plant and animal tissues, so the organic solvent extraction method is not very significant in the practical application of glutathione, and has not been widely used[2] .

2. Chemical Synthesis
The production of glutathione by chemical synthesis[3] is a chemical reaction in which glutamic acid, glycine and cysteine are chemically condensed to form glutathione, which is mainly used in the production of glutathione in the early stage. Currently, the production process has been more mature, but the steps are complicated, the operation process is complicated, and the trivial efficiency is low. It synthesizes the racemate of glutathione, which needs to be mixed with the racemate of glutathione using optical splitting reagents. The generation of diastereoisomers with different physicochemical properties for splitting ultimately leads to the difficulty of separation and low purity of the product, low production efficiency, and easy to cause environmental pollution. Therefore, it limits the wide application of this method.
3. Biosynthesis Method
There are two main forms of glutathione biosynthesis: fermentation and enzyme method. The common point is that there should be a good performance of the cell strain, and then use its more active enzyme system to synthesize glutathione under mild conditions; the biggest difference is that fermentation only needs to provide the nutrients required for microbial growth and metabolism, whereas the enzymatic method needs to provide a large amount of precursor amino acids and ATP to realize the accumulation of intracellular glutathione [4].

Biosynthesis of glutathione requires precursor amino acids mainly Glu, Cys and Gly. Meister et al. [5] proposed a γ-glutamyl cycle as shown in Figure 1, which emphasizes the central role of glutathione in amino acid uptake. In the biosynthetic pathway, glutathione is synthesized by the sequential action of two ATP-dependent ligases, i.e., γ-glutamylcysteine synthetase (GCL) and glutathione synthetase (GS)[6] .
Fig.1 Canonical γ-Glutamyl Cycle
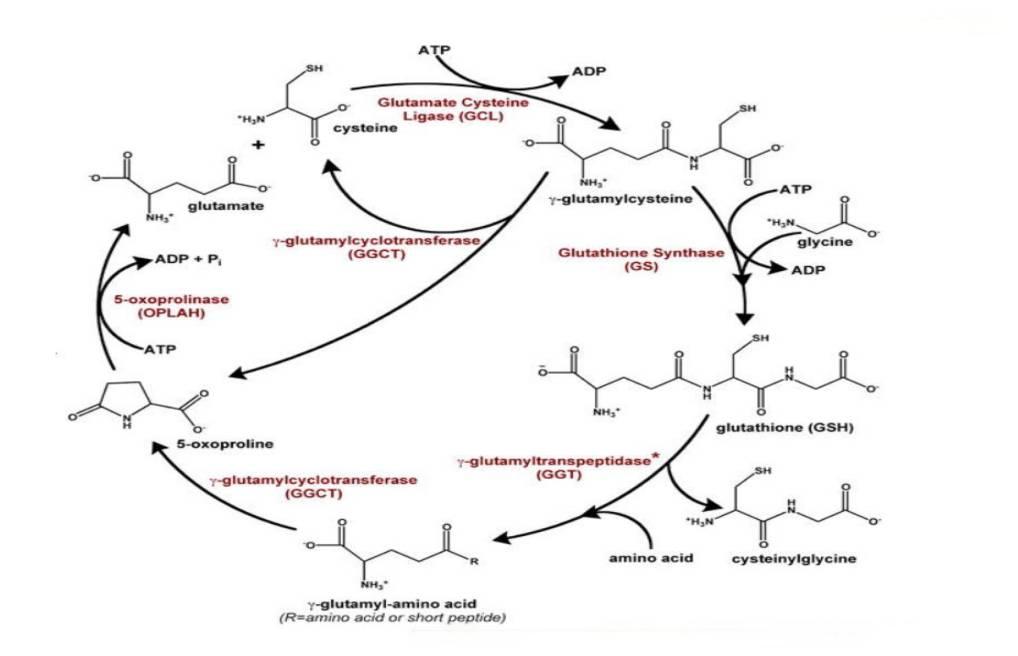
After biosynthesis comes the beginning of degradation of glutathione. In this cycle, degradation is carried out by γ-glutamyl transpeptidase (GGT), the only enzyme known at the time to degrade glutathione, which has a transpeptidic activity involving the transfer of γ-glutamyl groups to amino acids to form γ-glutamyl amino acids. The GGT activity called upon in the γ-glutamyl cycle is primarily a transpeptidase activity, and thus the enzyme's action is primarily transpeptidic, involving the transfer of amino acids to form γ-glutamyl amino acids, which are ultimately transported across membranes as γ-glutamyl amino acids. This was the initial understanding of glutathione biosynthesis and its metabolism.
In a later study, Bachhawat [53] initially concluded that the γ-glutamyl cycle was incorrect not only in terms of the functional role of amino acid transporter proteins but also in describing the synthesis and degradation of glutathione. This is also true in light of the newly emerging data on glutathione degradation. As these new findings have been explored and summarized, a new cycle has emerged, which we refer to as the “Glutathione Cycle[7] ”, as shown in Fig. 2.
Fig.2 Glutathione Cycle
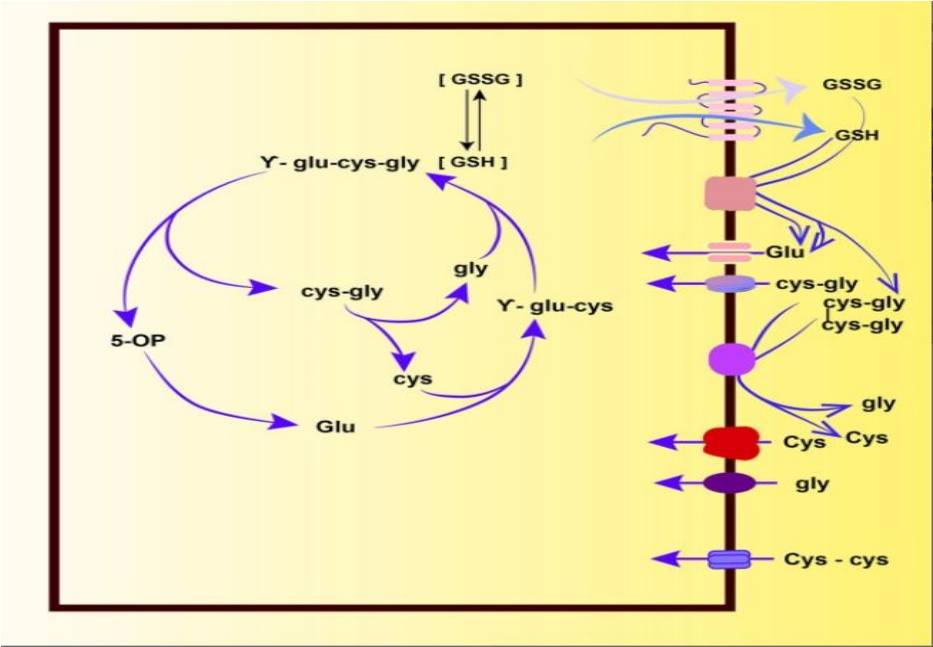
In the glutathione cycle, the first two steps are the biosynthesis of glutathione. The biosynthesis is carried out by two sequential enzymes, GCL catalyzes the formation of γ-glutamylcysteine from glutamate and cysteine in an ATP-dependent reaction. GS then catalyzes a second ATP-dependent reaction of γ-glutamylcysteine and glycine. Glutathione is then formed. It can be degraded by the cytoplasmic ChaC family of glutathione-specific degrading enzymes (γ-GCT) [13] to form 5-hydroxyproline and cysteinylglycine. Subsequently, 5-hydroxyproline and cysteinylglycine are cleaved by 5-hydroxyproline and cysteinylglycine peptidases, respectively, to form Glu, Cys, and Gly, which can be ploughed back to synthesize glutathione if required. Glutathione is formed via biosynthesis and can also form GSSG, with which it balances and determines the redox environment [8].
4. Enzymatic Synthesis of Glutathione
Enzymatic synthesis refers to the use of enzymes to catalyze the synthesis of glutathione from three amino acids. The enzyme-catalyzed method uses the three precursor amino acids as substrates, ATP as a functional substance, γ-glutamylcysteine synthetase (Glutathione I) and glutathione synthetase (Glutathione II), cofactors (Mg2+), and an appropriate pH environment. Currently, many studies have been reported on the enzymatic synthesis of glutathione, but in recent years, the main focus has been on the two-enzyme synthesis.
Xing Zhang et al.[9] synthesized glutathione enzymatically by constructing a multistage reaction of two enzymes (polyphosphate kinase (PPK) and glutathione bifunctional synthetase (glutathione F)), with polyphosphate as the energy donor, and optimized the expression conditions of the coupled enzymes (PPK and glutathione F), which resulted in a glutathione yield of 58±3.3 mmol/L. The enzyme synthesized glutathione by the enzymatic synthesis of PPK and glutathione F was also optimized. The glutathione yield reached (58±3.3) mmol/L. Enzymatic production of glutathione is characterized by high conversion rate and mild conditions. The lack of ATP supply and the high price of ATP are the biggest limiting factors for enzyme production.

In this study, Chen Yang et al [10] expressed an E. coli-derived PPK, which can be recycled for energy recycling, and solved the above problem. pET28a-glutathione f and pET28a-ppk vectors were constructed in a system containing polyphosphate kinase and transformed into E. coli BL21, respectively. A minimum of 20 mmol of ATP was required for the reaction to occur, after which ADP was generated and regenerated to obtain ATP for the continuous energy supply of the reaction, and the conversion rate of the substrate could reach 68.7% after 22 h. The reaction was carried out in a system containing a polyphosphate kinase.
5. Production of Glutathione by Fermentation Method
Fermentation refers to the use of microbial metabolism to convert low-priced nutrients into glutathione. Fermentation has become the most commonly used method for glutathione production due to the ease of cultivation of microorganisms, availability of nutrients, and simplicity of operation[11] .
Currently, yeasts, such as Saccharomyces cerevisiae, baker's yeast, and Pseudomonas syringae, are the most commonly used microorganisms for the production of glutathione, but most of the yeasts have a relatively low glutathione content. Therefore, selecting and breeding superior strains of yeasts, breeding wild strains by mutation and genetic engineering, as well as optimizing and regulating the culture conditions in the fermentation process are the hot topics in the research of the fermentation method. Kang et al.[12] isolated and selected dried Saccharomyces cerevisiae from Nuruk containing 25.53 µg/mg glutathione. Nisamedtinov et al[13] showed higher levels of glutathione in margaritas fermented with selected yeast strains. Random mutagenesis induced by UV irradiation of wild Saccharomyces cerevisiae strains resulted in a molecular mechanism of glutathione over-accumulation. The mutants accumulated glutathione at concentrations several times higher than their wild-type parent strain.

It has been shown that the addition of three precursor amino acids to yeast cell cultures increases glutathione levels to a certain extent. The batch addition of glutathione in Saccharomyces cerevisiae is more valuable than the sequential addition of cysteine as a method to promote glutathione production.14 Wen S et al.[15] investigated a two-step addition strategy as a suitable amino acid addition strategy: in the first step, cysteine was added after 2 h of incubation, followed by the addition of the three amino acids (glutamic acid, glycine, and serine) after 7 h. In shake flask cultures, the three precursor amino acids were added to the cells in the presence of glutamic acid, glycine, and serine, which were then added to the cells. In shake flask culture, the intracellular glutathione content was 55.2% higher than that without the addition of the three amino acids.
Wang Dahui et al. [16] investigated the effects of L-cysteine and L-methionine on the synthesis of glutathione in batch and flow-addition cultures, respectively, and showed that L-methionine enhanced the ability of yeast cells to synthesize glutathione during the growth phase, whereas L-cysteine significantly increased the content of intracellular glutathione in the yeast cells at the stage of almost stopping growth.
Based on this, we proposed a strategy to add amino acids to the yeast cells at the two stages of growth, which resulted in a glutathione yield of 1247.1 mg/L and an intracellular glutathione content of 24.1 mg/g. The results of the experiments were further improved. Wang Shuo et al.[17] showed that the addition of glucose, valine and L-cysteine to the culture medium during fermentation increased the total glu
References:
[1] Zhou Xiuqin. Special amino acid derivatives--glutathione[J]. Fermentation Science and Technology Newsletter, 2007, 36(2): 50-51.
[2] HUANG Jingchun, LIANG Libing. Production method of reduced glutathione and its application[J]. Light Industry Science and Technology, 2013, 1: 11-12.
[3] WANG Dahui, WEI Gongyuan. Prospects for the application of glutathione and current status of production research[J]. Chemical and Biological Engineering, 2004, 3: 10- 12.
[4] CHEN Jian, WEI Gongyuan, LI Yin, ZUO Guocheng. Production of glutathione by microbial fermentation[J]. Journal of Wuxi University of Light Industry, 2004, 5: 104- 110.
[5] Orlowski M, Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U SA. 1970 Nov; 67(3): 48-55.
[6] Kaur A, Gautam R, Srivastava R, et al. ChaC2, an enzyme for slow turnover of cytosolic Glutathione. J Biol Chem. 2017 Jan; 292(2): 638-651.
[7] Bachhawat AK, Kaur A. Glutathione degradation. antioxid redox signal. 2017 Nov; 27(15): 1200-1216.
[8] Bachhawat AK, Yadav S. The glutathione cycle: glutathione metabolism beyond the γ- glutamyl cycle. iUBMB Life. 2018 Jul; 70(7): 585-592.
[9] Zhang X, Cui XW, Li ZL, et al. Enzymatic production of glutathione based on energy cycle regeneration system[J]. Journal of East China Science and Technology (Natural Science Edition), 2020, 46(5): 119-124.
[10] CHEN Yang, WU Qiong, TAN Tianwei. Coupled ATP regeneration for enzymatic synthesis of glutathione[C]. 2013 Annual Meeting of the Chinese Society of Chemical Engineers. 2013.
[11] ZHAO Hong-Ling, GAO Yang, YIN Zhi-Feng, et al. Progress in the production of reduced glutathione by fermentation[J]. Journal of Chengde Medical College,2013, 30(6): 516-518.
[12] Kang S H, Kim H R, Kim J H, et al. Identification of wild yeast strains and analysis of their β-glucan and glutathione levels for use in makgeolli brewing . Mycobiology. 2014 Dec; 42(4): 1-7.
[13] Nisamedtinov I, Kevvai K, Orumets K, et al. Metabolic changes underlying the higher accumulation of glutathione in saccharomyces cerevisiae Appl Microbiol Biotechnol. 2011 Feb; 89(4).
2011 Feb; 89(4): 29-37.
[14] Hara K Y, Kiriyama K, Inagaki A, et al. Improvement of glutathione production by metabolic engineering the sulfate assimilation pathway of saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2012 Jun; 94(5): 3-9.
[15] Wen S H, Tao Z, Tan T. Utilization of amino acids to enhance glutathione production in Saccharomyces cerevisiae. 35(6-7): 501-507.
[16] Wang DH, Nie M, Wei GY. A high-yield method for glutathione fermentation based on two-stage amino acid addition[J]. Food Science, 2017, 38(22): 22-27
[17] WANG Shuo, XIE Yuewu, ZHANG Huiwen, et al. Optimization of fermentation and isolation and purification conditions of reduced glutathione[J]. Biotechnology Bulletin, 2013(11): 180-185.
[18] WAN Honggui, DENG Chunya, TAN Haitao, et al. Effects of two types of exogenous stimuli on glutathione production by fermentation of spent beer yeast[J]. Food and Fermentation Industry, 2014, 40(10): 54-57.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese