How to Produce Ubiquinone Coenzyme Q10 by Fermentation?
Coenzyme Q10 (ubiquinOne-10, COenzyme Q10, COQ10), also known as ubiquinone (hereinafter referred to as COQ10). Its molecular formula is C59 H90 O4 and molecular weight is 863. The structural formula is as follows:
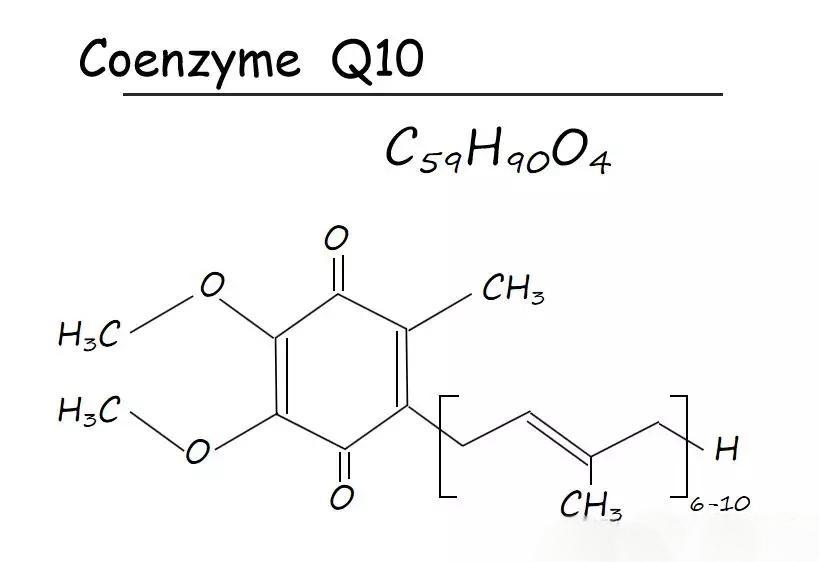
Ubiquinone Coenzyme Q10 is a fat-soluble quinone compound with orange-yellow crystals at room temperature and a melting point of 49 ℃, and it is odorless and tasteless [1].The physiological functions of COQ10 are mainly attributed to the redox properties of the quinone moiety and the physical properties of the isoprenoid side chain. Studies have shown that COQ10 in the reduced state and isoprene monomers with all trans structures have higher activity and pharmacological effects than COQ10 in the oxygenated state and isoprene monomers with all cis structures.
The role of COQ10 was first identified by MOOre et al. in 1940, but did not attract much clinical attention, and in 1957, Crane et al. purified COQ10 from bovine myocardium and measured its chemical structure, confirming that COQ10 actually plays an important role as a redox carrier in the respiratory transport chain of mammals.

Ubiquinone Coenzyme Q10 is known to be a fat-soluble electron carrier between NADH dehydrogenase, succinate dehydrogenase and bc complex in the respiratory chain, and it is a cellular energy-generating element, so it is a natural antioxidant and activator of cellular metabolism, and it is important in the treatment of cardiovascular diseases, and it can improve human immunity and treat human immune system diseases. As a natural antioxidant, it can be used in health and beauty care [2]. In China, the use of COQ10 capsules is increasing year by year, but the raw materials are basically dependent on imports, which requires the domestic production of large quantities of inexpensive COQ10 raw materials and preparations.
1 COQ10 Production Method
COQ10 is widely found in plant and animal tissues as well as microbial organisms. Microbial organisms generally contain high levels of COQ10, while plant and animal tissues contain low levels, making microorganisms a good source of COQ10.
Ubiquinone Coenzyme Q10 can be prepared by three methods: animal and plant tissue extraction, microbial fermentation and chemical synthesis. At present, animal and plant tissue extraction is mostly used in China. Overseas countries mostly use microbial fermentation, especially in Japan, where industrial production of COQ10 by microbial fermentation was realized as early as 1977.
The chemical synthesis method is characterized by harsh conditions and many steps; in addition, most of the isoprene monomers of COQ10 are cis-structures, which are not biologically active, and the content of by-products is high, so the purification cost is high. The method of animal and plant tissue extraction is mainly from the residue of pig heart after cytochrome C extraction. The content of COQ10 in animal tissues is low, with a yield of only 75 mg per kg of fresh pig heart [3], and the large-scale production is restricted by the limitation of raw materials and sources.

In contrast, the production of Ubiquinone Coenzyme Q10 by microbial fermentation has several basic advantages: (1) the fermentation product is natural, biologically active, and easily absorbed by the human body; (2) there is no limitation of raw materials, and the production capacity can be increased through scale-up. However, its limited content, low efficiency, and high production cost limit industrialization to a certain extent. If we can choose suitable strains for genetic modification, directional selection of strains with excellent performance, so as to increase the content of COQ10 in the bacterium, the production cost will be greatly reduced.
2 Advances in Microbial Fermentation for COQ10 Production
2 . 1 Selection of Strains
Despite the high content of COQ10 in microbial organisms in nature, the fermentation products are a mixture of various COQ10 congeners, which results in a high cost of COQ10 purification. Therefore, the selection of strains for the fermentation of COQ10 is of primary importance. The microorganisms used in the fermentation of COQ10 are listed in Table 1.
Table 1 Content of COQ10 in microbial organisms[4]
name of fungus | COQ Category | COQ Yield * |
Red bacterium R. capsulatus | COQ10 | 5.3 |
Rhodococcus sphaeroides | COQ10 | 5.3 |
Sulfur-loving small red oomycete R. su | COQ10 | 4.2 |
Swamp red pseudomonas Rp. palustris | COQ10 | 4.5 |
Rp. rubrum, the reddish red snail fungus | COQ10 | 6.3 |
Pseudomonas aeruginosa | COQ10 | 0 . 67 |
Throwing yeast sporobolomyces roseus | COQ10 | 0 . 51 |
Cryptococcus neoformans | COQ10 | 0 . 27 |
Black Powder Fungus ustilago zea | COQ10 | 0 . 20 |
From the above table, it can be seen that the content of COQ10 in the bodies of photosynthesizing bacteria (hereafter referred to as PSB) was generally high. In terms of taxonomic status, PSB belong to the phylum Bacteroidetes, which is divided into the subphylum Rhodobacteria and the subphylum Green Thiobacteria, and the former is divided into the families Rhodobacteriaceae and Rhodobacteriaceae; R. capsulatus and R. sphaeroides belong to the family Rhodobacteriaceae, which is one of the ideal choices for the COQ10-producing strains.
2 . 2 Genetic Modification of Strains
The Ubiquinone Coenzyme Q10 production capacity of wild-type strains is not sufficient to meet production needs, and can be genetically modified using conventional mutagenesis and genetic engineering techniques.
2 . 2 . 1 Breeding for Metabolic Regulation of COQ10
In 1976, Rudney proposed in an international conference on COQ10 that the microbial synthesis pathway of COQ10 is mainly divided into the biosynthesis routes of aromatic ring synthesis and isoprenyl side chain. According to the biosynthesis pathway of aromatic ring and isoprenyl side chain combined with the metabolic regulation mechanism of the bacteria, the selection pathway for the breeding of high yielding strains of COQ10 in industrial fermentation can be divided into the following:
(1) Selection and Breeding of Nutrient-Deficient Mutant Strains
OlsOn and Rudney [5] found that both carotenoids and COQ10 are anabolized by polyisoprene as precursors, and that reducing the production of carotenoids may promote the biosynthesis of COQ10. Therefore, the selection of a green mutant PSB strain could increase the COQ10 content. yOshida et al. [6] mutagenized R. sphae- roides Ky-4113, and obtained a green mutant strain with a 10-20% increase in COQ10 content compared with the wild strain.
(2) Selection and Breeding of Metabolic Antagonist-Resistant Mutant Strains
The removal of inhibitors on COQ10 synthesis or its related anabolism increased COQ10 levels. yOshida et al. [6] screened mutant strains for precursors, inhibitors, and their structural analogues (ethylthionine, L-methionine, methylnaphthoquinone, and daunorubicin) against COQ10 synthesis and obtained Agrobacterium tume『 aciens mutant strains with 10 to 20% higher levels than wild strains. aciens mutant strain was 10-20% higher than the wild strain.
Further combinatorial mutant strains, such as nutrient-deficient mutant strains and double mutant strains resistant to structural analogs, can be selected according to several routes to greatly increase the yield of the target product.
2 . 2 . 2 Constructing Genetically Engineered Strains
Using molecular biology technology to find the key enzyme gene of COQ10 production strain, and introducing this gene into the production strain by recombinant DNA technology, so as to increase the copy number of the key enzyme gene and express it efficiently, thus enhancing the ability to synthesize Ubiquinone Coenzyme Q10, this is the basic route for constructing the recombinant COQ10 fermentation strain.
The rate-limiting step in the biosynthesis of COQ in different organisms is the condensation of hydroxybenzoic acid with polyisoprene catalyzed by the enzyme paraben polyisoprene pyrophosphotransferase. Studies on this enzyme have shown that it has a relatively broad substrate specificity [7]. Based on this principle, the ubiA gene was cloned from E. coli and introduced into PSB, and the expression of this gene was enhanced to obtain a high-yield strain of COQ10.

On the other hand, since PSB is not a mature receptor for genetic engineering, the search for a metabolic pathway using E. coli as the receptor was turned to. The side chain lengths of the main components of COQ are controlled by genes (e.g., E. coli ispB, enzyme coq1, photosynthetic bacterium dds1, etc.), which are different in different cells of different organisms due to the different genes controlling the lengths of the side chains. E. coli is simple to cultivate at high density and has a well-established system for the expression of exogenous genes, but the main component of COQ synthesized by E. coli is COQ8.
Therefore, it is conceivable to clone the gene controlling the length of the side chain of COQ10 (dds1) from PSB and introduce it into E. coli cells, and at the same time inactivate ispB, the side chain control gene of COQ8, so as to realize the large-scale production of COQ10 in recombinant E. coli. Currently, ShOkuhin et al. showed that it is feasible to synthesize COQ10 in E. coli [8, 9].
2 . 3 Optimization of Fermentation Conditions
In addition to the application of metabolic control theory to select and breed high-yielding mutant strains or construct recombinant strains to improve fermentation yield, optimizing the fermentation conditions of producer bacteria is also an important and effective way to improve fermentation yield.
2 . 3 . 1 Optimization of the Culture Medium
In Ubiquinone Coenzyme Q10 fermentation, optimization experiments were carried out by selecting different sources of carbon, nitrogen, growth factors and inorganic salts to determine the composition of the culture medium. It has been shown that metal ions, especially Mg2+, Fe2+, and Mn2+, can promote the production of COQ10 by fermentation of R. sphaeroides. The addition of 12 . 2 mmOl/L MgSo4, 1 . 8 mmOl/L FeSo4 ·7H2 o,0 . 9 mmOl/L MnSo4 -7H2 o increased the COQ10 yield from 2.0 mg/g dry wt to 2.0 mg/g dry wt. 0 mg/g dry wt to 8 . 9 ~ 9 . 6 mg/g dry wt (Asahi Chemical Industry CO., Ltd., Ja- pan, 1981). In addition, precursors can significantly increase the product yield and, under certain conditions, control the flow of anabolic products in the bacterium. The precursors reported to be added in the production of COQ10 fermentation are p-hydroxybenzoic acid, mevalonic acid, isopentenol and geraniol [10].

2 . 3 . 2 Optimization of Culture Conditions
(1)Mixing-Aeration
The effect of stirring and aeration on CoQ10 production varies depending on the strain, with one promoting CoQ10 production [11] and the other inhibiting it [4, 12]. According to the existing studies, agitation and aeration are unfavorable to the production of CoQ10 by R. sphaeroides. sakato et al. investigated the effect of agitation and aeration on CoQ10 production using R. sphaeroides Ky8598 fermentation for the production of CoQ10 [13]. The results showed that the best bacterial growth was achieved when the redox potential (ORps) was - 150 mM, and the highest CoQ10 production was achieved when the ORps was - 200 mM, i.e., the restriction of oxygen supply was favorable to both bacterial growth and CoQ10 production.
On the basis of the above study, Yoshida et al. observed the effect of oxygen supply on the microstructure of R. sphaeroides by electron microscopy [6]. It was shown that the inner membrane of the cytoplasm of bacteria grown under oxygen-limited conditions was well developed and had a multilayered structure, where the photoreaction center of psB was located, which might lead to a higher content of CoQ10 than that of the bacteria grown under well-oxygenated conditions.
(2)Sunlight
Rhodobacter sphaeroides is capable of both specialized anaerobic bacterial photosynthesis and aerobic respiration and fermentation, and Car and ExCell have reported that CoQ10 production by psB is high under anaerobic conditions in the light, but decreases dramatically once the culture is switched to dark aerobic conditions [4].
(3)Incubation Time
Yoshida et al. found that the content of CoQ10 was higher when the bacteria were in the pre-stabilization period [6]. Zhu Xufen et al. also found that the content of CoQ10 in the bacteria increased with the increase of incubation time, and reached the highest level in the middle of the pre-stabilization period, and then began to decline [14].
2 . 4 CoQ10 Extraction from Microbial Cells
There are two methods for extracting CoQ10 from microbial cells: unsaponified and saponified. Unsaponifiable extraction has the advantage that it does not destroy CoQ10, although the amount of extract obtained is less than that obtained by saponification [15]. Saponification is the classical method for extracting fat-soluble substances, which is simple but costly and is being eliminated in modern industrial production [1, 16]. The recently proposed alkaline saponification method can completely eliminate the consumption of pyrogallic gallic acid and ethanol solvent, and the use of acid crushed cells directly saponified, so that the production cost of CoQ10 is greatly reduced, and thus it is possible to apply to industrialized mass production.
3 Outlook
At present, the price of CoQ10 products on the market is high. Especially in China, most of CoQ10 products are imported, which is due to the low fermentation content of strains and the low yield of isolation and purification. Therefore, in order to realize the industrial production of CoQ10 by microbial fermentation, it is necessary to use conventional microbial breeding methods and recombinant DNA technology to genetically modify the production strains, increase the content of intracellular CoQ10, and optimize the extraction route to reduce the cost of extraction and isolation.
Reference:
[1] Yunnan Zoological Research Institute. Isolation of coenzyme Q10 from pig heart residue for the preparation of cytochrome C[J]. Pharmaceutical Industry, 1976,(2):22 .
[2] Wu Zufang et al. Progress on the function of coenzyme Q10. [J] Journal of Ningbo University. 2001, 14(2):85-88.
[3] Yuan Yi. Extraction and purification of coenzyme Q10 (CytC) from pig heart [J]. Journal of Anhui Agricultural University, 1997, 24(2): 200 ~ 203.
[4] Carr NG aNd Exell G. ubiQuiNoNe CoNCeNtratioNs iN Athio- rhodaCeae growth uNder Various eNViroNmeNtal CoNditioNs [J]. rhodaCeae growth uNder Various eNViroNmeNtal CoNditioNs [J]. BioChim. J., 1965, 96: 688 ~ 692.
[5] OlsoN EO aNd RudNey H. BiosyNthesis of ubiQuiNoNe [J]. Vitam. Horm., 1983, 40: 1 ~ 43.
[6] Yoshida H et al. produCtioN of ubiQuiNoNe - 10 usiNg baC- terial [J]. J. GeN. Appl. MiCrobiol., 1998, 44: 19 ~ 26.
[7] El HaChimi Z, samuel O et al. BioChemiCal study oN ubiQuiNoNe biosyNthsis iN EsCheriChia Coli. 1. speCifiCity of para - hydrabeNzoate polypreNyltraNsferase [J]. BioChim- ie. , 56: 1239 ~ 1247.
[8] shokuhiN A. FormatioN of ubiQuiNoNe - 10. Jp11056372.
[9] Zhu X, yuasa M, Okada K, suzuki K, Nakagawa T. production of ubiQuiNoNe iN EsCheriChia Coli by expressioN of Various geNes respoNsible for ubiQuiNoNe biosyNthesis [J]. biosyNthesis[J].J. FermeNt. BioeNg., 1995, 79(5):493 ~ 95.
[10] Matsumura M, Kobayashi T, Aiba s. ANaerobiC production of ubiQuiNoNe - 10 by paraCoCCos deNitrifiCaNs [J].Eur. J. Appl. MiCrobiol. BioteChNol. 1983, 17(2):85 89.
[11] whistaNCe GR aNd Threifail DR. EffCt of aNerobiosis on the concentrention of demethylmeNaQuiNoNe, meNaQui- NoNe aNd ubiQuiNoNe iN EsCheriChia freuNdii , proteus mirabilis aNd AeromoNas puNCtata [ J ]. BioChem. J., 1968, 108: 505 ~ 507.
[12] urakami T aNd yoshida T. produCtioN of ubiQuiNoNe and baCterioChlorophyll a by RhodobaCter sphaeriodes aNd RhodobaCter sulfidophilus[ J]. J. FermeNt. BioeNg. , 1993, 76: 191 ~ 194.
[13] sakato K et al. AgitatioN - AeratioN studies on CoeNzyme Q10 produCtioN usiNg RhodopseudomoNas sphaeriodes [J]. BioteChNol. Appl. BioChem. 1992, 16: 19 ~ 28.
[14] ZHU Xufen, ZENG Yunzhong, et al. Exploration of ubiquinone species and synthesizing conditions in organisms[J]. Journal of Zhejiang University (Science Edition), 2000, 27(3): 324 ~ 328.
[15] Ouyang Pingkai, Hu Yonghong. Production of coenzyme Q10 and its application[J]. Progress of Chemical Industry, 1994, 4: 9 ~ 11.
[16] Ikeda T, Matsumoto T et al. FormatioN of ubiQuiNoNe by tobaCCo plaNt Cells iN suspeNsioN Culture[J]. phytoChem- istry, 1976, 15: 568 ~ 569.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






