Study on Nanoliposomal Coenzyme Q10 Preparation
Abstract: Coenzyme Q10 (Q10), also known as ubiquinone, can inhibit skin lipid peroxidation to delay skin aging, and has been added to cosmetics as an important anti-aging active ingredient. However, coenzyme Q10 is easy to decompose in the presence of light, and most of the topical preparations of coenzyme Q10 available on the market are ordinary emulsions or gels, which are poor in photostability and lack of slow and controlled release properties, and cannot effectively exert the efficacy of coenzyme Q10 in skin care. In this paper, we prepared a nanostructured lipid carrier of coenzyme Q10 (Q10-nanoliposomes), optimized the prescription and preparation process, investigated the stability of Q10-nanoliposomes and Q10 raw materials, and carried out in vitro release and transdermal studies on Q10-nanoliposomes.
1.1 Overview of Coenzyme Q10
1.1.1 Physicochemical Properties of Coenzyme Q10
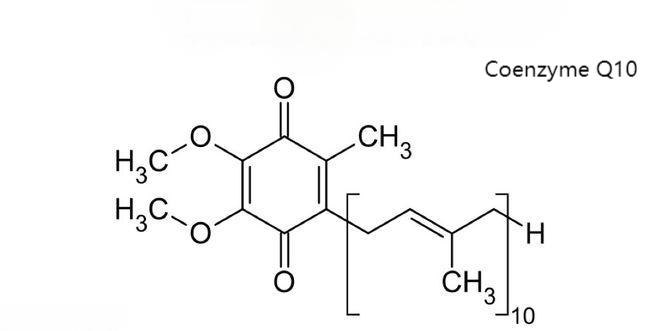
Coenzyme Q10 (Q10), also known as ubiquinone, is chemically known as 2,3-dimethyl-5-methyl-6-deca-isopentenylbenzoquinone. The molecular formula is C59H90O4 and the molecular weight is 863.36. The structural formula is shown in Figure 1-1. The structural formula is shown in Figure 1-1, where Q represents the quinone chemical group and 10 represents the number of isoprenoids in the tail. It is yellow or light orange-yellow crystalline powder at room temperature, odorless and tasteless, melting point is 48.0-52.0℃. It is unstable to light and decomposes easily into reddish substance when exposed to light, while it is more stable to temperature and humidity. Coenzyme Q10 is a fat-soluble vitamin-like substance, soluble in chloroform, carbon tetrachloride and benzene, soluble in acetone, petroleum ether and ethyl ether, slightly soluble in ethanol, insoluble in water and methanol because of its long isoprenoid side chain [1].
Figure 1-1 Structural Formula of Coenzyme Q10
1.1.2 Pharmacological Effects and Applications of Coenzyme Q10
Coenzyme Q10 was discovered in 1957 when it was isolated from mitochondrial lipids of bovine heart muscle[2] . Coenzyme Q10 is widely found in most eukaryotic organisms. Coenzyme Q10 is an important hydrogen transporter in the respiratory chain, is part of the electron transport chain, promotes cellular respiration, and is essential for ATP production. In the human body, 95% of the energy is produced through the respiratory chain [3,4]. Therefore, the highest levels of coenzyme Q10 are found in organs with a high energy demand, such as the heart, liver, and kidneys in animals, and in plants, it is mainly found in leaves and seeds [5,6]. In addition, coenzyme Q10 accelerates cellular metabolism and has antioxidant activity, inhibiting peroxidation [7].
Coenzyme Q10 has pharmacological effects such as scavenging free radicals [8-10], stabilizing biofilms to maintain calcium channel integrity [11], enhancing muscle immunity to prolong survival time [12], promoting learning and memory in animals [13], and promoting microcirculation [14], etc. Coenzyme Q10 has been clinically used in the treatment of cardiovascular diseases such as congestive heart failure [15-17] and angina pectoris [18], the prevention of heart surgery [19], migraine [20], chronic hepatitis [10], Parkinson's disease [21], and periodontal diseases [22]. It is currently used in the treatment of cardiovascular diseases such as congestive heart failure[15-17] , angina pectoris[18] , cardiac surgery[19] , migraine[20] , chronic hepatitis[10] , Parkinson's disease[21] , and periodontal diseases[22] .
The amount of coenzyme Q10 in the body changes with age, reaching a maximum at the age of 20 years, and then decreasing with age, falling to about 42% of the 20-year old level at the age of 77 years[7] , so it needs to be replenished from external sources. Due to its anti-cancer[23,24] and anti-fatigue[25] effects, coenzyme Q10 has been used in nutraceuticals and as an additive in food[26].
In addition, the antioxidant and pro-metabolic effects of coenzyme Q10 have been shown to be beneficial to the skin[27] . Coenzyme Q10 promotes electron transport in the respiratory chain of epithelial cells and ATP production, scavenges free radicals, and inhibits lipid peroxidation in the skin, thereby slowing down skin aging. Coenzyme Q10 is more effective than vitamin E and vitamin B in nourishing and revitalizing the skin[28] . With the increase of age, the decrease of coenzyme Q10 in the body will lead to easy aging of the skin, the formation of pigmentation and wrinkles, so more and more cosmetic products began to add coenzyme Q10 [29].
1.2 Progress in the Development of Topical Formulations of Coenzyme Q10
The Chinese Pharmacopoeia includes oral and injectable preparations of coenzyme Q10 [1], and the FDA has not yet approved the use of coenzyme Q10 as a drug for clinical treatment in the U.S. In 2004, the Japanese government firstly approved the use of coenzyme Q10 in skin care products. Nowadays, Coenzyme Q10 has become an important anti-aging active ingredient used in cosmetics. Famous domestic and foreign cosmetic companies have launched a series of skin care products containing coenzyme Q10, such as Beiersdorf launched Nivea Q10 face cream and eye cream, Shiseido launched Q10 moisturizing skin resurfacing cream, DHC listed Q10 youth regenerating beauty water and emulsion, in addition to Gossel, Mansurat, Avon, Korea Sokoban, etc. have launched cosmetics containing Q10, to seize the market share. In addition, Kose, Mansurat, Avon, and Korea Somang have all launched cosmetics containing Q10 to seize market share. The share of coenzyme Q10 skin care products in the international cosmetic market is increasing year by year.
At present, the State Intellectual Property Office of China has recorded 49 invention patents on coenzyme Q10, among which there are 4 patents related to cosmetics: a kind of coenzyme Q10 nano-microcapsule emulsion and its preparation method and application (200710304523.5); a kind of emulsion containing coenzyme Q10 and plant extracts and its preparation method (200710072068.0); a self-emulsifying composition of coenzyme Q10 and its preparation method and application (200910090001.9); a composition containing coenzyme Q10 for treating dermatological diseases and its preparation method (200510079650.0). Coenzyme Q10 self-emulsifying composition and its preparation method and application (200910090001.9); Composition containing Coenzyme Q10 for the treatment of skin diseases and its preparation method (200510079650.0). At present, there are 45 kinds of imported cosmetics containing coenzyme Q10 approved by SFDA and only 2 kinds of domestic cosmetics containing coenzyme Q10 on the market in China. It can be seen that the research and development of coenzyme Q10 cosmetics in China is still in the initial stage.
Coenzyme Q10 is chemically unstable and easy to decompose in the presence of light, which seriously affects its effectiveness in skin care and anti-aging. Currently, most of the coenzyme Q10 dosage forms on the market are ordinary emulsions or gels, which have poor stabilizing effect on coenzyme Q10, and do not have the performance of slow-release and controlled-release. Therefore, how to improve the stability of skin care active ingredients to extend their effective duration of action; promote the effective penetration of skin care active ingredients into the skin tissue, so that they can enter the skin care target site through the skin stratum corneum; as well as to achieve a slow and controlled release at the site of action, to give full play to the skin care efficacy of the active component, is the coenzyme Q10 skin care products as well as other similar cosmetic skincare products in the application of the problem to be solved urgently.
1.3 Overview of Nanostructured Lipid Carriers Research
In recent decades, nano drug delivery systems (NDDS) have been more and more deeply studied and widely applied. Nanoemulsions (NE), liposomes, cubic phase nanoparticles (Cubosome), solid lipid nanoparticles (SLN), nanostructured lipid carriers (nanostructured lipid carriers, nanoliposomes), and nanostructured lipid carriers (nanoliposomes) have been widely used in NDDS. Solid lipid nanoparticles (SLN), nanostructured lipid carriers (nanoliposomes) and other drug-carrying systems can be used for transdermal drug delivery and cosmetic applications [30-34].
SLNs are solid lipids used as carriers to encapsulate drugs, which are solid at room temperature, reducing the partitioning phenomenon with the external aqueous phase and greatly increasing the stability of the drugs. However, SLN prepared from one or several kinds of solid lipids are in high-energy α or β' configuration at the early stage of preparation, and in the process of long-term storage, they are transformed into low-energy and more orderly β configuration, which leads to the reduction of defective lattice and the transformation of highly ordered state, which will lead to the leakage of the drug. Moreover, the drug loading capacity of SLN is not high and the water content in the aqueous dispersion system is high [35].
Nanoliposomes were developed on the basis of SLN. It adds liquid lipids to solid lipids, which can also remain solid at room temperature [36,37]. The addition of liquid lipids disrupts the perfection of the lattice, thus forming many defective lattices, and drugs can be contained in the inter-lipid chains or defective lattices, so the drug loading capacity of nanoliposomes is higher than that of SLNs [38]. The incorporation of liquid lipids avoids drug leakage due to lattice rearrangement, so the stability of drug loading is greatly improved [39]. Moreover, because they are still in the solid state, nanoliposomes can overcome the disadvantages of SLN while inheriting the advantages of SLN, such as slow release and controlled release. Nanoliposomes can be applied to oral, injectable, transdermal, ocular, mucosal and other drug delivery routes, and have been widely studied and applied, and the preparation method is suitable for large-scale production, which is very promising for industrialization [40].

1.3.1 Lipid Materials for the Preparation of Nanoliposomes
The solid lipids for the preparation of nanoliposomes are the same as SLN, such as glycerol monostearate, glycerol tristearate, glycerol tristearate, fatty acids such as stearic acid, cholesterol, cetacean wax, etc. The selection of liquid lipids can improve the drug loading and stability of nanoliposomes. Reasonable selection of liquid lipids can improve the drug loading capacity and stability of nanoliposomes. When selecting liquid lipids, it is necessary to consider the solubility of the drug in them and the affinity of liquid lipids with solid lipids. Commonly used liquid lipids include caprylic triglyceride, oleic acid, isopropyl palmitate, vitamin E, isopropyl myristate, soybean oil and so on. Mixed lipid materials can also be used to increase the lattice defects and thus improve drug loading and stability [41].
1.3.2 Preparation of Liposome Nanoparticles
Nanoliposomes are developed on the basis of SLN, so the preparation methods of nanoliposomes are the same as those of SLN, such as high-pressure emulsification, ultrasonication, microemulsion, solvent dispersion, and melt emulsification[42] . In addition, the high-speed microjet method is a newly developed method. The microjet has a "Y" or "Z" shaped groove inside and utilizes the high shear and impact forces generated by the collision of fluids inside the chamber to pulverize particles down to the nanometer scale. The pressure of the high-speed microjet is higher than that of a high-pressure homogenizer, so the nanoparticles are smaller in size, less variable in size, and more stable and homogeneous. In addition, the use of organic solvents is avoided and the preparation time is short, which is suitable for industrialized mass production [43].
1.3.3 Advantages of Nanoliposomes for Cosmetic Applications
The use of carrier technology to improve the stability of skin care active ingredients and to achieve a long and slow release on the skin surface is a hot topic in cosmetic research. The most researched carrier systems include microemulsions, polymer microspheres/microcapsules, liposomes, solid lipid nanoparticles and nanostructured lipid carriers.
Microemulsion is an O/W system with clear appearance, thermodynamic and kinetic stability, and is suitable for various dosage forms, which is an ideal carrier. However, a large amount of surfactant is needed to stabilize the system, and the surfactant has certain toxicity and irritation effects, which may cause some adverse reactions to the skin, so it is not suitable for cosmetic use[44] . In addition, polymer microspheres/microcapsules also have potential biosafety problems of carrier materials[45] .
Liposome is a vesicle structure made of phospholipids, cholesterol, etc. Each layer is a lipid bilayer with a particle size ranging from 25 to 1000 nm, and liposome can have a multilayer structure. Since phospholipids have hydrophilic heads and hydrophobic tails, liposomes are amphiphilic, and water-soluble drugs can be encapsulated in their centers and between the membranes, while the bilayer membranes can be loaded with fat-soluble substances, which has good physiological compatibility and passive targeting. Liposomes were once a hot topic in cosmetic research, and Dior was the first to introduce liposome-containing cosmetics in 1986. However, due to the fluidity of the membrane, liposomes have the disadvantages of low encapsulation rate, easy leakage, poor storage stability, unsatisfactory delayed and controlled release, and unsuitable for industrialized large-scale production, which limit its application [32].
Nanoliposomes offer the advantages of the above mentioned drug delivery systems and improve upon their shortcomings. They are well suited for cosmetic applications, in particular:
(1) Improvement of Stability
Nanoliposomes are solid at room temperature, and there is no distribution of active molecules between the nanoparticles and the external aqueous phase, so the stability of unstable active ingredients can be improved. In addition, the solid lipid encapsulation can avoid the degradation of active substances by light and oxygen, thus enhancing the stability [46].
(2) Slow and Controlled Release
Nanolipid carriers have a slow and controlled release. Müller et al [47] found that SLN prepared by thermal homogenization has two release processes. The drug enriched in the SLN shell is first released abruptly and then the drug encapsulated in the shell is released slowly.Teeranachaideekul et al [48] also found that nanoliposomes have the same release characteristics. The initial burst release can increase the concentration of active ingredients on the skin in a short period of time and promote their penetration into the skin, while the subsequent slow release can continuously release the active substances to maintain the effective concentration to nourish the skin for a long period of time and better perform the skin care effect, which is a more ideal release behavior for cosmetics.
(3) Skin Targeting
Liposomal nanoliposomes are skin-targeted and can facilitate the passage of active ingredients through the stratum corneum into the epidermis and their retention in the deeper cells of the epidermis, thus exerting skincare effects[49] . The stratum corneum is a major obstacle to the entry of active ingredients into the skin, and Chen [50] et al. found that SLN could enter the epidermis through the interstitial space of the stratum corneum and hair follicle channels. In addition, the slow-release effect of SLN/nanoliposomes prevents the concentration of the drug from being too high to pass through the skin into the systemic circulation, so that the active ingredients can remain in the epidermis for a long time [51].
(4) Physiological Compatibility
The lipid materials used in the preparation of nanoliposomes are physiologically compatible lipids with good affinity to the skin, which can promote the penetration of active ingredients in the skin and effectively act on the deeper cells of the skin, and the lipids are biodegradable, safe and non-toxic [33].
(5) Closure Effect
Studies have shown that nanoparticles smaller than 400 nm have an occlusive effect, which can form a closed film on the surface of the skin, slow down the evaporation of skin water, promote the absorption of active ingredients in skin care, and have good moisturizing properties. The smaller the nanoparticle size and the higher the concentration, the stronger the occlusive effect [52].
(6) Light Scattering Effect
Nanoparticles with high crystallinity have a light scattering effect, which can reflect UV rays, protect the skin from damage, and slow down skin photoaging [47]. Currently, nanosunscreen cosmetics containing SLN and nanoliposomes have been marketed in Europe and the United States.
(7) High Drug Loading Capacity and Good Storage Stability
Nanoliposomes containing liquid lipids have a higher drug loading capacity than SLNs and liposomes, and are stable and less prone to drug leakage.
2 Studies on the Prescription and Preparation Process of Q10 Nanolipids
2.1 Introduction
Nanostructured lipid carriers are a new generation of lipid nanodrug carriers developed on the basis of solid lipid nanoparticles. Nanoliposomes not only have high drug loading capacity, but also have good slow and controlled release properties, which can stabilize the active substance, and also have skin closure effect, which is very suitable for cosmetic applications [33].
Coenzyme Q10 is a fat-soluble vitamin-like substance, yellow to orange-yellow crystalline powder; odorless and tasteless; easily decomposed by light. It is soluble in trichloromethane, benzene, acetone, ether or petroleum ether, very slightly soluble in ethanol and insoluble in water. Coenzyme Q10 is an important hydrogen transporter in the cellular respiratory chain, activating cellular respiration and accelerating the production of ATP. Q10 is present in most human tissues, with the highest levels found in the liver, heart, kidneys and pancreas. Coenzyme Q10 has been shown to penetrate deep into the skin and can be used in cosmetics to promote skin metabolism and inhibit lipid peroxidation. However, due to its unstable chemical nature, it is easy to be decomposed in the presence of light, which seriously affects its effective performance in skin care [7].
In this paper, Q10 nanolipids were prepared using physiologically compatible lipids to improve the photostability of Q10, promote the effective penetration of skincare active ingredients into skin tissues, and give full play to the skincare efficacy of Q10.
In this chapter, the prescription and preparation process of Q10 nanolipids were investigated. Response surface design was used to select the prescription of Q10 nanolipids by using the particle size as the evaluation index, and the preparation process was optimized by investigating the effects of high shear speed and time, high-pressure micro-jet pressure and the number of cycles on the particle size. The prepared Q10 nanolipids were characterized by TEM, DSC and XRD.
2.2 Materials and Instruments
2.2.1 Materials
Coenzyme Q10 Raw Material Drug (Q10 , Pharmaceutical Grade, Xinchang Pharmaceutical Factory, Zhejiang Pharmaceutical Co;)
Labrafac Lipophile WL1349 (MCT, Caprylic Capric Triglyceride, Gattefosse, France); Tego Care 450 (Polyglyceryl-3 Methylglucose Distearate, Goldschmidt, Germany);
PreciroLATO-5 (ATO-5, a mixture of mono-, diglycerides and triglycerides of stearic acid and palmitic acid, Gattefosse, France);
Anhydrous ethanol (analytically pure, Sinopharm Chemical Reagent Co., Ltd.); all others are commercially available analytically pure or chromatographically pure reagents.
2.2.2 Instruments
Zetasizer laser particle sizer (Nano-ZS90, Malvern, UK);
Circulating vacuum pump (SHZ-D Ⅲ , Gongyi IYUHUA Instrument Co., Ltd.); M-100PCE High-pressure micro-jet (Microfluidics, USA);
Collector-type constant temperature heating magnetic stirrer (DF-101S, Zhengzhou Great Wall Science, Industry and Trade Co., Ltd.); rotary evaporator (RE-52, Shanghai Yarong Biochemical Instrument Factory);
Tecnai G2 20 transmission electron microscope (FEI, The Netherlands); electronic balance (Beijing Sartorius Instrument System Co., Ltd.);
High Shear Mixing & Emulsifying Machine (BME100L , Shanghai Weiyu Mechanical & Electrical Manufacturing Co;)
Agilent 1100 HPLC system: G1310A pump, G1314A UV detector, G1326-AA105 autosampler, Agilent Chromatography Workstation (Agilent, USA);
Chromatographic column: Elite ODS2 C18 column 250 mm×4.6 mm Hypersil (5 μm particle size); Labconco 6L freeze-drying system (Labconco, USA);
Ultrasonic cell pulverizer (JY98-III, Shanghai Xinzhi Institute of Biotechnology, China); Ultrafiltration tube (30 KDa, Millipore, USA);
DSC-7 differential thermal analyzer (Perkin-Elmer, USA);
D/MAX III B Powder Diffractometer (Rigaku Corporation, Japan);
0.45 μm microporous membranes and membrane filters (Shanghai Xingya Purification Material Factory), etc.
2.3 Experimental Methods and Results
2.3.1 Physicochemical Properties of Coenzyme Q10 APIs
Coenzyme Q10 is yellow to orange-yellow crystalline powder; odorless and tasteless; easily decomposed by light. It is soluble in trichloromethane, benzene, acetone, ether or petroleum ether, very slightly soluble in ethanol and insoluble in water. Melting point is 48-52 ℃ [1].
2.3.2 Design and Optimization of Prescriptions
2.3.2.1 Lipid Selection
The particle size, encapsulation rate and drug loading capacity of nanostructured lipid carriers are highly dependent on the lipid. Nanoliposomes were prepared using Labrafac Lipophile WL 1349 (MCT) as a liquid lipid, Tego Care 450 as a surfactant, and ATO-5 and Hexadecanoic acid hexadecyl ester (Cadmium hexadecanoate), which is more commonly used in cosmetic industry, as solid lipids, to compare the differences in particle size, PDI, and sense of use.
Weigh 6.00 g of solid lipid, 1.65 g of MCT, 2.00 g of Tego Care 450 and 2.80 g of Coenzyme Q10. The lipids and drugs were dissolved in 10 mL of anhydrous ethanol and transferred to a pear-shaped flask. The beaker was washed thoroughly with appropriate amount of anhydrous ethanol and transferred to a pear-shaped flask. The beaker was washed thoroughly with appropriate amount of anhydrous ethanol and transferred to a pear-shaped flask. In addition, take the prescribed amount of Tego Care 450 and add it to distilled water, and heat it up in a water bath at 85 ℃ to produce the aqueous phase. The aqueous phase was poured into the oil phase, and the two phases were mixed well by magnetic stirring at 85℃ for half an hour, and then the product was circulated twice with a high-speed shear emulsifier at 6000 rpm for 4 min and a high-pressure micro-jet at 1200 bar. The results are shown in Table 2-1:
Table 2-1 Differences in particle size, PDI and sense of use of nanoliposomes prepared from different solid lipids.
Samples | Particle size (nm) | PDI | feeling of use |
Prescription of ATO-5 as a solid lipid | 244.8 | 0.128 | Has a greasy feeling and feels dry on the skin when left to dry |
Prescription with hexadecanoic acid hexadecyl ester as a solid lipid | 168.1 | 0.171 | Reduced greasiness and comfortable to use |
The particle size of nanoparticles made from ATO-5 solid lipid was found to be larger than that made from hexadecanoic acid solid lipid under the same conditions, and the feel of the nanoparticles was not good, so hexadecanoic acid was chosen to be the backbone material of Q10-nanoliposomes.
2.3.2.2 Prescription Design
According to the Box-Benhnken experimental design principle, the three factors that have a great influence on the preparation, namely hexadecanoic acid hexadecyl ester, MCT, and the dosage of Tego Care 450, were set in the ranges of 3.00-6.00 for hexadecanoic acid hexadecyl ester, 0.30-3.00 for MCT, and 1.00-3.00 for Tego Care 450, according to the reference [48]. 3.00, MCT 0.30-3.00, Tego care 450 1.00-3.00, and a three-factor, three-level response surface analysis experiment was designed. The design prescription is shown in Table 2-2.
Table 2-2 Prescriptions based on the Box-Benhnken principle
Sixteen acid sixteen Ester (%) | MCT (%) | Tego Care 450 (%) | Q10 (%) | H2 0 (%) | |
1 | 6.00 | 1.65 | 2.00 | 2.80 | 87.55 |
2 | 3.00 | 3.00 | 1.00 | 2.80 | 90.20 |
3 | 6.00 | 0.30 | 2.00 | 2.80 | 88.90 |
4 | 6.00 | 1.65 | 3.00 | 2.80 | 86.55 |
5 | 6.00 | 1.65 | 1.00 | 2.80 | 88.55 |
6 | 9.00 | 3.00 | 2.00 | 2.80 | 83.20 |
7 | 6.00 | 3.00 | 2.00 | 2.80 | 86.20 |
8 | 3.00 | 0.30 | 3.00 | 2.80 | 90.90 |
9 | 6.00 | 1.65 | 2.00 | 2.80 | 87.55 |
10 | 9.00 | 1.65 | 2.00 | 2.80 | 84.55 |
11 | 6.00 | 1.65 | 1.00 | 2.80 | 88.55 |
12 | 6.00 | 1.65 | 2.00 | 2.80 | 87.55 |
13 | 6.00 | 1.65 | 2.00 | 2.80 | 87.55 |
14 | 3.00 | 0.30 | 1.00 | 2.80 | 92.90 |
15 | 9.00 | 1.65 | 3.00 | 2.80 | 83.55 |
16 | 9.00 | 3.00 | 3.00 | 2.80 | 82.20 |
17 | 3.00 | 0.30 | 2.00 | 2.80 | 91.90 |
2.3.2.3 Particle Size and Zeta Potential Determination
The Q10-NCL aqueous dispersion was diluted 300 times with ultrapure water, and the particle size and PDI of the Q10-nanoliposome aqueous dispersion were measured by laser particle sizing instrument at an angle of 90o and a temperature of 25 ℃, and repeated 11 times. Appropriate amount of Q10-nanoliposome aqueous dispersion was added into a U-shaped electrophoresis cell, and the conductivity was adjusted to over 50 μs/cm with 0.9% saline, and the zeta potential was measured at a temperature of 25 ℃. The zeta potential was measured at a temperature of 25°C. The changes in particle size were measured on the day of preparation, after 10 days, and after 40 days, and the results are shown in Table 2-3.
Table 2-3 Changes in particle size, PDI, zeta potential and particle size for different prescriptions
Hexadecanoic acid hexadecyl ester (%) | MCT (%) | Tego Care 450 (%) | Q10 (%) | size (nm) | PDI | Zeta Potential (mV) | After 10 days particle size (nm) | After 40 days particle size (nm) |
6.00 | 1.65 | 2.00 | 2.80 | 166.3 | 0.174 | -22.9 | 170.0 | 164.6 |
3.00 | 3.00 | 1.00 | 2.80 | 199.1 | 0.084 | -27.5 | 194.0 | 197.6 |
6.00 | 0.30 | 2.00 | 2.80 | 161.8 | 0.182 | -31.1 | 162.8 | 159.3 |
6.00 | 1.65 | 3.00 | 2.80 | 149.3 | 0.181 | -20.4 | 145.3 | 141.5 |
6.00 | 1.65 | 1.00 | 2.80 | 187.2 | 0.157 | -42.2 | 193.3 | 202.3 |
9.00 | 3.00 | 2.00 | 2.80 | 183.5 | 0.199 | -49.6 | 188.6 | 185.5 |
6.00 | 3.00 | 2.00 | 2.80 | 175.2 | 0.135 | -25.6 | 180.0 | 176.5 |
3.00 | 0.30 | 3.00 | 2.80 | 177.3 | 0.121 | -25.4 | 179.4 | 180.5 |
6.00 | 1.65 | 2.00 | 2.80 | 158.6 | 0.128 | -22.4 | 162.3 | 163.8 |
9.00 | 1.65 | 2.00 | 2.80 | 176.0 | 0.203 | -35.1 | 174.8 | 174.1 |
6.00 | 1.65 | 1.00 | 2.80 | 202.6 | 0.143 | -25.8 | 199.7 | 203.7 |
6.00 | 1.65 | 2.00 | 2.80 | 159.1 | 0.123 | -23.1 | 163.2 | 162.3 |
6.00 | 1.65 | 2.00 | 2.80 | 153.8 | 0.128 | -24.2 | 156.2 | 156.2 |
3.00 | 0.30 | 1.00 | 2.80 | 173.0 | 0.170 | -29.5 | 173.4 | 174.6 |
9.00 | 1.65 | 3.00 | 2.80 | 162.7 | 0.176 | -35.9 | 160.9 | 156.7 |
9.00 | 3.00 | 3.00 | 2.80 | 158.7 | 0.188 | -24.0 | 155.4 | 159.1 |
3.00 | 0.30 | 2.00 | 2.80 | 140.9 | 0.131 | -5.6 | 139.5 | 143.5 |
2.3.2.4 Determination of Drug Loading Capacity
Coenzyme Q10 is slightly soluble in ethanol, while the lipid materials for the preparation of nanoparticles are soluble in ethanol, so ethanol was chosen as the emulsifier. 0.1 g of Q10-nanoliposome aqueous dispersion was sucked into a 50 mL volumetric flask, and the volume was fixed with ethanol. After the flocculent precipitate was completely dissolved by ultrasonication for 20 min, the drug loading capacity was determined by HPLC using octadecylsilane-bonded silica gel as filler, the mobile phase: methanol:ethanol=3:7, the flow rate was 1 mL/min, the detection wavelength was 275 nm, and the injection volume was 20 μL.
2.3.2.5 Determination of Encapsulation Rate
The encapsulation rate of Q10-nanoliposomes was determined by ultrafiltration. A 30 kDa ultrafiltration tube was used, 1 mL of Q10-nanoliposome aqueous dispersion was added, and centrifuged at 5000 rpm for 30 min. The Q10 content in the supernatant was determined by HPLC method, and the encapsulation rate was calculated according to the following formula, and the results of the encapsulation rate and the drug loading capacity are shown in Table 2-4.
Table 2-4 Measurement results of encapsulation rate and drug loading of different prescriptions
Hexadecanoic acid hexadecyl ester (%) | MCT (%) | Tego Care 450 (%) | Q10 (%) | H2 0 (%) | Encapsulation rate (%) | Load (%) | |
1 | 6.00 | 1.65 | 2.00 | 2.80 | 87.45 | 99.7 | 2.53 |
2 | 3.00 | 3.00 | 1.00 | 2.80 | 90.20 | 99.9 | 2.68 |
3 | 6.00 | 0.30 | 2.00 | 2.80 | 88.90 | 97.2 | 2.58 |
4 | 6.00 | 1.65 | 3.00 | 2.80 | 86.55 | 99.9 | 2.50 |
5 | 6.00 | 1.65 | 1.00 | 2.80 | 88.55 | 96.3 | 2.53 |
6 | 9.00 | 3.00 | 2.00 | 2.80 | 83.20 | 99.9 | 2.63 |
7 | 6.00 | 3.00 | 2.00 | 2.80 | 86.20 | 99.9 | 2.27 |
8 | 3.00 | 0.30 | 3.00 | 2.80 | 90.90 | 95.6 | 2.40 |
9 | 6.00 | 1.65 | 2.00 | 2.80 | 87.55 | 100.0 | 2.46 |
10 | 9.00 | 1.65 | 2.00 | 2.80 | 84.55 | 100.0 | 2.39 |
11 | 6.00 | 1.65 | 1.00 | 2.80 | 88.55 | 97.9 | 2.35 |
12 | 6.00 | 1.65 | 2.00 | 2.80 | 87.55 | 99.9 | 2.62 |
13 | 6.00 | 1.65 | 2.00 | 2.80 | 87.55 | 100.0 | 2.25 |
14 | 3.00 | 0.30 | 1.00 | 2.80 | 92.90 | 95.8 | 2.51 |
15 | 9.00 | 1.65 | 3.00 | 2.80 | 83.55 | 99.8 | 2.38 |
16 | 9.00 | 3.00 | 3.00 | 2.80 | 82.20 | 99.9 | 2.41 |
17 | 3.00 | 0.30 | 2.00 | 2.80 | 91.90 | 96.7 | 2.56 |
2.3.2.6 Response Surface Analysis
From Table 2-3 and Table 2-4, it can be seen that there is not much difference in the encapsulation rate, drug loading capacity, and the change of particle size in different prescriptions at different times. Therefore, we chose the particle size as the dependent variable, and carried out the response surface analysis through the design-expert, and the results are shown in Table 2-5.
Table 2-5 Response surface analysis results
considerations | variance (statistics) | df | mean square | F-value | P-value | |
mould | 4101.65 | 9 | 455.74 | 6.96 | 0.0090 | statistically significant |
A | 378.43 | 1 | 378.43 | 5.78 | 0.0472 | |
B | 49.74 | 1 | 49.74 | 0.76 | 0.4123 | |
C | 1996.29 | 1 | 1996.29 | 30.49 | 0.0009 | |
AB | 9.94 | 1 | 9.94 | 0.15 | 0.7085 | |
AC | 96.03 | 1 | 96.03 | 1.47 | 0.2652 | |
BC | 23.82 | 1 | 23.82 | 0.36 | 0.5654 | |
A2 | 18.64 | 1 | 18.64 | 0.28 | 0.6102 | |
B2 | 150.36 | 1 | 150.36 | 2.30 | 0.1735 | |
C2 | 1004.04 | 1 | 1004.04 | 15.33 | 0.0058 | |
residual | 458.35 | 7 | 65.48 | |||
lost proposal | 260.08 | 3 | 86.69 | 1.75 | 0.2952 | insignificant |
net error | 198.27 | 4 | 49.57 | |||
total deviation | 4560.00 | 16 |
Where A is the amount of hexadecanoic acid hexadecyl ester, B is the amount of MCT, and C is the amount of Tego Care 450.
As shown in Table 2-5, the F-value of Model is 6.96, p-value is 0.009, which means that the model is constructed with significant difference, and only 0.09% of this significance may come from the interference of external conditions. p-value less than 0.05 means that the effect of this item on the results is significant, and greater than 0.10 means that it is not significant, so A, C, C2 are the significant influencing factors. The F-value of the misfit term is 1.75 and the p-value is 0.2952, which means that the misfit term is not significant and the fit is high. Figure 2-1 shows the response surface of particle size under the interaction of different influencing factors.
Derived response surface equations:
Grain size = 188.82401 + 9.08800 x A + 4.42116 x B - 57.33553 x C - 1.15834 x A x
B-4.89541×A×C-6.01464×B ×C+0.58637×A2+5.26515×B2+19.02422×C2
The hypothesized optimal prescription was: hexadecanoic acid hexadecyl ester 3.00%, MCT 1.09%, Tego Care 450 2.07%, Q10 2.80%, water 91.04%, and the hypothesized particle size was 147.5 nm.
2.3.3 Optimization of the Preparation Process
2.3.3.1 Selection of Preparation Process
There are many methods to prepare nanoliposomes, such as high-pressure emulsification, sonication, microemulsification, solvent dispersion, and melt emulsification[42] . Thin-film ultrasonication was proposed to be used for the preparation of small amount of liposomes due to its simplicity and suitability for small-dose production. However, it was found that the amount of lipids used was too large, and lipids were precipitated during sonication, so the method was changed to high-pressure microjetting. The nanoparticles prepared by the high-pressure microjet method were smaller in size, and the differences in particle size were not large and stable.
2.3.3.2 Selection of Preparation Temperature
The melting point of hexadecanoic acid hexadecyl ester is between 52-56 ℃, and the melting point of coenzyme Q10 is between 48-52 ℃, in order to make the drug and lipid intermixing the preparation temperature should be higher than the melting point of 20-30 ℃, so 85 ℃ was chosen as the preparation temperature.
2.3.3.3 Selection of Oil Phase Preparation Methods
Two methods were used to prepare the oil phase:
① Weigh the solid lipid, liquid lipid and coenzyme Q10, add ethanol and stir, pour into a pear-shaped flask at 75 ℃ for 20 min, wait until the ethanol completely evaporated and then poured into the aqueous phase mixing.
② Weigh solid lipids, liquid lipids and coenzyme Q10 85 ℃ water bath for 30min, wait until the lipids and coenzyme Q10 completely melted into the aqueous phase mixing.
Comparing the two methods of oil phase preparation, the oil film formed after suspension and evaporation in the first method was not uniform, some solids adhered to the flask wall, and some substances remained in the beaker after dissolving with ethanol in the beaker and then transferring to the flask, which made it difficult to ensure the accuracy, especially for the small amount of MCT. Since the melting point of coenzyme Q10 is between 48-52 ℃ and is stable to high temperature, and the melting point of hexadecanoic acid hexadecyl ester is between 52-56 ℃, it can be melted after 30 min of water bath at 85 ℃. The second method is easier and more accurate, so the second method was chosen to prepare the oil phase.
2.3.3.4 Selection of High Shear Speed and Time
The rotational speed and time of high shear will affect the particle size and PDI of the produced nanoparticles. 4 rotational speeds of 5000 rpm, 6000 rpm, 7000 rpm and 8000 rpm were selected to shear the nanoparticles for 1min, 2min, 3min, 4min, 5min, 8min, 12min and 15min respectively to compare the changes of the particle size. The results are shown in Figure 2-2.
From the experimental results, it can be seen that the larger the high shear speed, the smaller the particle size. In order to improve the preparation efficiency, the high shear speed of 8000 rpm was chosen. From the data in Figure 2-2, it can be seen that the length of time at 8000 rpm does not have much effect on the particle size, so the high shear time was chosen to be 1 min.
2.3.3.5 Selection of High-Pressure Microjet Pressure and Number of Cycles
In the preparation of nanostructured lipid carriers, the pressure and the number of cycles of the high velocity microjet have a great influence on the particle size and PDI of the nanoparticles produced. The pressure of the high pressure microjet was chosen to be 1000 bar, 1200 bar, and 1600 bar for 1, 2, 3, 4, and 5 cycles, respectively, to investigate the effect on the particle size and to determine the optimal process conditions. The results are shown in Figure 2-3.
The higher the pressure of the high-speed microjet, the smaller the particle size. However, excessive pressure can cause damage to the instrument itself, and the nanoparticles prepared at 1600 bar were smaller than those prepared at 1200 bar for the same number of cycles, but the difference was not significant. In order to prolong the lifetime of the instrument, 1200 bar for 3 cycles was chosen.
The finalized process conditions were water bath agitation of the aqueous and oil phases at 85°C for 20 min, high shear speed of 8000 rpm for 1 min, and high pressure microjet at 1200 bar for 3 cycles. The Q10-nanoliposomes were prepared by this process and the particle size, PDI, zeta potential, encapsulation rate and drug loading were measured and the measured particle size was (151.7±2.31) nm (Figure 2-4), which is close to the predicted size, indicating that the response surface equation is accurate and the optimization was successful. zeta potential was (-44.1±1.68) mV (Figure 2-5). The higher the zeta potential, the greater the charge repulsion between the particles and the more stable the formulation. The encapsulation rate was 100% and the drug loading was 2.51%.
2.3.4 Transmission Electron Microscopy (TEM) of Q10-nanoliposomes
The microscopic morphology of Q10-nanoliposomes was observed by TEM. The freshly prepared Q10-nanoliposomes were diluted 300 times with ultrapure water, ultrasonically dispersed for 20 min, and 1 drop was taken on a copper grid, stained with 1% phosphotungstic acid solution, dried at room temperature, and then observed under TEM. The pictures are shown in Figure 2-6.
2.3.4 Transmission Electron Microscopy (TEM) of Q10-nanoliposomes
The microscopic morphology of Q10-nanoliposomes was observed by TEM. The freshly prepared Q10-nanoliposomes were diluted 300 times with ultrapure water, ultrasonically dispersed for 20 min, and 1 drop was taken on a copper grid, stained with 1% phosphotungstic acid solution, dried at room temperature, and then observed under TEM. The pictures are shown in Figure 2-6.
2.3.5 Examination of the Physical State of Q10 in Q10-nanoliposomes
2.3.5.1 Differential Scanning Calorimetric Analysis (DSC)
The lyophilized powder of hexadecanoic acid hexadecyl ester, Q10, physical mixture and Q10-nanoliposome was placed in a 40 μL aluminum dish and tested under argon protection. The gas flow rate was 50 mL/min, the scanning rate was 5 ℃/min, and the scanning temperature range was 0 ℃-90 ℃. The temperature and energy precision of the DSC instrument were calibrated by using indium as the standard material. The results are shown in Figure 2-7.
The thermal absorption peaks of hexadecanoic acid hexadecyl ester (53.43 ℃) and coenzyme Q10 (55.33 ℃) appeared at 45.88 ℃ and 52.45 ℃ in the physical mixture according to the ratio of prescription, and the lyophilized powder of Q10 nanoliposomes showed only one absorption peak (49.59 ℃). It is assumed that the interaction between the lipids and the nanoparticles resulted in the change of the physical phase, and the intensity of the thermal absorption peaks decreased significantly, indicating the formation of a new physical phase.
2.3.5.2 X-ray Powder Diffraction (XRD) Analysis
Cu-Kα radiation was used with a voltage of 40 kV, a tube current of 50 mA, a scanning range of 3o - 65o(2θ), and a scanning angle of 10o(2θ)/min, and the results are shown in Figs. 2-8.
As shown in Figure 2-8, the diffraction peaks of Q10 and hexadecanoic acid hexadecyl ester are significant in the physical mixture, but the peak shapes of the blank nanoliposomes and Q10 nanolipids are almost the same, and there is no obvious Q10 diffraction peaks, and all the diffraction peaks are greatly weakened, which indicates that the Q10 is encapsulated in nanoliposomes and exists in an amorphous state, which is in agreement with the DSC results.
In this experiment, the microscopic morphology of Q10 nanolipids was observed by TEM, and the obtained electron microscope images showed that the nanoparticles in the aqueous dispersion of Q10 nanolipids were round or ellipsoidal, and the size of the particles was in the range of 100 nm, which was smaller than the value measured by laser particle sizer, and it was presumed that it was related to the hydration layer on the surface of the nanoparticles.The results of the DSC and XRD analyses showed that the Q10 was encapsulated in the nanolipids in the amorphous state and existed in the amorphous state. DSC and XRD analyses showed that Q10 was encapsulated in nanoliposomes and existed in amorphous state.
References:
[1] Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China (2010 Edition), Beijing: Chemical Industry Press, 2010.
[2] Crane F, Hatefi Y, Lester R, et al. Isolation of a quinone from beef heart mitochondria[J]. Biochimica et Biophysica Acta, 1957, 25 (1): 220-221
[3] Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function[J]. Biochimica et Biophysica Acta, 1995, 1271 (1): 195-204
[4] SANG Yanshuang, WEI Minji. Biochemical mechanism and clinical application of coenzyme Q10[J]. China Medical Journal, 2006, 7: 371-373
[5] Aberg F, Appelkvist EL, Dallner G, et al. Distribution and redox state of ubiquinones in rat and human tissues[J]. Archives of biochemistry and biophysics, 1992, 295 (2): 230- 234
[6] Shindo Y, Witt E, Han D, et al. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin[J]. The Journal of investigative dermatology, 1994, 102 (1): 122-124
[7] Crane FL. Biochemical Functions of Coenzyme Q10 [J]. Journal of the American College of Nutrition, 2001, 20: 591-598
[8] YANG Xueyi, SU Yangang, CHEN Haozhu. Pharmacology and clinical application of coenzyme Q10[J]. Chinese Journal of Pharmacology, 1994, 10(2): 88-91
[9] SUN Xiaofang, HAN Yanmei, DU Jinduo et al. Anti-lipid peroxidation effects of coenzyme Q10 in aging mice[J]. Chinese Journal of Practical Chinese and Western Medicine, 2004, 4(17): 3112-3113
[10] QIAN Xue, WANG Zuqiao, KORAN Ping et al. Pharmacology and application of coenzyme Q10[J]. Food and Drugs, 2006, 8:16-19
[11] Wang Chunlin. Application of coenzyme Q10 in the treatment of heart disease[J]. New Medicine, 1991, 22 (7): 377-379
[12] XU Caiju, MENG Jia, FU Jianyun et al. Role of coenzyme Q10 in immunomodulation[J]. China Health Inspection Journal, 2007,17: 222-224
[13] YAO Wen-Bing, WANG Hua, SHI Jun et al. Study on the effect of coenzyme Q10 on learning memory in animals[J]. Journal of China Pharmaceutical University, 2004, 35(1): 73-76
[14] ZHANG Guoping, KIM Huiming, MORI Masao et al. Experimental study on the improvement of microcirculatory disorders by coenzyme Q10 in rats[J]. China Circulation, 2005, 9(1): 33-35
[15] YAO Shuyuan, REN Jianghua, CAO Maoyin. Protective effect of coenzyme Q10 on ischemia-reperfusion myocardium[J]. Journal of Wuhan University, 2004, 25(1): 34-37
[16] Tran M, Mitchell TM, Kennedy VT, et a1.Role of coenzyme Ql0 in chronic heart failure, angina and hypertension[J]. Pharmacotherapy, 2001, 21(7): 797-806
[17] XU Bao-Feng, JI Wei-Hua. Treatment of refractory heart failure in pulmonary heart disease with the vasodilator coenzyme Q10[J]. Huaxia Medical Science, 2000, 13(4): 423-425
[18] MA Jianfang, LIU Xiaopeng, LI Dangsheng, et al. Effect of coenzyme Q10 on free radicals in elderly patients with coronary heart disease[J]. Journal of Cardiovascular Rehabilitation Medicine, 2000, 9(5): 52-53
[19] Jeejeebhoy F, Keith M, Freeman M, et a1. Nutritional supplementation with Myo Vive replete essential cardiac myocyte nutrients and reduces left ventricular size in patients with left ventricular dysfunction[J]. American Heart Journal, 2002, 143(6): 1092-1100
[20] Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial[J]. Neurology, 2005, 64 (4): 713-5
[21] ZHAO Chunyu, ZHAO Baodong, WANG Yajun et al. The role of coenzyme Q10 in Parkinson's disease[J]. Chinese Journal of Neurology, 2003, 36 (4): 314
[22] McRee JT, Hanioka T, Shizukuishi S, et al. Therapy with coenzyme Q10 for patients with periodontal disease[J]. Journal of public health dentistry, 1993, 43 (5): 659-666
[23] Portakal O, Ozkaya O, Boza B, et a1. Coenzyme Ql0 and antioxidant status in tissues of breast cancer patients[J]. Clinical Biochemistry, 2000, 33(4): 279-284
[24] Lockwood K, Mesegarard S, Wu Zu. Apparent partial remission cancer in hJ sh risk[J]. Molecular Aspects of Medicine, 1994, 15: 231-240
[25] Wang Qirong, Gou Bo, Yang Zeyi et al. Effects of antioxidant supplementation on the physical function of middle-distance runners [J]. Chinese Journal of Sports Medicine, 2005, 24(1): 125
[26] ZHU Qingzhe, XIA Shuqin, XU Shiyin. Anti-fatigue effects of coenzyme Q10 nanoliposome-fortified sports drinks in mice[J]. Food and Machinery, 2007, 23: 44-48
[27] U. Hoppe, J. Bergemann, W. Diembeck, et al. Coenzyme Q10, a cutaneous antioxidant and energizer [J]. Biofactors, 1999: 371-378
[28] ZHOU Quan, TIAN Guoxiang. Application of coenzyme Q10 in dermatology and cosmetics[J]. Medicine Herald, 1997,16:32-33
[29] YE Qing, ZHANG Binhong. Properties of coenzyme Q10 and its application in cosmetics[J]. Flavor and Fragrance Cosmetics, 1999: 32-34
[30] Mason TG, Wilking J, Meleson K, et al. Nanoemulsions: formation, structure, and physical properties[J]. Journal of Physics: Condensed Matter, 2006, 18: 635
[31] Cevc G. Lipid vesicles and other colloids as drug carriers on the skin[J]. Advanced Drug Delivery Reviews, 2004, 56: 675-711
[32] Torchilin VP. Recent advances with liposomes as pharmaceutical carriers[J]. Nature Reviews Drug Discovery, 2005, 4: 145-160
[33] Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products[J]. International Journal of Pharmaceutics, 2009, 366: 170-184
[34] Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems[J]. Advanced Drug Delivery Reviews, 2001, 47: 229-250


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese