What Is the Use of Turmeric Extract Curcumin in Livestock Breeding?
Curcumin is the active ingredient in turmeric. As a plant extract, it is widely found in the rhizomes of perennial plants in the ginger family, such as Curcuma longa, Curcuma aromatica, and Curcuma domestica [1]. The amount of curcumin contained in turmeric from different origins varies greatly, and its content is usually between 2% and 9% [2]. Curcumin was first discovered in 1815 and first chemically characterized in 1910 [3]. In the past few decades, research on curcumin has focused on its medicinal properties, such as antioxidant, anti-inflammatory, lipid-lowering, chemopreventive and potential chemotherapeutic properties [4].
According to an analysis of the results of a Web of Science search for “curcumin”, papers in the field of animal research only account for 2.75% of all documents (15,462). Therefore, research on curcumin in animal production is still in its infancy. With people's growing awareness of environmental protection and the safety of livestock products, problems such as improper use of antibiotics are becoming increasingly prominent. Turmeric extract, with its advantages of being of natural origin, having no residues, and possessing physiological functions such as anti-oxidation and anti-inflammation, has become a green plant-derived additive with considerable potential.

However, at present, the application and promotion of curcumin is not extensive, and the main reason for this is that the dosage and mechanism of action of curcumin in the production of different types of livestock and poultry are not yet clear. Therefore, this paper aims to review recent domestic and foreign research on curcumin, summarize its physiological functions and the application effects and possible mechanisms in livestock and poultry production, with a view to providing theoretical reference for the development and utilization of curcumin.
1 Physical and chemical properties of curcumin
Curcumin (molecular formula C 21 H20 O 6 ) is a crystalline orange-yellow powder with a melting point of 183 °C. It is very insoluble in water and soluble in organic solvents. It is stable in acidic and neutral environments, but extremely unstable in alkaline conditions [5]. In its chemical structure, curcumin has an α, β-unsaturated-β-diketone group and phenolic hydroxyl and methoxy groups on the two benzene rings [6].
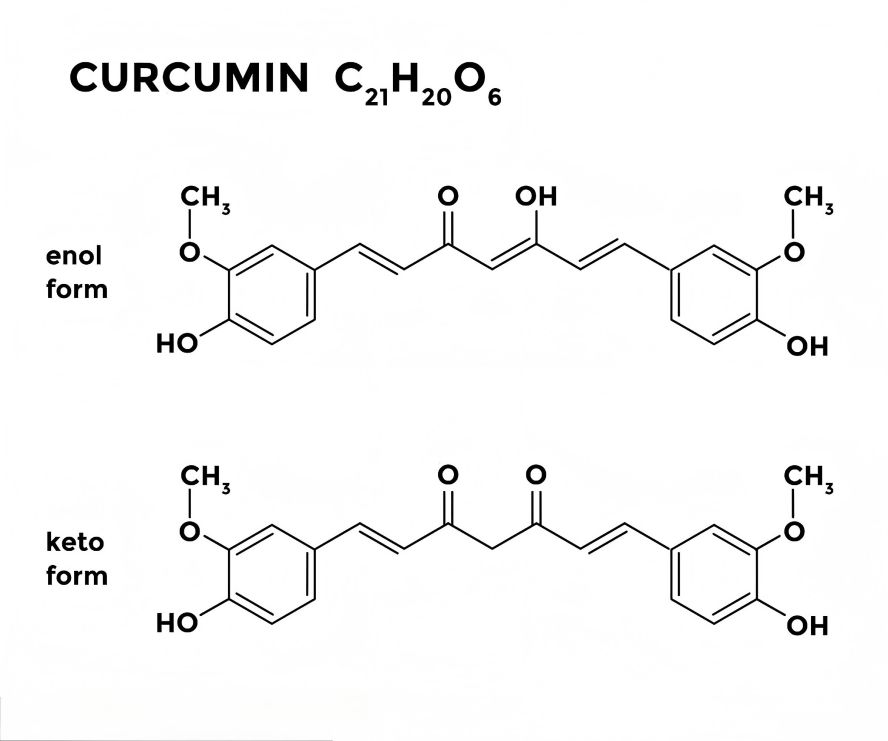
2. Physiological functions and mechanisms of curcumin
2.1 Antioxidant effects
Free radicals are a type of highly oxidizing group produced by the body during metabolic processes. They have physiological functions such as regulating cell growth, but excessive accumulation of free radicals can damage the body. To remove excessive free radicals in animals in a timely manner, a variety of antioxidant feed additives have emerged. As a natural antioxidant, curcumin has received widespread attention from scholars from all walks of life.
2.1.1 Curcumin's own antioxidant properties in the regulation mechanism for inhibiting lipid peroxidation
Studies have shown that curcumin has strong antioxidant properties, and its strong antioxidant properties come from its own structure. The phenolic structure in curcumin can capture free radicals to form highly stable quinone substances [7]. In addition, Osawa et al. [8] and Sugiyama et al. [9] reported that after curcumin is absorbed from the intestine, it undergoes hydrogenation in cells to form the highly antioxidant tetrahydrocurcumin, which binds with free radicals and degrades into another substance with antioxidant activity, 2'-methoxypropiic acid. This substance can further bind with free radicals, thus exhibiting a dual antioxidant function. However, there is still some debate about the core group of curcumin's antioxidant effect. Jovanovic et al. [10] believe that the core group is the β-diketone unit; Priyadarsini et al. [6] believe that it is mainly the phenolic hydroxyl group, with the methoxy group synergistically promoting the antioxidant effect; while Miriyala et al. [11] believe that it is attributed to the combination of the phenolic group and methoxy group with the 1,3 dione binding of the diene system.
Studies have shown that in an oxidative stress model established with 2,2'-azobis(2-methylpropionamidine) dihydrochloride (AAPH), the addition of curcumin can inhibit the lysis and apoptosis of human red blood cells [12] and chicken red blood cells [13]. The reason for this may be that curcumin is fat-soluble, so it can enter the cell membrane, capture free radicals, inhibit free radical-mediated lipid peroxidation, and protect the membrane structure in red blood cells. Studies have also shown that adding curcumin significantly relieves the swelling of the mitochondria in the breast muscle of broilers exposed to chronic heat stress [14].
Mitochondria produce a large amount of reactive oxygen species (ROS) during metabolic processes [15], so they are also the first structures in the body to suffer from free radical damage. Oxidative damage mainly targets unsaturated fatty acids in DNA and membrane structures [16]. Waseem et al. [17] found in a cisplatin-induced oxidative damage model test in rats that curcumin significantly reduced lipid peroxidation levels and protein carbonyl content in mitochondria, reducing stress damage. Trujillo et al. [18] also found that curcumin alleviated the reduction of fibronectin and kidney tight junction proteins caused by oxidative stress. In addition, Dorta et al. [19] found that natural flavonoids can specifically accumulate in mitochondria after absorption, thereby protecting the body. It can be inferred that curcumin can protect the body from oxidative damage by trapping free radicals in mitochondria and thereby inhibiting peroxidation damage to lipids in mitochondria.
2.1.2 Induces the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) – antioxidant responsive element (ARE)
The antioxidant response element (ARE) is an important regulatory element in the body's response to ROS damage and the production of various antioxidant proteins. Nrf2 can activate ARE [20]. Under normal physiological conditions, Nrf2 is present in the cytoplasm and is eventually degraded; in the case of oxidative stress, Nrf2 changes conformation and enters the cell nucleus, where it binds to the antioxidant original ARE, activating the expression of mRNAs and proteins such as antioxidant enzyme genes and detoxification enzymes, and enhancing the body's antioxidant capacity [21].
Studies have shown that curcumin powder can upregulate the expression of the liver Nrf2 protein downstream genes, such as the quinone oxidoreductase (NQO1) and heme oxygenase 1 (HO-1), in arsenic-induced oxidative damage, thereby affecting Nrf2 expression and alleviating oxidative damage in the body [22]. Sahin et al. [23] also found that curcumin can alleviate stress damage by regulating the Nrf2/HO-1 pathway under heat stress conditions in quails. It has also been found that after oxidative stress is produced, the addition of curcumin can significantly increase cell stress conditions, curcumin can alleviate stress damage by regulating the Nrf2/HO-1 pathway. It has also been found that after oxidative stress is produced, adding curcumin can significantly increase the content of glutathione (GSH) in cells, thereby exerting an antioxidant effect [24]. A study using Nrf2 knockout mice found that the absence of the Nrf2 gene reduced the GSH content of liver cells [25].
Therefore, the increase in GSH content by curcumin may also be related to the activation of the Nrf2 pathway. At the same time, in the study by Zheng et al. [26], it was found that the addition of curcumin can also directly induce the up-regulation of mRNA and protein expression of glutathione cysteine ligase (GCL), a GSH synthesis limiting enzyme, thereby promoting the synthesis of GSH in the body and achieving the effect of improving the body's antioxidant capacity. However, some scholars believe that curcumin may not be an absolute antioxidant. In vitro test results show that the ROS level increased significantly during the process of increasing the curcumin concentration from 1 μmol/L to 25 μmol/L, which is believed to be related to the mitochondrial enzyme activated by curcumin to produce ROS [27].
In summary, the antioxidant regulation of curcumin is mainly achieved through two pathways: on the one hand, curcumin exerts its function by using its own structural antioxidant properties; on the other hand, curcumin can act as an inducer to promote the expression of related genes in the antioxidant signal pathway, promote the production of antioxidant proteins and enzymes, and improve the body's antioxidant capacity.
2.2 Anti-inflammatory effect
Studies have shown that the cyclooxygenase (COX) and lipoxygenase (LOX) enzymes in the body catalyze the formation of arachidonic acid to produce various inflammatory mediators such as prostaglandins, which cause inflammatory reactions. Studies have found that curcumin has an antagonistic effect on COX and LOX, which can limit the production of inflammatory mediators and achieve an anti-inflammatory effect [28]. In addition, nitric oxide (NO) is an important signaling molecule involved in inflammatory responses. The addition of curcumin can inhibit the catalytic effect of inducible nitric oxide synthase in the conversion of L-arginine to NO [29]. In addition, the nuclear factor kappa B (NF-κB) signaling pathway is an important signaling pathway related to inflammation. Excessive inflammatory mediators can activate NF-κB, dissociate it from the inhibitor κB (IκB), enter the nucleus, and activate the expression of inflammatory mediator genes, exacerbating the inflammatory response [30]. Tumor necrosis factor-alpha (TNF-α) is an important inflammatory mediator that activates NF-κB. necrosis factor-alpha (TNF-α) is an important inflammatory mediator that activates NF-κB.
According to Peng Jinghua et al. [31], curcumin pretreatment of Kupffer cells (KC) can significantly reduce the levels of interleukin (IL)-1β and IL-6 produced by lipopolysaccharide (LPS) stress, and also has a significant inhibitory effect on the protein expression of TNF-α. Reu- ter et al. [32] also found in their research that curcumin can inhibit many TNF-α activation pathways in cells.
In summary, the main ways in which curcumin exerts its anti-inflammatory effect focus on two aspects: on the one hand, it inhibits the production of inflammatory mediators by inhibiting the activity of enzymes that produce inflammatory mediators, thereby reducing the production of inflammatory mediators and thus inhibiting the inflammatory response; on the other hand, it inhibits inflammatory factors such as TNF-α, preventing their activation of the NF-κB signaling pathway, thereby reducing the expression of inflammatory factors and inhibiting the inflammatory response.
2.3 Lipid-lowering effect
As early as 1971, it was found that curcumin has a lipid-lowering effect [33]. Subsequent studies have also shown that curcumin can lower plasma cholesterol, triglycerides, low-density lipoprotein and apolipoprotein B levels and increase plasma high-density lipoprotein and liver apolipoprotein A expression compared to the control group when mice are fed a high-cholesterol diet and treated with curcumin for 18 weeks [34]. Curcumin can inhibit weight gain caused by a high-fat diet. Male C57BL/6 mice were fed a high-fat diet with 22% fat content and 500 mg/kg for 12 weeks. It is believed that this is related to curcumin activating the main switch of energy metabolism and fatty acid β-oxidation in adipocytes, adenosine monophosphate-activated protein kinase (AMPK). In vitro experiments have also shown that curcumin can activate carnitine palmitoyl transferase 1 (CPT-1) to promote β-oxidation, while it inhibits enzymes involved in lipid biosynthesis such as glycerol-3-phosphate transferase 1 (GPAT1) and acyl-coenzyme A carboxylase have been shown to inhibit the enzymes involved in lipid biosynthesis, such as glycerol-3-phosphate transferase 1 (GPAT1) and acyl-coenzyme A carboxylase [35].
2.4 Antibacterial and insecticidal effects
As early as 1949, studies on the antibacterial effects of curcumin were conducted, and it has been shown that curcumin has an inhibitory effect on Staphylococcus aureus, Microsporum canis, Salmonella paratyphi and Mycobacterium tuberculosis [36]. Studies have shown that adding 100 μmol/L curcumin to an in vitro culture test can reduce Escherichia coli by 80% [37]. Lüer et al. [38] also found that Staphylococcus aureus and Pseudomonas aeruginosa had a 100% mortality rate when exposed to 100 μmol/L curcumin. Enterococcus faecalis can achieve a 80% mortality rate after 4 hours of treatment with 200 μmol/L curcumin. It can be seen that curcumin has an inhibitory effect on both Gram-positive bacteria (Staphylococcus aureus and Enterococcus faecalis) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa). According to Tyagi et al. [39], 106 CFU/mL of Staphylococcus aureus was exposed to 100 μmol/L curcumin, and after 2 hours, it was completely killed. It was found by labeling with two transilluminating fluorescent probes, propidium iodide and calcein, that 94% to 98% of the cell membranes of Staphylococcus aureus were damaged and leaky. This indicates that the antibacterial mechanism of curcumin is to destroy the structure of the bacterial membrane.
Bazh et al. [40] found that curcumin has a certain insecticidal effect, and the insecticidal effect is related to its concentration and treatment time. Khalafalla et al. [41] showed that the infectivity of C. sporogenes was reduced by 41.6% and 72.8% at curcumin concentrations of 100 and 200 μmol/L, respectively, compared to the control group, and that curcumin doses below 400 μmol/L did not show negative effects on infected cells. Therefore, curcumin can be considered as a potential anti-insect agent.
3 Application of curcumin in animal feeding
3.1 Application in poultry production
Curcumin has the effect of improving poultry production performance and immunity. Studies have shown that adding 200 and 250 mg/kg of curcumin can increase the weight gain of broilers by 4.48% and 1.59%, respectively, and reduce the feed conversion ratio by 7.39% and 6.40%, respectively [42], with the 200 mg/kg dose being extremely effective [43]. Platel et al. [44] reported that the addition of 5 g/kg curcumin to the rat diet significantly increased pancreatic enzyme activity. Therefore, the mechanism by which curcumin promotes the growth performance of broilers may be to improve feed digestibility by stimulating the production of intestinal digestive enzymes or enhancing the activity of digestive enzymes.

At the same time, studies have shown that NO, as an important product of L-arginine decomposition [45], is an important inhibitory neurotransmitter in the intestine, which inhibits gastrointestinal motility [46]. Combined with the analysis of the physiological function of curcumin in inhibiting inflammation, curcumin may inhibit the production of NO by inhibiting the enzymatic activity of L-arginine conversion to NO, thereby promoting gastrointestinal motility and increasing feed intake. In addition, Zhang et al. [14] found that the addition of curcumin is beneficial to alleviate the effects of stress on broiler production. Therefore, relieving stress damage may also be an important reason for improving growth performance.
Studies have shown that the addition of curcumin can significantly increase the thymus index [47], spleen index [48], antibody [47] and total protein levels [48] of broilers. The index of immune organs reflects the state of development of immune organs. The mechanism by which curcumin promotes their development may be through the increase in total protein levels, which improves the body's absorption and utilization of protein and provides conditions for the healthy growth of immune organs; while the increase in antibody levels is related to curcumin's promotion of thymus development, which affects the differentiation and maturation of T lymphocytes, and in turn enhances the process by which T lymphocytes stimulate B lymphocytes to produce antibodies.
Curcumin also has the function of improving meat color and freshness [49-50] and improving the nutritional value of muscles [42, 51]. The mechanism is related to the strong antioxidant properties of curcumin. In poultry muscle, myoglobin, which is mainly responsible for meat color regulation, may oxidize and cause discoloration of the muscle [52]. Muscle contains high concentrations of polyunsaturated fatty acids, which are easily attacked by free radicals, leading to lipid peroxidation and the accumulation of peroxide products, which ultimately affect meat quality and nutritional composition [53]. Curcumin, which has strong antioxidant properties, can effectively prevent excessive oxidation of myoglobin and prevent the rapid oxidative necrosis of muscle cells in fresh meat. At the same time, curcumin improves the nutritional value of chicken meat by regulating the fat metabolism and deposition of broiler chickens.
3.2 Application in pig production
Studies have shown that the addition of curcumin can improve the digestibility of pig feed [54] and its function in production performance [55], and its effect is even significantly better than that of quinolones [56-57]. The mechanism may be related to the regulatory effect of curcumin on the intestine. In a study by Xun et al. [56] on piglets challenged with Escherichia coli, it was found that the addition of curcumin significantly increased the ratio of the height of the jejunal villi to the depth of the crypts, improved the jejunal epithelial mucosal morphology, and repaired the damage caused to the intestine by Escherichia coli. It can be considered that the protection of the intestinal tract by curcumin provides conditions for the digestion and absorption of nutrients by the body and the improvement of productive performance. At the same time, the antibacterial and insecticidal functions of curcumin and its ability to reduce the effects of stress are also possible mechanisms by which curcumin improves the productive performance of pigs.
Studies have shown that curcumin also has a regulating effect on pork quality [58].
Zhu Guoqiang et al. [58] found that after adding 300 and 400 mg/kg of curcumin to the feed, the lean meat rate and muscle pH were significantly increased, the backfat thickness and muscle drip loss were significantly reduced, and the post-slaughter meat quality and muscle quality were significantly improved. The regulation of the lean meat rate and backfat thickness may be achieved by activating glutathione peroxidase, inhibiting the oxidative process of the pentose phosphate pathway, and thus affecting lipid synthesis. On the other hand, it may promote lipid metabolism and excretion through the lipid-lowering function of curcumin. The pH in muscle can change the charge of proteins. After slaughter, the pH of fresh meat will decrease and reach the isoelectric point. The proteins in the muscle will coagulate and shrink, producing more free water and drip loss. Therefore, adding curcumin can increase the pH of fresh meat, which can reduce drip loss to a certain extent, while maintaining the freshness of the meat with its antioxidant properties.

3.3 Application in ruminants
There have been few reports on ruminants, and they are limited to improving the quality of semen and semen preservation in breeding animals. Studies have shown that the addition of curcumin during semen preservation can significantly improve the antioxidant capacity of sperm before freezing and after thawing [59], mitochondrial activity and sperm motility, and ensure sperm quality [60]. This is because the sperm structure contains a high concentration of unsaturated fatty acids, which are easily damaged by free radicals [61]. Curcumin may use its lipid solubility to enter the interior of the sperm, bind to the excess free radicals produced during the freezing and thawing process, protect the mitochondrial membrane and sperm membrane structure, and ensure sperm quality.
4 Summary
In summary, curcumin has important physiological functions, and as a feed additive, it can improve the production performance of livestock and poultry and enhance the quality of livestock products. However, there is currently limited research on the application of curcumin in livestock production, and the types of livestock studied are relatively limited, and the mechanism of action is unclear. Currently, “antibiotic-free farming” is a global trend to address the safety issues caused by the abuse of antibiotics. Therefore, there is an urgent need to further study the appropriate additive amount of curcumin in various livestock and poultry, the effect and regulatory mechanism on different livestock and poultry species, and to lay a theoretical foundation for the application of curcumin in animal husbandry.
Reference:
[1 ] SHARMA R A,GESCHER A J,STEWARD W P. Curcumin : the story so far[J].European Journal of Cancer,2005,41 ( 13) : 1955-1968.
[2 ] KALYAN S,SHARMA P K,GARG V K,et al. Re- cent advancement in chitosan based formulations and its pharmaceutical application[J].Der Pharmacia Sini- ca,2010,1 ( 3) : 195-210.
[3 ] MILOBEDZKA J ,KOSTANECKI V,LAMPE V . Structure of curcumin[J].Chemische Berichte,1910, 43 : 2163-2170.
[4 ] ESATBEYOGLU T,HUEBBE P,ERNST I M A,et al.Curcumin-from molecule to biological function[J]. Angew andte Chemie : International Edition,2012,51 (22) : 5308-5332.
[5 ] GOEL A,KUNNUM AKKARA A B,AGGARWAL B B.Curcumin as “Curecumin ” : from kitchen to clinic [J].Biochemical Pharmacology,2008,75 ( 4) : 787 -809.
[6 ] PRIYADARSINI K I,MAITY D K,NAIK G H,et al. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcu- min[J].Free Radical Biology and Medicine,2003,35 (5) : 475-484.
[7 ] KANEKO T,BABA N.Protective effect of flavonoids on endothelial cells against linoleic acid hydroperox- ide-induced toxicity [J].Bioscience,Biotechnology,and Biochemistry,1999,63 (2) : 323-328.
[8 ] OSAWA T,SUGIYAMA Y,INAYOSHI M ,et al.An- tioxidative activity of tetrahydrocurcuminoids[J].Bio- science,Biotechnology,and Biochemistry,1995,59 (9) : 1609-1612.
[9 ] SUGIYAMA Y,KAWAKISHI S,OSAWA T.In- volvement of the β-diketone moiety in the antioxida- tive mechanism of tetrahydrocurcumin[J].Biochemi- cal Pharmacology,1996,52(4) : 519-525.
[10] JOVANOVIC S V,STEENKEN S,BOONE C W,et al.H-atom transfer is a preferred antioxidant mecha- nism ofcurcumin[J].Journal of the American Chemi- cal Society,1999,121 (41) : 9677-9681.
[11] MIRIYALA S,PANCHATCHARAM M ,RENGARA- JULU P.Cardioprotective effects of curcumin[M]/ / AGGARWAL B B,SURH Y J,SHISHODIA S.The molecular targets and therapeutic uses of curcumin in health and disease.US: Springer,2007.
[12] DENG S L,CHEN W F,ZHOU B,et al.Protective effects of curcumin and its analogues against free radi- cal-induced oxidative haemolysis of human red blood cells[J].Food Chemistry,2006,98( 1) : 112-119.
[13] ZHANG J F,HOU X,AHMAD H,et al.Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes[J].Food Chemistry,2014,145 : 57-65.
[14] ZHANG J F,HU Z P,LU C H,et al.Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mi- tochondrial pathway[J].Journal of Animal Science, 2015,93 (4) : 1656-1665.
[15] TORGOVNICK A,SCHIAVI A,MAGLIONI S,et al. Healthy aging : what can we learn from Caenorhabditis elegans? [J].Zeitschrift für Gerontologie und Geriat- rie,2013,46(7) : 623-628.
[16] SHIH C M ,WU J S,KO W C,et al.Mitochondria- mediated caspase-independent apoptosis induced by cadmium in normal human lung cells[J].Journal of Cellular Biochemistry,2003,89(2) : 335-347.
[17] WASEEM M ,PARVEZ S.Mitochondrial dysfunction mediated cisplatin induced toxicity: modulatory role of curcumin[J].Food and Chemical Toxicology,2013, 53 : 334-342.
[18] TRUJILLO J,MOLINA-JIJ6N E,MEDINA-CAM - POS O N,et al.Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions : relation to oxidative stress[J].Food & Function,2015,7 ( 1 ) : 279-293.
[19] DORTA D J,PIGOSO A A,MINGATTO F E,et al. The interaction of flavonoids with mitochondria : effects on energetic processes[J].Chemico-Biological Interactions,2005,152(2 /3) : 67-78.
[20] ITOH K,WAKABAYASHI N,KATOH Y,et al. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of N rf2 in response to electrophiles [J].Genes to Cells,2003,8(4) : 379-391.
[21] Wang Tian, Zhang Jingfei. Physicochemical properties, antioxidant function and application of curcumin in broiler production [J]. Journal of Animal Nutrition, 2014, 26 (10): 3101-3107.
[22] GAO S,DUAN X X,WANG X,et al.Curcumin atten- uates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of N rf2 pathway,promotion of arsenic methylation and urinary excretion[J].Food and Chemical Toxicology,2013, 59 : 739-747.
[23] SAHIN K,ORHAN C,TUZCU Z,et al.Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of N rf2 / HO-1 pathway in quail[J]. Food and Chemical Toxicology,2012,50( 11) : 4035-4041.
[24] NAKAI N,MURATA M ,NAGAHAM A M ,et al.Ox- idative DNA damage induced by toluene is involved in its male reproductive toxicity [J].Free Radical Re- search,2003,37( 1) : 69-76.
[25] CHAN J Y,KWONG M.Impaired expression of gluta- thione synthetic enzyme genes in mice with targeted deletion of the N rf2 basic-leucine zipper protein[J]. Biochimica Et Biophysica Acta : Gene Structure and Expression,2000,1517( 1) : 19-26.
[26] ZHENG S Z,FU Y M ,CHEN A P.De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell ( HSC ) activation[J].Free Radi- cal Biology and Medicine,2007,43 ( 3) : 444-453.
[27] SANDUR S K,ICHIKAWA H,PANDEY M K,et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin ( diferu- loylmethane) [J].Free Radical Biology and Medi- cine,2007,43 (4) : 568-580.
[28] RAO C V . Regulation of COX and LOX by curcumin [J]/ /AGGARWAL B B,SURH Y J,SHISHODIA S . The molecular targets and therapeutic uses of curcumin in health and disease.US: Springer,2007.
[29] NISHINO H,TOKUDA H,SATOMI Y,et al.Cancer prevention by antioxidants[J].Biofactors,2004,22 ( 1 /2 /3 /4) : 57-61.
[30] POMERANTZ J L,BALTIMORE D.Two pathways to NF-κB[J].Molecular Cell,2002,10(4) : 693-695.
[31] Peng Jinghua, Cao Jianmei, Wang Xiaoning, et al. Inhibitory effect of curcumin on the secretion of inflammatory cytokines by Kupffer cells induced by endotoxin lipopolysaccharide [J]. Chinese Journal of Integrated Traditional and Western Medicine for Digestion, 2006, 14 (4): 211-214.
[32] REUTER S,CHARLET J,JUNCKER T,et al.Effect of curcumin on nuclear factor κB signaling pathways in human chronic my elogenous K562 leukemia cells [J].Annals of the New York Academy of Sciences, 2009,1171 ( 1) : 436-437.
[33] PATIL T N,SRIN IVASAN M.Hypocholesteremic effect of curcumin in induced hypercholesteremic rats [J].Indian Journal of Experimental Biology,1971,9 (2) : 167-169.
[34] SHIN S K,HA T Y,MCGREGOR R A,et al.Long - term curcumin administration protects against athero- sclerosis via hepatic regulation of lipoprotein cholesterol metabolism [J].Molecular Nutrition Food Re- search,2011,55( 12) : 1829-1840.
[35] EJAZ A,WU D Y,KWAN P,et al.Curcumin inhibits adipo genesis in 3T3-L1 adipocytes and ang iogenesis and obesity in C57 / BL mice[J].Journal of Nutrition, 2009,139(5) : 919-925.
[36] SCHRAUFST TTER E,BERNT H.Antibacterial ac- tion of curcumin and related compounds[J].Nature, 1949,164(4167) : 456-457.
[37] RAI D,SINGH J K,ROY N,et al.Curcumin inhibits FtsZ assembly : an attractive mechanism for its antibac- terial activity[J].Biochemical Journal,2008,410( 1) : 147-155.
[38] L ER S,TROLLER R , AEBI C.Antibacterial and an- tiinflammatory kinetics of curcumin as a potential an- timucositis agent in cancer patients[J].Nutrition and Cancer,2012,64(7) : 975-981.
[39] TYAGI P,SINGH M ,KUMARI H,et al.B actericidal activity of curcumin Ⅰ is associated with damaging of bacterial membrane[J].PLoS One,2015,10 ( 3 ) : e0121313.
[40] BAZH E K A,EL-BAHY N M.In vitro and in vivo screening of anthelmintic activity of ginger and curcu- min on Ascaridia galli [J].Parasitology Research, 2013,112( 11) : 3679-3686.
[41] KHALAFALLA R E,M LLER U,SHAHIDUZZA- MAN M ,et al.Effects of curcumin ( difer-uloylmeth-ane) on Eimeria tenella sporozoites in vitro[J].Para- sitology Research,2011,108(4) : 879-886.
[42] Zhu Guoqiang, Hou Fengqin. Effects of curcumin on daily weight gain, lipid metabolism, and meat quality of broiler chickens [J]. Feed Expo, 2007(2): 49-51.
[43] Cui Yan, Zhu Guoqiang, Hou Fengqin, et al. Effect of curcumin on the production performance and biochemical indicators of broilers [J]. Animal Husbandry and Veterinary Medicine, 2010, 42(8): 44-46.
[44] PLATEL K,SRIN IVASAN K.Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats [J].International Journal of Food Sciences and Nutrition,1996,47( 1) : 55-59.
[45] GRASA L,ARRUEBO M P,PLAZA M A,et al.A downregulation of nNOS is associated to dysmotility evoked by lipopolysaccharide in rabbit duodenum[J]. Journal of Physiology & Pharmacology,2008,59( 3) : 511-524.
[46] Luo Shuang, Yu Xiaoling, Li Hongjing. The effect of curcumin on gastrointestinal motility in mice and its mechanism of action [J]. Qilu Medical Journal, 2012, 27 (5): 430-431.
[47] Hu Zhongze, Jin Guangming, Wang Like, et al. Effect of curcumin on the production performance and immune function of broilers [J]. Grain and Feed Industry, 2004 (10): 44-45.
[48] Zhou Luli, Zhou Hanlin, Wang Dingfa. Effects of turmeric extract on growth performance, blood biochemical indicators and immune organ index of guinea fowls [J]. Feed Industry, 2016, 37(18): 5-8.
[49] ZHANG J F,HU Z P,LU C H,et al.Effect of various levels of dietary curcumin on meat quality and antioxi- dant profile of breast muscle in broilers[J].Journal of Agricultural & Food Chemistry,2015,63 ( 15) : 3880-3886.
[50] Zhu Guoqiang, Wang Bin, Hou Fengqin, et al. Effects of curcumin on the production performance and meat quality of broilers [J]. Feed Industry, 2009, 30(13): 8-10.
[51] Hu Zhongze, Wang Like, Wen Aiyou. The effect of curcumin on the fat metabolism of Anhui yellow chickens [J]. Grain and Feed Industry, 2008 (4): 29-30, 33.
[52] SUM AN S P,JOSEPH P.Myoglobin chemistry and meat color[J].Annual Review of Food Science and Technology,2013,4( 1) : 79-99.
[53] FINKEL E.The mitochondrion : is it central to apoptosis? [J].Science,2001,292(5517) : 624-626.
[54] Lu Na, Qiu Jingyun, Ying Zhixiong, et al. Effects of dietary supplementation with different levels of curcumin on the performance, digestibility and blood indicators of weaned piglets [J]. Journal of Animal Science and Biotechnology, 2017, 38(1): 30-35.
[55] Zhao Chunping, Xun Wenjuan, Hou Guanyu, et al. Effects of curcumin on growth performance and antioxidant capacity in piglets challenged with Escherichia coli [J]. Journal of Animal Science and Biotechnology, 2015, 36(7): 24-27.
[56] XUN W J,SHI L G,ZHOU H L,et al.Effects of cur- cumin on growth performance,jejunal mucosal membrane integrity,morphology and immune status in weaned piglets challenged with enterotoxigenic Esche- richia coli [J].International Immunopharmacology, 2015,27( 1) : 46-52.
[57] Zhou M, Zhang J, Shen S, et al. Study on the effect of curcumin in fattening pigs [J]. Chinese Journal of Cereals, Oils and Foodstuffs, 2014, 29(3): 67-73.
[58] Zhu Guoqiang, Zhang Weitao, Chen Tao, et al. Effect of curcumin additive on carcass GP, meat quality and blood biochemical indicators of finishing pigs [J]. Feed Industry, 2013 (16): 9-12.
[59] SHAH S A H,ANDRABI S M H,QURESHI I Z .Freezability of water buffalo bull ( Bubalus bubalis ) spermatozoa is improved with the addition of curcu- min ( diferuoyl methane) in semen extender[J].An- drologia,2016.
[60] TVRD E,TUIMOV E,KOV IK A,et al.Curcumin has protective and antioxidant pro-perties on bull spermatozoa subjected to induced oxidative stress [J].Animal Reproduction Science,2016,172 : 10-20.
[61] ANDRABI S M.Factors affecting the quality of cry o- preserved buffalo (Bubalus bubalis) bull spermatozoa [J]. Reproduction in Domestic Animals,2009,44 ( 3) : 552-569.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






