What Is the Medicinal Use of Turmeric Powder?
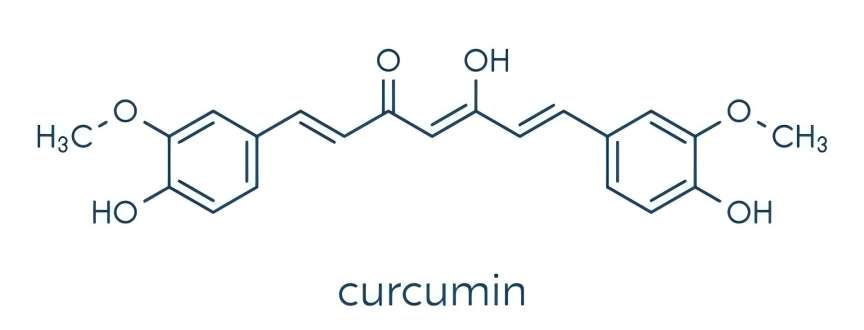
Curcumin is a natural phenolic active ingredient in the rhizome of turmeric and is the main phenolic substance extracted from the rhizome of turmeric. Turmeric can be divided into three curcuminoids, namely bisdemethoxycurcumin, demethoxycurcumin and ferulic acid methyl ester (Figure 1). The three curcuminoids have similar structures and similar pharmacological effects, including antibacterial, antioxidant, anti-apoptotic, anti-tumor and anti-metastasis effects [1,2]. Turmeric is used as an herbal remedy for a variety of ailments [3] and is also used as a spice, food coloring and preservative. Curcumin is not only used as an anti-inflammatory agent, but also to treat gastrointestinal disorders such as indigestion, flatulence, diarrhea and stomach and duodenal ulcers [4,5].
1 Chemical composition of curcumin
Curcumin is a polyphenolic compound with a diketone structure. It contains a special 1,7-dioxane skeleton and consists of two o-methylated and β-diketone phenols. Due to the presence of multiple active groups such as phenolic hydroxyl, carbonyl and double bonds in the curcumin molecule, the chemical properties of curcumin are relatively active. It has been reported that the stronger antioxidant properties of curcumin may be due to the chelation of transition metals between the o-methoxy and diketophorol [6]. Curcumin contains two tautomers: the keto and enol forms. The former is stable in both the solid and liquid phases and appears reddish-brown in color; the latter biologically active component is present in acidic and neutral conditions in the form of the diketone 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione and appears bright yellow, so it can be used as a chemical acid indicator [7,8].
Studies have shown that curcumin can exert a variety of beneficial effects in the gastrointestinal tract, such as preventing reflux esophagitis, Barrett's esophagus, and non-steroidal anti-inflammatory drug (NSAID)-induced gastric mucosal damage. Curcumin can prevent the invasion of gastric cancer cells and the proliferation of various carcinogenic factors, such as p23 and human epidermal growth factor receptor 2. In recent years, curcumin has received considerable attention in the treatment of oxidative stress and inflammation-related diseases, including gastritis, gastric ulcers and gastric cancer. Curcumin can also be used to improve age-related diseases such as atherosclerosis, diabetes, cardiovascular disease and chronic kidney disease. Turmeric derivatives have been found to alleviate chronic inflammation such as arthritis, uveitis and inflammatory bowel disease [9]. Curcumin has been identified as having strong antibacterial properties, with broad-spectrum antimicrobial effects against a range of bacteria [10]. Curcumin has been found to be effective in treating Helicobacter pylori when used alone or in combination with other existing antibiotics. Curcumin has been shown to be a broad-spectrum antimicrobial agent in both in vitro and in vivo studies, and it has been shown to have a synergistic effect with certain antibiotics when used as an adjunct therapy [11].
This review aims to analyze the chemical composition of curcumin and highlight its various pharmacological effects.
2 Pharmacological effects of curcumin
2.1 Curcumin's effect against Helicobacter pylori
Helicobacter pylori was first discovered in 1983. It is a spiral-shaped, highly mobile Gram-negative pathogenic bacterium measuring 0.6 hm 3.5 hm. Helicobacter pylori is a prokaryotic human carcinogen recognized by the World Health Organization and is a primary human pathogen [12]. In China, the infection rate of H. pylori is about 56%. Many upper gastrointestinal diseases are related to Helicobacter pylori, such as gastric cancer, dyspepsia, gastritis, peptic ulcers and upper gastrointestinal diseases [13]. The high pathogenicity of H. pylori is mainly due to its various virulence factors, such as urease, vacuolating cytotoxin A (VacA), cytotoxin-associated gene A (CagA) and -glutamyl transpeptidase (HpGGT).
2.1.1 Helicobacter pylori infection
CagA is a Helicobacter pylori protein and an important virulence factor of H. pylori. During chronic H. pylori infection, CagA is directly delivered to target cells (e.g., gastric epithelial cells) by the type IV secretion system (T4SS) encoded by the cag pathogenicity island [14, 15]. The CagA protein can function as an eukaryotic Gab1 adaptor protein and help activate some intracellular pathways, such as cell proliferation and migration [16, 17]. After delivery to the host cell, CagA undergoes tyrosine phosphorylation at its characteristic sequence (Glu-Pro-Ile-Tyr-Ala) EPI-YA motif [18]. The phosphorylated CagA protein plays a very important role in triggering cell signaling pathways and causing cell lesions.
CagA-induced biological activity can be divided into three categories: scattering/hummingbird phenotype, disruption of tight junctions between cells, and activation of several transcription factors that control cell proliferation, inflammation, and survival [19]. Therefore, through CagA-dependent pathways, H. pylori modifies the intracellular signals of target cells and promotes its infection and pathogenicity. Therefore, H. pylori infection is an important factor leading to gastritis.
Phosphorylation of the CagA protein and activation of downstream signaling pathways are the main ways in which the CagA protein exerts its pathogenic effects, and the site of phosphorylation is the tyrosine residue of the CagA protein EPIYA motif. Once CagA enters the host cell, it is immediately phosphorylated by the non-receptor tyrosine kinase Src. 2 to 4 hours after infection, the Src kinase can be inactivated by feedback inhibition of the phosphorylated CagA protein with which it is bound. At this time, another non-receptor tyrosine kinase, Abl, is activated to continue phosphorylating CagA[20]. Phosphorylation of different sites of EPIYA causes the degree of cell shape change is also different, which indicates that the phosphorylation of CagA is strictly controlled during H. pylori infection. The interaction between CagA and target proteins results in the activation of abnormal signal pathways, thereby disrupting cell function [21].
The tyrosine kinase receptor encoding gene (Mesenchymal epithelial transition factor, MET) is a proto-oncogene. The transmembrane receptor protein MET encoded by it has tyrosine kinase activity, so MET is a member of the receptor tyrosine kinase family. Helicobacter pylori induces proinflammatory and malignant precancerous reactions through CagA-MET signaling, induces epithelial cell movement, and activated MET uses CagA as an adaptor protein, which then induces CagA phosphorylation, leading to downstream phospholipase C (PLC) and MAPK pathways to reduce Helicobacter pylori-induced cell movement [22]. CagA can also activate MET signaling in a non-phosphorylated manner in which case a conserved motif in the C-terminal region of CagA called the conserved repeat phosphoinositide-independent activity (CRPIA) motif may interact with the activated MET, leading to activation of PI3K/Akt signaling, which promotes cell migration and motility, ultimately leading to cell proliferation and inflammatory responses, resulting in the proliferation of gastric cancer cells after Helicobacter pylori infection [23]. Therefore, both the phosphorylated and non-phosphorylated forms of CagA can interact with MET and stimulate downstream signaling pathways in gastric cancer progression.
In addition, H. pylori infection-induced MET activation affects immune cells surrounding gastric cancer cells. Helicobacter pylori infection in gastric cancer cells has been reported to increase the production of exosomes containing active forms of MET [24]. Exosomes are extracellular signaling bodies that are widely produced under normal physiological conditions and mediate communication between eukaryotic cells. Tumor cells can transfer MET-inducing factors such as IL-6, Akt, and TNF-a through exosomes, inducing MET in neighboring tumor cells and thus inducing carcinogenic effects on the stomach.
2.1.2 Antibacterial effect of curcumin
The role of curcumin in Helicobacter pylori infection is mainly to inhibit Helicobacter pylori-induced nuclear factor-KB, nuclear factor-activated B cell K-light chain enhancement (NF-KB), activation-induced cell death (AICD), interleukin-8 (IL-8), MMP-3 and MMP-9 in host epithelial cells, thereby preventing inflammatory reactions [25], see Figure 2. Studies have shown that curcumin is more effective against COX-2 and TXA2 than against COX-1. Curcumin can inhibit the activity of COX-2 and TXA2 but does not affect the activity of COX-1. The anti-inflammatory effect of curcumin may be achieved by inhibiting major inflammatory mediators such as cyclooxygenase (COX-1 and COX-2), lipoxygenase (LOX), tumor necrosis factor (TNF2), interferon (IFN2) and inducible nitric oxide synthase (iNOS) [26]. Among these, COX-2, LOX and iNOS are important enzymes that mediate inflammatory responses [27]. Studies have shown that curcumin affects the phosphorylation of cytosolic phospholipase A2 and reduces the expression of COX-2 by blocking arachidonic acid metabolism, while inhibiting the catalytic activity of 5-LOX.
In Helicobacter pylori, the components of the type IV secretion system transport the CagA protein into gastric epithelial cells. Subsequently, the CagA protein is phosphorylated. This phosphorylated CagA interacts with the host phosphatase SHP-2, causing rearrangements of the cytoskeleton and resulting in morphological changes in the host cell [28].
Studies have shown that H. pylori exposed to curcumin for 6 hours can lead to reversible growth inhibition of the bacteria, effectively reducing the translocation of CagA [29,30]. By reducing the translocation of CagA, it can reduce cytoskeletal rearrangements, leading to almost complete inhibition of CagA phosphorylation [31,32]. These findings are related to the inhibitory effect of curcumin on H. pylori growth. A large number of experimental studies in mice have shown that oral administration of curcumin can significantly inhibit gastric inflammation caused by H. pylori infection [33,34]. Many studies have emphasized the effect of curcumin on H. pylori. Due to its strong antibacterial activity, curcumin can effectively inhibit bacterial growth, thus providing a new way for the treatment of H. pylori in the field of disease treatment [35]. Therefore, curcumin may be an effective drug for the prevention and treatment of H. pylori infection.
2.2 Anti-cancer effects of curcumin
Studies have shown that compared with patients infected with CagA-negative H. pylori isolates, colonization with CagA-positive H. pylori isolates is associated with an increased risk of severe gastritis, peptic ulcer disease and gastric cancer [36]. Helicobacter pylori can initiate abnormal activation pathways of cell signals. Phosphorylated CagA can induce the up-regulation of -enolase expression by activating the ERK/MAPK signaling pathway. -Enolase is a new tumor-associated protein that is involved in the process of tumor unlimited proliferation, causes cell autophagy and induces the occurrence of gastric cancer [37,38]. This further proves the important role of phosphorylated CagA in the mechanism of H. pylori-induced gastric cancer.
The anti-inflammatory activity of curcumin is similar to that of non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin. Ulcers caused by non-steroidal anti-inflammatory drugs (NSAIDs) are a complex process involving inhibition of prostaglandin synthesis in the gastrointestinal tract, resulting in increased gastric acid secretion, reduced bicarbonate mucus secretion, reduced trophic effect on epithelial mucosa [39], as well as increased microvascular permeability, imbalance of nitric oxide and production of free radicals. These anti-inflammatory drugs block prostaglandin synthesis by inhibiting the activity of cyclooxygenase (COX), which increases gastric acid secretion, leading to mucus depletion and increased damage to the mucosal wall [40]. Their anti-inflammatory and anticancer properties are mediated by inhibiting COX-2, LOX and iNOS, producing cytokines such as IFN- and TNF-, and activating transcription factors such as NF-KB and AP-1 [41,42]. Therefore, drugs that interfere with the signal pathways involved in COX-2 transcription can also reduce inflammation and the occurrence of tumors.
Further studies have shown that arachidonic acid metabolites from the LOX pathway play an important role in growth-related signal transduction, which means that intervention in these pathways should be useful for preventing cancer progression [43]. Curcumin exhibits strong antioxidant and anticancer properties by regulating the expression of genes activated by activator protein (AP-1) and nuclear factor KB (NF-B). Since inflammation is closely related to tumor promotion, curcumin is expected to exert chemopreventive effects on cancer development due to its strong anti-inflammatory properties [44]. Potential anti-cancer mechanisms of curcumin include inhibition of NF-KB and COX-2 (elevated levels of COX-2 are associated with various cancers); inhibition of arachidonic acid metabolism via lipoxygenase, which removes free radicals produced by this pathway; suppression of cancer cell growth due to reduced expression of inflammatory cytokines such as IL-1β, IL-6 and TNF-; and down-regulation of enzymes that mediate inflammation and tumour cell proliferation, such as protein kinase C [45]. Therefore, curcumin can prevent, inhibit tumor growth and promote apoptosis of tumor cells through multiple signal pathways.
2.3 Antioxidant effect of curcumin
Curcumin is an effective antioxidant in the upper digestive tract and a scavenger of nitrogen compounds [46,47]. The antioxidant effect of curcumin is mainly reflected in the removal of active oxygen free radicals, inhibition of lipid peroxidation, increased activity of superoxide dismutase (SOD) and catalase (CAT), etc., and a significant inhibitory effect on lecithin lipid peroxidation and induced DNA oxidative damage. Helicobacter pylori causes gastric damage through inflammatory mediators, which leads to the production of large amounts of oxygen free radicals and reactive oxygen species. After lysosomal membrane rupture, the body's hydrolases are released into the cells, thereby hydrolyzing the entire cell and causing damage to gastric epithelial cells [48]. This damage can lead to constriction of the veins and arteries of the gastric mucosa, resulting in congestion, inflammation and tissue damage. Experiments have shown that the protective effect of curcumin on indomethacin-induced gastric mucosal damage in rats depends on the down-regulation of curcumin-mediated pro-inflammatory cytokine (IL-6, TNF-) expression, and histological damage is significantly reduced.
Among them, curcumin inhibits apoptosis and increases mucosal epithelial resistance, thereby increasing gastric mucosal cell differentiation. Curcumin is a unique antioxidant containing many functional groups, including phenolic hydroxyl groups, methoxy groups and 1,3-β-diketones, as determined by chemical structure analysis. Curcuminoids can undergo nucleophilic addition via various complex mechanisms, which confers their antioxidant properties [49,50]. Curcumin is unstable under alkaline conditions, but its stability increases significantly under acidic conditions. In an in vivo study, curcumin was compared with lansoprazole, a proton pump inhibitor (PPI) that is the recommended standard drug for the treatment of gastrointestinal diseases such as gastroesophageal reflux disease (GERD) [51]. It was found that curcumin can effectively prevent the formation of acute mixed reflux esophagitis, reduce neutrophil infiltration, and reduce its severity. Although curcumin is less effective than the proton pump inhibitor (PPI) lansoprazole in inhibiting acid reflux esophagitis, it is effective in preventing bile acid reflux esophagitis. This protective mechanism of curcumin in the esophagus can be attributed to the antioxidant properties of curcumin.
3 Conclusion
Analysis of the chemical structure and pharmacological effects of curcumin shows that it has anti-inflammatory, anticancer and antioxidant biological effects. As research continues, the overall efficacy of curcumin in treating various diseases such as gastric inflammation and cancer is expected to be promising. The role and mechanism of curcumin powder in the development and progression of diseases will provide new ideas for the prevention and treatment of various diseases, which is of great significance for the diagnosis and prevention of diseases.
Reference:
[ 1] LIU W H, YUAN J B, ZHANG F, et al. Curcumin inhibits pro- liferation, migration and invasion of gastric cancer cells via wnt3a/p-catenin/EMT signaling pathway[J]. China Journal of Chinese Materia Medica, 2019, 44( 14): 3107-3115.
[2] IVY B R, JúlLIA S V, CARLA V G, et al. Polymorphisms of the IL-6, IL-8 and IL-10 genes and the risk ofgastric pathology in patients infected with Helicobacter pylori[J]. Journal of Micro- biology, Immunology and Infection, 2017, 50(2) : 153-159.
[3] HE C Y, CHEN M Y, LIU J Y, et al. Host genetic factors re- spond to pathogenic step-specific virulence factors of Helico- bacter pylori in gastric carcinogenesis[J]. Mutation Research- Reviews in Mutation Research, 2014, 759( 1): 14-26.
[4] NANJUNDASWAMY S, JAYASHANKAR J, RENGANA- THAN R R A, et al. Pyridine coupled pyrazole analogues as lethal weapon against MRSA: an in-vitro and in-silico approach [J]. Microbial Pathogenesis, 2022, 166: 105508.
[5]NAUMANN M, SOKOLOVAO, TEGTMEYER N, et al. Heli- cobacter Pylori: a paradigm pathogen for subverting host cell sig- nal transmission[J]. Trends in Microbiolgy, 2016, 25(4): 316-328.
[6] SANAEI M J, SHIRZAD H, SOLTANI A, et al. Up-regulated CCL18, CCL28 and CXCL13 expression is associated with the risk of gastritis and peptic ulcer disease in Helicobacter Pylori infection[J]. The American Journal of the Medical Sciences, 2020, 361( 1): 43-54.
[7] GORGIEVAO S, TRCEK J. Bacterial cellulose: production, modification and perspectives in biomedical applications[J]. Nanomaterials, 2019, 9( 10): 1352.
[8] WANG J, TAVAKOLI J, TANG Y. Bacterial cellulose produc- tion, properties and applications with different culture methods- a review[J]. Carbohydrate Polymers, 2019, 219: 63-76.
[9] KHAN H, KADAMA, DUTT D. Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus[J]. Carbohy- drate Polymers, 2020, 229(22) : 115513-115523.
[ 10] CHENG H, XU T. Macrophage polarization in the develop- ment and progression of ovarian cancers: an overview[J]. Fron- tiers in Oncology, 2019, 9: 421.
[ 11] RUFFELL B, COUSSENS L M. Macrophages and therapeutic resistance in cancer[J]. Cancer Cell, 2015, 27(4): 462-472.
[ 12] BISWAS S K, MANTOVANI A. Macrophage plasticity and interaction with iymphocyte subsets: cancer as a paradigm[J]. Nature Immunology, 2010, 11( 10): 889-896.
[ 13] CAO X, CHEN J, LI B, et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy[J]. Science Advances, 2022, 8( 11): l91-201.
[ 14] WENG WH, GoelA. Curcumin and colorectal cancer: An up- date and current perspective on this natural medicine[J]. Sem- inars in Cancer Biology, 2022, 80: 73-86.
[ 15] TURCHI R, TORTOLICI F, Monica B, et al. Low sulfur ami- no acid, high polyunsaturated fatty acid diet inhibits breast cancer growth[J]. International Journal of Molecular Scien- ces, 2022, 24( 1) : 249.
[ 16] LIM, GUO T, LIN J, et al. Curcumin inhibits the invasion and metastasis of triple negative breast cancer via Hedgehog/Gli 1 signaling pathway[J]. Journal of Ethnopharmacology, 2022, 283: 114689.
[ 17] WANG N Z, FENG T, LIU X N, et al. Curcumin inhibits mi- gration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/ AKT/mTOR signaling pathway[J]. Acta Pharmaceutica, 2020, 70(3): 399-409.
[ 18] GHASEMI F, SHAFIEE M, ZARRIN S, et al. Curcumin in- hibits NF-kB and Wnt/p-catenin pathways in cervical cancer cells[J]. Pathology-Research and Practice, 2019, 215 ( 10): 152556.
[ 19] DA W, ZHANG J, ZHANG R, et al. Curcumin inhibits the ly- mphangiogenesis of gastric cancer cells by inhibiton of HMGB1/VEGF-D signaling[J]. International Journal of Im- munopathology and Pharmacology, 2019,33: 3107-3115.
[20] KHONCHE A, BIGLARIANO, PANAHIY, et al. Adjunctive therapy with curcumin for peptic ulcer: a randomized control- led trial[J]. Drug Research, 2016, 66(8) : 444-448.
[21] JIANG C, LUO P. Nrf2/ARE is a key pathway for curcumin- mediated protection of TMJ chondrocytes from oxidative stress and inflammation[J]. Cell Stress and Chaperones, 2020, 25(3): 395-406.
[22] JIANG X, LI S, QIU X, et al. Curcumin inhibits cell viability and increases apoptosis of SW620 human colon adenocarcin- oma cells via the caudal type Homeobox-2 (CDX2)/Wnt/ - Catenin Pathway[J]. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 2019, 25: 7451-7458.
[23] KWIECIEN S, MAGIEROWSKI M, MAJKA J, et al. Curcu- min: a potent protectant against esophageal and gastric disor- ders[J]. International Journal ofMolecular Sciences, 2019, 20 (6): 1477.
[24] LATHAM A, SRINIVASAN P, KEMEL Y. Microsatellite in- stability is associated with the presence of lynch syndrome pan-cancer[J]. Journal of Clinical Oncology, 2019, 37 (4): 286-295.
[25] LIU J, ZHANG J, REN L, et al. Fine particulate matters in- duce apoptosis via the ATM/P53/CDK2 and mitochondria ap- optosis pathway triggered by oxidative stress in rat and GC-2spd cell[J]. Ecotoxicology and Environmental Safety, 2019, 180: 280-287.
[26] YU Y T, TIAN L Q, XIAO Y Y, et al. Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: A systematic review and meta-analy- sis of randomized controlled trials[J]. Annals ofNutrition and Metabolism, 2018, 73( 1): 62-73.
[27] NI Y, NI L, ZHU F, et al. Adipose yissue macrophage pheno- types and characteristics: the key to insulin resistance in obesity and metabolic disorders[J]. Obesity, 2020, 28(2): 225-234.
[28] JEFFRY A, ANDREW M J, BODDY AV. Curcumin as a cli- nically-promising anti-cancer agent: pharmacokinetics and drug interactions[J]. Expert Opin on Metabolism and Toxicology, 2017, 13(9) : 953-972.
[29] ZHANG J L, ZHANG J, ZHANG R, et al. Implications of im- munoglobulin G depositin glomeruli in Chinese patients with diabetic nephropathy[J]. Clinical and Experimental Pharma- cology and Physiology, 2020, 47(6) : 919-926.
[30] FENG J, LU S Y, OU B, et al. The role ofJNK signaling pat- hway in obesity-driven insulin resistance[J]. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 2020, 13: 1399-1406.
[31] XIAO H, KANG S. The role ofthe gut microbiome in energy balance with a focus on the gut-adipose tissue axis[J]. Fronti- ers in Genetics, 2020, 11(7): 297.
[32] HERSOUG L, MOLLER P, LOFT S. Role of microbiota-de- rived lipopolysaccharide in adipose tissue inflflammation, adipocyte size and pyroptosis during obesity[J]. Nutrition Re- search Reviews, 2018, 31(2): 153-163.
[33] RACHDAOUI N. Insulin: the friend and the foe in the devel- opment oftype 2 diabetes mellitus[J]. International Journal of Molecular Sciences, 2020, 21(5) : 1770.
[34] MOHAMAD A, VAHIDEH HZ. IL-6 signalling pathways and the development oftype 2 diabetes[J]. Inflflammopharmacology, 2018, 26(3) : 685-698.
[35] AKASH M S H, REHMAN K, Liaqat A. Tumor necrosis fac- tor-alpha: role in development of insulin resistance and pat- hogenesis of type 2 diabetes mellitus[J]. Journal of Cellular Biochemistry, 2018, 119( 1): 105-110.
[36] VOLPE C M O, VILLAR D, PAULA M, et al. Cellular death, reactive oxygen species (ROS) and diabetic complications[J]. Cell Death and Disease, 2018, 9(2): 119.
[37] WANG J, WANG H. Oxidative stress in pancreatic beta cell regeneration[J]. Oxidative Medicine and Cellular Longevity, 2017, 120(9): 193-201.
[38] HESARI A R, MITRA A, ALIREZA S, et al. Chemopreven- tive and therapeutic potential of curcumin in esophageal can- cer: current and future status[J]. International Journal of Cancer, 2019, 114(6): 1215-1226.
[39] KARBALAEI M, TALEBI B. Clinical relevance ofthe CagA and VacA s1m1 status and antibiotic resistance in Helicobac- ter pylori: a systematic review and meta-analysis[J]. BMC In- fectious Diseases, 2022, 22( 1): 573.
[40] COVER T L, LACY D B, OHI M D. The Helicobacter pylori cag type IV secretion system[J]. Trends in Microbiology, 2020, 28(8): 682-695.
[41] UMA R B, ROUTHUN K, KUMAR A. Multifaceted roles of plant derived small molecule inhibitors on replication cycle of SARS-CoV-2[J]. Microbial Pathogenesis, 2022, 168: 105512.
[42] KARKHAN A. Helicobacter pylori evasion strategies of the host innate and adaptive immune responses to survive and de- velop gastrointestinal diseases[J]. Microbiological Research, 2018, 218: 49-57.
[43] MENTIS A, BOZIKI M, GRIGORIADISN. Helicobacter py- lori infection and gastric cancer biology: tempering a double- edged sword cell[J]. Cellular and Molecular Life Sciences, 2019, 76( 13): 2477-2486.
[44] PEREIRA M J. Helicobacter Pylori infection, the gastric microbiome and gastric cancer[J]. Advances in Experimental Medicine and Biology, 2019, 1149: 195-210.
[45] TANG X Q, BIH, FENG J Q, et al. Effect of curcumin on mul- tidrug resistance in resistant human gastric carcinoma cell Line SGC7901/VCR[J]. Acta Pharmacologica Sinica, 2005, 26(8): 1009-1016.
[46] PIWOCKA K, BIELAK M A, EWA S. Curcumin induces cas- pase-3-independent apoptosis in human multidrug-resistant cells[J]. Annals ofthe New York Academy of Sciences, 2002, 973: 250-254.
[47] PAL S, TANYA D, GAURISANKAR S. Curcumin selectively induces apoptosis in seregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner[J]. Journal of Biological Chemistry, 2005, 280(20): 20059-20068.
[48] CHEN Y, KUO T C. Induction of HSP70 gene expression by modulation of Ca(12) Ion and cellular p53 protein by curcu- min in colorectal carcinoma cells[J]. Molecular Carcinogen- esis, 1996, 17(4) : 224-234.
[49] KATO K, ITO H, KAMEI K, et al. Stimulation of the stress- induced wxpression of stress proteins by curcumin in cultured cells and in rat tissues in vivo[J]. Cell Stress and Chaperones, 1998, 3(3) : 152-160.
[50] SUNDAR D K S, HOURELD N N, MHEIDI A, et al. Thera- peutic potential and recent advances of curcumin in the treatment of aging-associated diseases[J]. Molecules, 2018, 23(4): 835.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese