What Is the Emulsification and Encapsulation of Curcumin Powder?
Curcumin is a natural active ingredient extracted from the dried rhizomes of turmeric, Curcuma longa L., tumeric, Curcuma domestica, and galangal, Alpinia officinarum, of the ginger family, Zingiberaceae. It has a wide range of pharmacological effects, is low in toxicity and well tolerated [1]. Curcumin was first isolated from Curcumalonga L. in 1870 as a low-molecular-weight polyphenol compound, and its chemical structure was elucidated as dihydroferulic acid in 1910 [2].
Curcumin is a natural pigment permitted for use in China's food additive standards [3], and its coloring power is greater than that of other natural pigments and synthetic lemon yellow. Curcumin also has many physiological effects, such as antioxidant, lipid-lowering, anti-atherosclerosis [4], anti-inflammatory [5], anti-aging [6], anti-tumor [7] and a series of other biological pharmacological activities, with very few toxic side effects on the human body. It has a very broad application prospect. However, due to its poor physicochemical stability and low in vivo bioavailability, it is often necessary to use a large amount to achieve the effective dose (when taken orally, 10 g to 12 g is required to detect trace amounts of curcumin in the body), which greatly limits the promotion of curcumin in the fields of functional health food and medicine. Emulsifying and encapsulating curcumin can solve some of the problems associated with its poor water solubility and instability. This article focuses on the properties of curcumin, the preparation of emulsions and encapsulates, and the research progress and development prospects of their stability.

1 Structure and function of curcumin
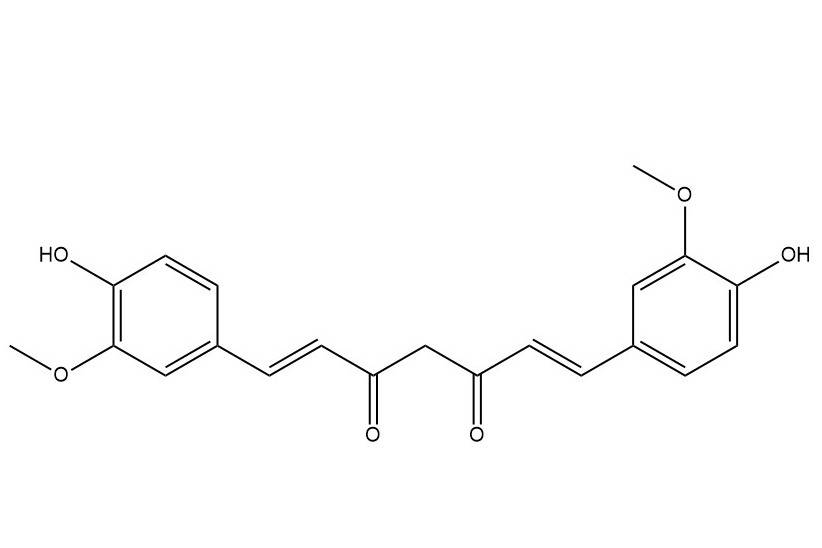
Curcumin has the molecular formula C21H20O6, molecular weight 368.39, and melting point 180 ℃~183 ℃. It is an orange-yellow crystalline powder with a slightly bitter taste. It is insoluble in water and ether, soluble in ethanol and propylene glycol, and easily soluble in glacial acetic acid and alkali solution. Curcumin is reddish brown under alkaline conditions and light yellow under acidic conditions. It has strong coloring power and stains proteins well. It is particularly sensitive to light and must be stored away from light. Its maximum absorption peak is near a wavelength of 425 nm [8]. The main components of curcuminoids are curcumin (60%–70%), demethoxycurcumin (20%–27%), and bisdemethoxycurcumin (10%–15%). The molecular structures of the three are are shown in Figure 1 [9]. Among them, curcumin (3-methoxy-4-hydroxy-phenyl-1,6-heptadiene-3,5-dione) is the main active ingredient and is a polyphenolic compound with a β-diketone functional group [10].
Curcumin is stable to reducing agents, has strong coloring properties, and once colored, is not easily faded. However, it is sensitive to light and heat and easily forms chelates with iron ions. Zn2+, Fe2+, Fe3+, sucrose, and maltose have a color-enhancing effect on curcumin, while tartaric acid, citric acid, sodium benzoate, and Cu2+ have a color-fading effect. K+, Na+, Mg 2+, and vitamin C have no significant effect on curcumin [11]. Because curcumin has two hydroxyl groups at each end of the molecule, a conjugation effect occurs under alkaline conditions where the electron cloud is displaced. Therefore, when the pH is greater than 8, curcumin changes from yellow to red. Modern chemistry uses this property to make it an acid-base indicator.
Curcumin is an orange-yellow, alcohol-soluble compound that is not only a universal coloring agent but also has many nutritional values [12]. Due to the presence of multiple carbon-carbon double bonds in the structure of curcumin, its chemical properties are very unstable, and it is prone to oxidative degradation under light and heat conditions. At the same time, the unsaturated structure gives it strong antioxidant activity and free radical scavenging ability, so it has certain physiological activity and can effectively capture and remove active oxygen free radicals in the body. Most studies have shown that curcumin can reduce oxidative stress. This is because curcumin inhibits lipid and protein oxidation by inhibiting the carbonyl groups of formaldehyde and proteins, and it also stimulates the activity of various antioxidant enzymes, including superoxide dismutase and various oxidase enzymes [13].
Curcumin's many physiological functions, such as its anticancer and immunomodulatory functions after binding to albumin [14], its sun protection effect by reducing UV-induced apoptotic changes in human keratinocytes and human epidermal cancer cells [15-16], and its ability to reduce the incidence of specific cancers [17], are all closely related to its antioxidant properties. Some studies in recent years have shown that curcumin even has an antidepressant effect in patients with major depression [18-19].
Due to the poor stability and solubility limitations of curcumin, it can be encapsulated using various food gels such as modified starch, cyclodextrin, gum arabic, and chitosan, as well as various protein-peptide compounds such as zein, wheat protein hydrolysate, soy protein hydrolysate, and egg white powder. It can also be prepared as a liquid crystal system using a surfactant, or as a nanoemulsion, to reduce its degradation and loss during preparation and storage, improve its water solubility and bioavailability, and increase its value for application development.
2 Curcumin emulsion
2.1 Properties of food emulsions
An emulsion is a dispersion system formed by a liquid dispersed in the form of liquid droplets in another liquid with which it is immiscible. Emulsions are generally opaque and milky white in appearance[20]. Emulsions can be classified according to the spatial position of the water and oil phases as either oil-in-water or water-in-oil. An emulsion in which the water phase is the external phase and the oil phase is the internal phase is called an oil-in-water emulsion (O/W type), and vice versa an emulsion is called a water-in-oil emulsion (W/O type) [21]. According to this classification, several important food emulsions are listed in Table 1 [22].
Emulsions can be divided into conventional emulsions and nanoemulsions according to particle size. Conventional emulsions have an average droplet size of between 100 nm and 100 μm. These emulsions are thermodynamically unstable systems. This is due to the large surface tension at the oil-water interface and the strong light scattering effect, as the droplet size is similar to the wavelength of light, so the emulsion is generally not transparent [23]. Nanoemulsions can be regarded as small droplets contained in traditional emulsions, with an average particle size of 10 nm to 100 nm [24]. The identification method for emulsions is also very simple. One common method is dilution, which involves diluting the emulsion with water. If the two phases are miscible, the continuous phase must be the aqueous phase, and the emulsion is therefore an oil-in-water emulsion. If they are not miscible, the emulsion is an oil-in-water emulsion. Another method is the dyeing method, which involves adding a small amount of dye to the oil phase before emulsification. After emulsification, the droplets are observed under a microscope. If the droplets are colored, it is an oil-in-water emulsion; if the continuous phase is colored, it is a water-in-oil emulsion. Similarly, the dye can be dissolved in the water phase for observation [25].
Emulsions are usually thermodynamically unstable systems that become unstable as storage time increases, as shown in Figure 2 [26]. Examples include gravitational separation, flocculation, coalescence, and Oswald ripening [27-28].

2.2 Preparation methods for food emulsions
For general food-grade emulsions, the preparation process usually involves preparing the emulsifying object as the oil phase, dissolving the emulsifier in water to form the water phase, and then pouring the oil phase into the water phase and subjecting it to various treatments, including simple heat treatment, ultrasonic emulsification, homogenization and ultra-high pressure homogenization, and nano-grinding [29]. The specific principles, advantages and disadvantages are shown in Table 2.
Emulsions are widely used in the food, beverage, pharmaceutical and cosmetics industries to encapsulate, protect and deliver functional ingredients such as alcohol-soluble pigments, vitamins, preservatives and many other functional factors. In the food industry, food-grade emulsions are attracting increasing attention. Many food ingredients and functional ingredients that were previously restricted in their application are now being added to foods (health foods) as emulsion carriers. This can improve food quality and bioavailability without affecting the stability of the food system [35]. As an alcohol-soluble substance, curcumin is difficult to dissolve in an aqueous system. It can be made into an oil-in-water emulsion or microcapsule using various emulsification and encapsulation techniques such as shear homogenization, nano-grinding, and spray drying to change its solubility, thereby increasing the breadth and depth of its use.

3 Preparation of curcumin emulsions and complexes
3.1 Preparation of curcumin nanoemulsions
Zeng Qinghan et al. [36] used medium chain triglycerides (MCT) as the oil phase and lecithin as the emulsifier to prepare curcumin nanoemulsions with different oil phase concentrations using high-pressure homogenization. The effect of different oil phase concentrations on the stability of curcumin nanoemulsions was studied by storing the emulsions at 4, 25 and 55 °C for 30 days. It was found that increasing the oil phase concentration can increase the curcumin encapsulation rate, average particle size and zeta potential of the curcumin nanoemulsion, but also reduce the centrifugal stability and thermal stability of the emulsion. Specifically, when the oil phase concentration is low (5 %, 10 %), the curcumin nanoemulsion has high stability, and the curcumin retention rate reaches 48.50 % and 48.99 % respectively. At the same time, the particle size of the emulsion increases by 0.79 % and 15.78 % respectively. The physical and chemical stability is best when stored at 4 °C, and the curcumin loss rate after 30 d was only 14.98 %.
Yao Yanyu et al. [37] used Tween 80 as an emulsifier to continue exploring the effects of different oil phases (canola oil, linseed oil and medium-chain triglycerides) on the physicochemical properties and storage stability of curcumin nanodispersions prepared by high-pressure homogenization. The results were consistent with previous studies. It was found that compared with canola oil and linseed oil, the curcumin nanoemulsion prepared with medium-chain triglycerides as the oil phase had a smaller average particle size, a higher entrapment amount (2.44 mg/mL), and better centrifugal stability, but slightly poorer thermal stability. In the storage test, the curcumin nanoemulsion prepared with medium-chain triglyceride as the oil phase had good physical and chemical stability, and the curcumin content and average particle size did not change significantly. The study concluded that medium-chain triglyceride can be used as a good oil phase to prepare oil-in-water curcumin nanoemulsions with good physical and chemical stability, providing theoretical guidance for expanding the application of curcumin in the food industry.
Wu Minhui et al. [38] established four (protein, polysaccharide, small molecule synthetic emulsifier, phospholipid) stable curcumin emulsion delivery systems through high-pressure microjet homogenization. The effect of different homogenization pressures, homogenization times, and emulsifier concentrations on the stability of curcumin emulsions was studied using a Lumisizer stability analyzer, with particle size as the index of investigation. The results showed that among the four emulsifiers, Tween-80 had the greatest effect on the particle size of the emulsion, followed by whey protein, then lecithin and gum arabic. When preparing a stable curcumin emulsion system, the homogenization pressures required for Tween-80, whey protein, lecithin and gum arabic were 40, 60, 40 MPa and 20 MPa, respectively; the number of homogenization times were 6, 4, 4 and 2, respectively; and the mass fractions were 2 %, 2 %, 4 % and 4 %, respectively.
Foreign researchers Kharat et al. [39] also used a high-pressure microjet to prepare water-in-oil nanoemulsions loaded with curcumin, and then investigated the effects of the type (gum arabic, saponin, Tween-80, sodium caseinate) and amount of antioxidant on the preparation and stability of the nanoemulsions. It was found that the addition of gum arabic to the nanoemulsion resulted in the fastest decrease in surface loading compared to that of saponin and Tween-80, as well as sodium caseinate. In other words, a large amount of gum arabic is required to prepare a stable emulsion. It can be seen from the storage test that high pH (7.0) and high temperature (55 °C) conditions can accelerate the degradation of curcumin, and in the emulsion with the addition of saponin, the curcumin content decreases the fastest, which is likely due to its ability to promote peroxidation. At the same time, the use of excessive emulsifier does not significantly reduce the degradation of curcumin.
3.2 Preparation of curcumin cyclodextrin complexes
The use of biopolymers such as proteins or polysaccharides to encapsulate curcumin is a research hotspot in recent years, mainly because the use of food-grade biopolymers can produce commodities with wider commercial value, and biopolymers can improve various properties of curcumin.
Cyclodextrin (CD) is a water-soluble, non-reducing, acid-stable, white, non-toxic, edible, porous oligosaccharide that is extracted from starch-containing raw materials such as corn or potatoes using catalytic enzymes. It is of purely plant origin, does not cause allergic reactions and has no E-number. Common cyclodextrins are α-CD, β-CD and γ-CD, which are composed of 6, 7 or 8 glucose units linked by 1,4-glycosidic bonds [40].
The unique feature of the cyclodextrin molecule is its cyclic three-dimensional structure: a hydrophobic cavity can form inside the cyclodextrin molecular structure, which can absorb lipophilic molecules with compatible sizes and shapes as “objects”. Its hydrophilic surface ensures the tolerance of the molecule in an aqueous system. There are various methods for verifying whether a cyclodextrin complex has formed, such as ultraviolet, circular dichroism, infrared, X-ray diffraction, differential scanning calorimetry, etc. With the rapid development of computer technology, molecular simulation methods are also being used more frequently [41]. In the food industry, cyclodextrins can provide a new purely plant-based option for stabilizing oil-in-water emulsions.
Due to the extremely hydrophobic nature of curcumin, its absorption rate is low and its bioavailability is extremely low. If the curcumin dosage is increased, not only will production costs increase, but the problem of bioavailability will not be well resolved. Germany's Wacker Company was the first to develop a curcumin complex with γ-cyclodextrin, CAVACURMIN R. Not only does it have a high curcumin content (>15%), but it also has good free-flowing properties, small and uniform particle sizes, and can be well dispersed in water. Animal experiments and in vitro and in vivo experiments in humans have shown that the water solubility, bioavailability and antioxidant properties of this product have been greatly improved [42].
Some domestic researchers have also conducted some research on curcumin cyclodextrin inclusion complexes. For example, Li Yi et al. [43] prepared a cyclodextrin inclusion complex of curcumin (CCIC) using the milling method, and used microscopic observation, differential scanning calorimetry and infrared spectroscopy to verify the formation of the inclusion complex. Solubility was also used as an evaluation index. A three-factor three-level orthogonal design was used to explore the three factors that had a greater influence on the preparation of the inclusion compound: the feeding ratio, grinding time and grinding temperature. This was used to optimize the preparation process of CCIC. The experiment found that under the optimal process conditions, that is, when the encapsulation feed ratio (molar ratio) is 1:1, the grinding temperature is 40 °C, and the encapsulation time is 1.5 h, the solubility of curcumin is 3.82×104 times higher than that of the free drug. Luo Jianchun et al. [44] also used the milling method to prepare (curcumin hydroxypropyl-β-cyclodex- trin, CurcHD), the structure of which is shown in Figure 3.
The absorption rate constant (Ka) and effective permeability (Papp) of the curcumin hydroxypropyl-β-cyclodextrin complex and curcumin in each intestinal segment (duodenum, jejunum, ileum and colon) in rats were determined using ultraviolet-visible spectrophotometry. It was found that the solubility of curcumin hydroxypropyl-β-cyclodextrin complex in water is 33.68 times that of curcumin, and the absorption of curcumin hydroxypropyl-β-cyclodextrin complex in each intestinal section of rats is significantly higher than that of curcumin. Supercritical carbon dioxide (SC-CO2) is a new method for preparing inclusion compounds that has emerged in recent years [45]. Zhang Zhiyun et al. [46] used supercritical CO2 to prepare curcumin hydroxypropyl-β-cyclodextrin complexes. The testers used a single factor method and a Box-Behnken response surface design method with solubility as the evaluation index to optimize the preparation process of the complex, and obtained a curcumin cyclodextrin complex with high solubility.

The results showed that the optimal preparation process for the inclusion complex was an inclusion temperature of 57 °C, an inclusion time of 2 h, a pressure of 24 MPa, and a drug-to-hydroxypropyl-β-cyclodextrin molar ratio of 0.96:1. The solubility of curcumin in the resulting inclusion complex was 34.24 μg/mL, which is about 400 times that of curcumin powder. It is precisely because cyclodextrin has a special three-dimensional ring structure that is “internally hydrophobic and externally hydrophilic” that when cyclodextrin non-covalently encapsulates the poorly soluble drug curcumin into its hydrophobic cavity, it not only improves the water solubility of curcumin, but also improves the defect of curcumin being easily decomposed by light.
There is still a lot of research being done internationally on curcumin complexes. For example, the relevant literature [47] reports the use of co-precipitation, freeze-drying and solvent evaporation to complex curcumin with β-cyclodextrin. The peak shift of the curcumin aromatic ring was observed using Fourier infrared spectroscopy and Fourier Raman spectroscopy to verify the construction of the complex by the co-precipitation method. In addition, the disappearance of energy bands associated with aromatic rings, as detected using photoacoustic spectroscopy and X-ray diffraction, also proved the formation of the complex.
Popat et al. [48] used a novel, scalable spray dryer to prepare highly water-soluble (3 mg/mL) curcumin-γ-cyclodextrin hollow spheres. These hollow spheres were then embedded in a positively charged biodegradable chitosan shell to form nanoparticles. These nanoparticles were then characterized using transmission electron microscopy, scanning electron microscopy, drug loading and in vitro release. After in vitro testing, it was found that the CUR-CD-CS nanoparticles exhibited excellent in vitro release properties and high cytotoxicity, with an apoptosis mortality rate approaching 100%. This indicates that cyclodextrin not only improves the solubility of curcumin, but also the rate of absorption by cells. This finding provides new ideas for subsequent researchers, that is, the design of a reasonable biodegradable natural biomaterial as a next-generation hydrophobic drug curcumin delivery nanocarrier has great potential.
4 Innovative applications of curcumin
As the turmeric market grows, major brands are also stepping up their market presence, launching increasingly diverse products that go far beyond the standard capsule supplements to meet the different needs of consumers. At present, a variety of water-soluble and oil-soluble curcumin products have been developed at home and abroad, and curcuminoids of various shades have been produced through compounding, which have been widely used in pasta, beverages, fruit wine, candy, pastries, canned food, fruit juice and cooking dishes [9, 49]. They are used as compound condiments in chicken essence compound seasonings, puffed seasonings, instant noodles and puffed noodle products, instant food seasonings, hot pot seasoning sauce, paste-like flavorings, seasoned pickles, beef jerky products, etc.
Zhang Baojun et al. [50] added curcumin to instant noodles, which not only brings out the physiological functions of curcumin and is beneficial to the human body, but also adds a natural bright color to the instant noodle dough, which can enhance people's appetite. More importantly, curcumin is the cheapest among natural pigments, which can further reduce costs and enhance market competitiveness, and has broad prospects for promotion. A British dairy company launched a Greek-style yogurt in 2019 that is lactose-free and flavored with mango and turmeric [51].
Turmeric has long been a staple of keto dieters' homemade recipes due to its significant anti-inflammatory properties. Major brands are now launching keto soups with turmeric. In March 2019, a new bone broth hit the market: the main ingredients are lemon, turmeric and MCT oil bone broth (grass-fed butter and coconut oil). Turmeric has also penetrated the growing beverage market. In August 2018, a foreign beverage company innovatively launched a mango lychee juice that contains 200 mg of curcumin. The product also contains piperine to improve the bioavailability of curcumin. Curcumin products are growing and diversifying [52]. It is expected that with the continuous advancement of scientific research and technological development, curcumin products will have broad development prospects.
5 Conclusion
Curcumin powder is a natural polyphenolic compound with a wide range of proven bioactive properties. In addition to its use as a food additive (e.g. as a coloring agent and antioxidant), it is also used to treat various diseases. In recent years, the use of emulsions to encapsulate, protect and deliver fat-soluble functional ingredients (such as oil-soluble flavor substances, vitamins, preservatives, nutrients and drugs) has attracted increasing attention in the food, beverage and pharmaceutical industries. With the development of nanotechnology, research on curcumin nanoparticles has gradually deepened, and the encapsulation of curcumin in different materials has made the particle size smaller and more uniform, the stability higher, and its performance continuously optimized. In addition, in order to reduce production costs, designing a more economical method for manufacturing nano-curcumin particles is a problem that must be faced in industrial production. In addition, in order to apply curcumin to nano-scale drugs for the prevention and treatment of various diseases and nano-scale additives in food, there is still an urgent need to study and evaluate the toxicological safety of curcumin applications.
References:
[1] Di Jianbin, Gu Zhenlun, Zhao Xiaodong, et al. Research progress on the antioxidant and anti-inflammatory effects of curcumin [J]. Chinese Herbal Medicine, 2010, 41(5): 854-857
[2] Yu Meirong, Jiang Fusheng, Ding Zhishan. Research progress of curcumin [J]. Chinese Herbal Medicine, 2009, 40(5): 828-831
[3] National Health and Family Planning Commission of the People's Republic of China. Food safety national standard food additive curcumin: GB 1886.76-2015 [S]. Beijing: China Standards Press. 2015
[4]Ganjali S, Blesso C N, Banach M, et al. Effects of curcumin on HDL functionality[J]. Pharmacological Research, 2017, 119: 208-218
[5]Fadus M C, Lau C, Bikhchandani J, et al. Curcumin: An age-old an- ti-inflammatory and anti-neoplastic agent[J]. Journal of traditional and complementary medicine, 2017, 7(3): 339-346
[6]Lima C F, Pereira-Wilson C, Rattan S I S. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox sig- naling: Relevance for anti-aging intervention[J]. Molecular nutrition & food research, 2011, 55(3): 430-442
[7]Goel A, Kunnumakkara A B, Aggarwal B B. Curcumin as“ Cure- cumin”: from kitchen to clinic[J]. Biochemical pharmacology, 2008, 75(4): 787-809
[8] Zhou Ming, Li Zeyang, Wang Huan. Physicochemical properties, extraction technology and nutritional and health effects of curcumin [J]. Feed and Animal Husbandry, 2013(6): 5-7
[9] Yuan Peng, Chen Ying, Xiao Fa, et al. Bioactivity of curcumin and its application in food [J]. Food Industry Science and Technology, 2012, 33(14): 371-375
[10] Qi Lili, Wang Jinbo. Study on the stability of curcumin monomers [J]. Food Industry Science and Technology, 2007(1): 181-182
[11] Fan Shuqi, Zhang Baojun, Li Chunlin. Natural curcumin and its application in fruit and vegetable drinks [J]. China Food Additives, 2002(5): 57-59,78
[12] Han Ting, Mi Heming. Research progress on the chemical composition and pharmacological activity of turmeric [J]. Journal of the Chinese People's Liberation Army, 2001(2): 95-97
[13] Abrahams S, Haylett W L, Johnson G, et al. Antioxidant Effects of Curcumin in Models of Neurodegeneration, Ageing, Oxidative and NITROSATIVE Stress: A Review[J]. Neuroscience, 2019(406): 1-21
[14] AAravind S R, Krishnan L K. Curcumin-albumin conjugates as an effective anti -cancer agent with immunomodulatory properties [J]. International immunopharmacology, 2016, 34: 78-85
[15] Rabinovich L, Kazlouskaya V. Herbal sun protection agents: Human studies[J]. Clinics in dermatology, 2018, 36(3): 369-375
[16] Grumezescu A . Surface Chemistry of Nanobiomaterials[M]. Amster- dam:Elsevier, 2016: 359-392
[17] Rodrigues F C, Anilkumar N V, Thakur G. Developments in the Anticancer Activity of Structurally Modified Curcumin: An Up-to - Date Review[J]. European journal of medicinal chemistry, 2019,177: 76-104
[18] Lopresti A L, Maes M, Maker G L, et al. Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study[J]. Journal of affective disorders, 2014, 167: 368-375
[19] Lopresti A L, Drummond P D. Efficacy of curcumin, and a saffron/ curcumin combination for the treatment of major depression: A ran- domised, double-blind, placebo-controlled study[J]. Journal of af- fective disorders, 2017, 207: 188-196
[20] Xu Duxia, Cao Yanping, Han Fu. Research progress in testing methods for the stability of food emulsions [J]. Food Industry Science and Technology, 2014, 35(21): 365-370
[21] Berton-Carabin C, Schron K. Towards new food emulsions: Design- ing the interface and beyond [J]. Current Opinion in Food Science, 2019(27): 74-81
[22] Jiao Xueshun. Natural food emulsifiers and emulsions: composition, properties, preparation, processing and application [M]. Beijing: Science Press, 1999: 66-69
[23] G Mason, J N Wilking, K Meleson,et al. Nanoemulsions: Formation, structure, and physical properties[J]. Journal of Physics Condensed Matter, 2006, 18(41): 635
[24] Tadros T, Izquierdo P, Esquena J, et al. Formation and stability of nano-emulsions[J]. Advances in colloid and interface science,2004, 108: 303-318
[25] Zhao Guoxi. Physical Chemistry of Surfactants [M]. Beijing: Peking University Press, 1984: 102-104
[26] Xiao Jinxin. Principles of Surfactant Application [M]. Beijing: Chemical Industry Press, 2015: 90-93
[27] Eva Blomberg, Evgeni Poptoshev, Per Claesson. Surface Forces and Emulsion Stability[M]// Johan Sjglom. Encyclopedic Handbook of Emulsion Technology. New York:Marcel Dekker, Inc,2001: 33-34
[28] McClements D J. Food emulsions: principles, practices, and tech - niques[M]. Melbourne:CRC press, 2015: 34-38
[29] Yang Lei, Qiu Dan, Wang Zuoyang, et al. Research progress of food-grade oil-in-water emulsions [J]. Journal of Ningbo Institute of Technology, 2013, 25(1): 43-48
[30] Solans C, Solé I. Nano-emulsions: formation by low-energy meth- ods[J]. Current opinion in colloid & interface science, 2012, 17(5): 246-254
[31] Gheisari S M M M, Gavagsaz-Ghoachani R, Malaki M, et al. Ultra - sonic nano-emulsification-A review[J]. Ultrasonics sonochemistry, 2018,52: 88-105
[32] Piorkowski D T, McClements D J. Beverage emulsions: Recent de - velopments in formulation, production, and applications [J]. Food Hydrocolloids, 2014, 42: 5-41
[33] Chen C C, Wagner G. Vitamin E nanoparticle for beverage applica - tions[J]. Chemical Engineering Research and Design, 2004, 82(11): 1432-1437
[34] Zhang Wenjin, Wen Yanjun, Li Linzheng, et al. A curcumin preparation method: CN102283373A[P]. 2011-12-21
[35] Mao Lijun, Xu Honggao, Gao Yanxiang. High-pressure homogenization technology and food emulsions [J]. Food and Machinery, 2007(5): 146-149
[36] Zeng Qinghan, Ma Peihua, Tai Kedong, et al. Effect of oil phase concentration on the stability of curcumin nanoemulsions [J]. Food Industry Science and Technology, 2017, 38(22): 17-21
[37] Yao Yanyu, Ma Peihua, Zeng Qinghan, et al. Effect of oil phase type on the stability of curcumin nanoliposomes [J]. Food Science and Technology, 2017, 42(9): 238-242
[38] Wu Minhui, Wang Lei, He Mei. Effect of high-pressure microjet homogenization on the stability of curcumin nanomicelles [J]. Chinese Journal of Food Science, 2018, 18(5): 51-57
[39] Kharat M, Zhang G, McClements D J. Stability of curcumin in oil - in-water emulsions: Impact of emulsifier type and concentration on chemical degradation[J] . Food research international, 2018, 111: 178-186
[40] Song Lexin, Meng Qingjin, You Xiaozeng. Cyclodextrins and cyclodextrin complexes [J]. Journal of Inorganic Chemistry, 1997, 13(4): 368-374
[41] Cao Xinzhi, Jin Zhengyu. Preparation method of cyclodextrin inclusion compounds [J]. Food Industry Science and Technology, 2003(10): 158-160
[42] Purpura M, Lowery R P, Wilson J M, et al. Analysis of different in - novative formulations of curcumin for improved relative oral bioavailability in human subjects[J]. European journal of nutrition, 2018, 57(3): 929-938
[43] Li Y, Mei H, Zhao C, et al. Construction and optimization of curcumin cyclodextrin molecular inclusion compounds [J]. Journal of Food and Biotechnology, 2017, 36(11): 1197-1202
[44] Luo J C, Guo Q, Zhang M, et al. In vitro intestinal absorption of curcumin-hydroxypropyl-β-cyclodextrin complex in rats using a unidirectional perfusion method [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(6): 957-962
[45] Bounaceur A, Rodier E, Fages J. Maturation of a ketoprofen/β-cy- clodextrin mixture with supercritical carbon dioxide[J]. The Journal of supercritical fluids, 2007, 41(3): 429-439
[46] Zhang Zhiyun, Zhang Wei, Wang Lihong, et al. Preparation of curcumin hydroxypropyl-β-cyclodextrin complex using supercritical CO2 [J]. Chinese Journal of Pharmaceutical Industry, 2014, 45(12): 1147-1150
[47] Mangolim C S, Moriwaki C, Nogueira A C, et al. Curcumin – β-cy- clodextrin inclusion complex: stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application[J]. Food chemistry, 2014, 153: 361- 370
[48] Popat A, Karmakar S, Jambhrunkar S, et al. Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity[J] . Colloids and Surfaces B: Biointerfaces, 2014, 117: 520-527
[49] Niu Shengyang, Hao Fengge, Xu Qiuya. Research progress on the extraction and application of curcumin [J]. Journal of Henan University of Science and Technology (Natural Science Edition), 2008, 36(4): 58-61
[50] Zhang Baojun, Zhang Wei. The physiological functions of curcumin and its application in instant noodles [J]. China Food Additives, 2001(4): 37-38,26
[51]Convenience Store website.Tims Dairy launches London-themed yo - gurts[EB/OL].[2019-3-22].https://www.kurokin.uk/work/tims-dairy
[52] Zyn website.Zyn voted product of the year 2019 [EB/OL].[2019-2- 9].


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






