What Is the Use of Tagatose Powder in the Food Field?
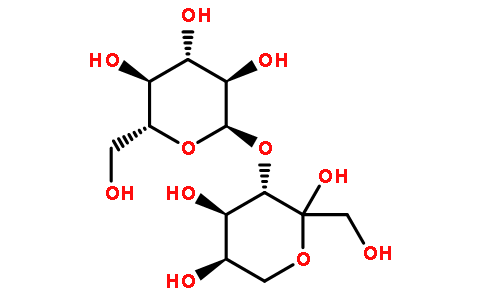
Tagatose (see Figure 1) is an enantiomer of fructose at the C-4 chiral carbon atom with a molecular weight of 180∙16u and CAS 87- 81-0. It is a good low-energy food sweetener and filler, and has various physiological effects such as inhibiting hyperglycemia, improving intestinal flora, and being non-cariogenic. In 2001, the US FDA approved tagatose as GRAS.
1 Properties and functions of tagatose
Pure tagatose is a white, odorless, non-crystalline substance with a melting point of 134°C and a glass transition temperature of 15°C. It is highly water-soluble and, when dissolved in water, causes the boiling point to rise and the freezing point to fall. However, it does not absorb heat, so it does not produce a cooling effect. Tagatose is hygroscopic and has good stability under acidic conditions. It can exist stably in the pH range of 3–7. It is prone to Maillard browning and can caramelize at lower temperatures [1].
Tagatose is 92% as sweet as sucrose and is a good filling sweetener. Its sweetness profile is similar to sucrose and it does not have any unpleasant off-flavors or aftertastes. Relatively speaking, the sweetness of tagatose stimulates faster than sucrose and is similar to fructose. In addition, tagatose has a good synergistic effect with strong sweeteners, including cyclamate, saccharin, aspartame, acesulfame, stevia, neotame and sucralose [2].
The tagatose ingested by the body is not completely absorbed by the small intestine. The tagatose absorbed by the small intestine is metabolized via the liver via the glycolytic pathway. Unabsorbed tagatose enters the large intestine directly, where it is almost completely fermented by the microbial flora. The short-chain fatty acids produced by this fermentation are almost completely reabsorbed and metabolized by the body. Based on many relevant studies, the US FDA has confirmed that tagatose can be labeled with an energy value of 6280∙2J/g on nutrition labels [1].
Tagatose is widely found in nature, and is present in some foods (e.g. sterilized milk, UHT milk, milk powder, hot cocoa, various cheeses, some types of yogurt, baby formula) and some plants and medicines [3].
Tagatose has a low absorption rate in the body and does not cause significant changes in blood glucose levels, making it suitable for people with diabetes. Studies have shown that tagatose does not cause significant changes in fasting blood glucose and insulin levels in healthy subjects and type 2 diabetes patients, and can significantly inhibit the increase in blood glucose caused by glucose intake in diabetes patients [4], but has no significant effect on insulin sensitivity. It has also been reported in patents that tagatose can alleviate and improve the symptoms of diabetes and inhibit the occurrence of various complications [5]. The mechanism by which tagatose suppresses elevated blood glucose may be that, in addition to having a low absorption rate, tagatose also inhibits the absorption of glucose in the small intestine.
Only 20% of the tagatose ingested by the body is absorbed by the small intestine. The vast majority of tagatose enters the colon directly, where it is selectively fermented by the microbial flora, promoting the growth of beneficial bacteria and inhibiting the growth of harmful bacteria, which has a significant effect on improving the intestinal flora and is a good prebiotic [6]. At the same time, the fermentation of tagatose also produces a large amount of beneficial short chain fatty acids (SCFA). In particular, butyric acid is a good energy source for colon epithelial cells and is considered to have a good effect in inhibiting colon cancer, inhibiting intestinal pathogenic bacteria (such as Escherichia coli, etc.) and promoting the growth of beneficial bacteria such as lactic acid bacteria. Some studies have found that the minimum dosage of tagatose to have a significant prebiotic effect is 7.5 g/d.
Studies have shown that tagatose does not lower the pH of dental plaque and does not cause tooth decay [1]. It is effective in inhibiting dental plaque and eliminating bad breath, so it is widely used in oral products to inhibit dental caries, gingivitis and other dental diseases, eliminate bad breath and clean teeth. On December 2, 2002, the US FDA issued a statement confirming that tagatose is not fermented by oral bacteria and does not cause tooth decay based on the results of many scientific studies.
Other studies have shown that tagatose can appropriately and continuously reduce body weight in healthy subjects and type 2 diabetes patients [1]. According to patent reports, tagatose is also very beneficial for promoting blood health [7] and can help increase the chance of pregnancy in female rats and promote maternal and embryonic health [8]. In addition, tagatose can also enhance the sensitivity of cells to toxins and significantly inhibit the toxic effects of cocaine, nitrofurantoin, etc. on liver cells [9].
A large number of safety and toxicology tests have shown that tagatose is safe and non-toxic. On April 11, 2001, the US FDA approved tagatose as GRAS for use in food. Later, Australia and New Zealand also approved the use of tagatose in food. However, excessive consumption of tagatose may still cause mild gastrointestinal discomfort, such as flatulence and diarrhea, which may be mainly due to the body's impaired absorption of tagatose. In June 2001, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) approved tagatose as a food additive with an ADI of 0–80 mg/kg·d [1].
2 Production technology of tagatose
Tagatose is generally produced from galactose through chemical or enzymatic isomerization. Galactose can be obtained by hydrolyzing lactose. Some studies have also used galactitol as a raw material and oxidized it biologically to produce tagatose. However, galactitol is relatively expensive and is not suitable for industrial production at present.
2.1 Chemical synthesis of tagatose
The chemical synthesis of tagatose uses galactose as the raw material and mainly includes two steps: isomerization and acid neutralization [10]. First, a soluble alkali metal salt or alkaline earth metal salt is used as a catalyst to isomerize galactose with a metal hydroxide to form a metal hydroxide-tagatose complex intermediate precipitate. The intermediate complex is then neutralized with an acid to obtain the final product tagatose. Galactose is obtained by hydrolyzing lactose.
The isomerization of galactose is the key step in the chemical synthesis of tagatose. For cost reasons, the metal hydroxide reactant is preferably Ca (OH) 2 or a mixture of Ca (OH) 2 and NaOH. Generally, it is added as a water-soluble slurry made from mixing Ca(OH)2 with water, or as a product of hydration after adding lime (CaO) mixed with water. A catalyst of alkali metal salt (or alkaline earth metal salt) is usually CaCl2, and the amount used is about 1% to 5% of the molar number of galactose. The isomerization reaction should be carried out under alkaline and low temperature conditions, controlled within the range of pH>10 and -15 to 40°C.
The purpose of acid neutralization is to form insoluble metal salts and release tagatose from the complex intermediate. The remaining ions are removed by ion exchange resin. H2SO4, H3PO4 or HCl can be used for acid neutralization, with CO2 being the best. The progress of acid neutralization is controlled according to the pH of the reaction system. When the pH is <7, the neutralization reaction is complete.
During the addition of acid, the temperature of the reaction system should be controlled below 25°C to avoid adverse side reactions. Finally, the tagatose is crystallized from the reaction solution and filtered out.
For example, add 10∙0kg lactose and 40L deionized water to a 230L stainless steel reaction vessel, mix well, and raise the temperature to 50°C. Add lactase and hydrolyze for 6 hours until the hydrolysis is basically complete to obtain a lactose hydrolysate containing 45% glucose, 45% galactose and 10% lactose. After cooling the lactose hydrolysate to 25°C, sequentially add 154 g of CaCl2 and a Ca (OH)2 aqueous solution (2.0 kg of Ca (OH)2 plus 2.5 L of water). Then, add an appropriate amount of 10% NaOH solution to adjust the pH to 12.5. After reacting for 3 hours, the reaction mixture becomes thick and begins to form a precipitate. The precipitate is filtered and centrifuged to obtain a pasty filter cake. 25 L of water is mixed with the filter cake to form a suspension. Then, an appropriate amount of CO2 was introduced to neutralize the solution to a final pH of 6.5. During the neutralization process, the filter cake dissolved, and the final product tagatose and the CaCO3 precipitate were formed. The reaction solution was purified by centrifugation, deionization, and crystallization to obtain tagatose. HPLC analysis showed that the yield of tagatose could reach 47.6%.
2.2 Enzyme synthesis of tagatose
Studies have shown that L-arabinose isomerase (AraA, EC 5∙3∙1∙4) has catalytic activity for the isomerization of L-arabinose and D-galactose with similar three-dimensional conformations, and can isomerize L-ribulose and D-tagatose, respectively [11,12].
Lactobacillus fermentum, Lactobacillus pentosus, Lactobacillus mannitopous, Lactobacillus buchneri, Lactobacillus brevis, Lactobacillus pentoaceticus, Lactobacillus lycopersici and other species of Lactobacillus, Aerobacter aerogenes, Bacillus amyloliquefaciens, Bacillus subtilis, Candida utilis, (Aerobacter aerogenes), medical ring-shaped rod bacteria (Bacillus amyloliq uefaciens), Bacillus subtilis, Candida utilis, Clostridium acetobutylic um), ( Escherichia coli), Erw inia cativosa ,( Mycobacterium),( Salmonella typhimurium),( Pediococcus , Pediococcus pentosaceous)、 ( A rthrobacter),can both be fermented to produce AraA 。 Using L-arabinose as a carbon source and fermenting at pH 5.5–7.0 and 30–40°C, the enzyme L-arabinose isomerase can be obtained.
Depending on the source of AraA, the optimal conditions for isomerization vary. Isomerization is usually carried out at 20–80°C and pH 4.0–9.0, preferably at 50–70°C and pH 5.5–7.0. Some mutant strains can also produce L-arabinose isomerase that can isomerize at temperatures up to 100°C. A study has shown that the AraA coding gene from Thermotoga neapolitana can be cloned, recombined, and expressed in Escherichia coli to obtain a recombinant AraA with very high thermal stability [13].
The concentration of D-galactose significantly affects the rate and conversion rate of the isomerization reaction. When the concentration of the raw material D-galactose is high, the Michaelis constant Km of the enzymatic reaction process for the conversion of D-galactose to tagatose is usually high, so the yield of tagatose is also high. If the concentration of the raw material D-galactose is low, the yield of tagatose depends on the strain of the producing enzyme.
Figure 2 shows the process flow for the preparation of tagatose from lactose permeate (obtained by ultrafiltration of cheese whey or cow's milk, containing 2% to 6% lactose, 0.2% to 0.4% protein, 0.2% to 0.6% salt and trace amounts of fat) [14]. Lactose permeate is removed by ultrafiltration (1) to remove proteins, and then passes through a storage tank (2), where it is desalted and concentrated by reverse osmosis (3). The concentrated liquid is separated by microfiltration (4) to remove high molecular weight substances (bacteria, i.e., insoluble proteins), and then hydrolyzed by immobilized lactase (5) to a mixture of glucose and galactose (glucose: galactose is about 1:1). The lactose hydrolysate is fermented in a semi-continuous process (6), where glucose is fermented by yeast or bacteria to produce ethanol, which is recovered by a vacuum pump (15) and then distilled (16). Alternatively, the liquid can be centrifuged (7) to obtain a cell-free liquid, and the ethanol can be recovered by distillation (16), while the microbial cells are returned to the fermenter (6).
The ethanol recovered by distillation is pumped into a storage tank (7) as a by-product. The unfermented galactose is isomerized (8) to obtain a mixture of galactose and tagatose. The tagatose crude liquid is then separated by passing it through a cation exchange column (9) and selectively eluting it with deionized water (10). The un-isomerized galactose is returned to the isomerization column (8) for another round of isomerization. The tagatose crude liquid is concentrated by evaporation (11), crystallized (12), filtered, dried, and then the finished product is obtained. During the crystallization process, an appropriate amount of ethanol and tagatose seed crystals are introduced to facilitate crystallization. The ethanol is filtered and recovered, and then returned to the crystallization tank (12) for recycling.
2.3 Bioconversion of galactitol to tagatose
Studies have shown that Acetic acid bacteria can bioconvert galactitol to tagatose [15]. Studies have shown that Acetobacter sp∙ produces tagatose at a low yield of only 3–35 mg/L, while Gluconobacter sp∙ oxidizes galactitol to tagatose at a high yield of 100–160 mg/L. Among them, Gluconobacter MIM 1000/9 has the highest yield of tagatose, oxidizing 5 g/L galactitol to tagatose in 24 h to reach 158 mg/L. Moreover, no by-products of galactose and fructose were found.
To increase the yield of tagatose, the amount of added galactitol was gradually increased in the medium to induce the G ∙ oxydans DSM 2343 strain to gradually adapt to higher concentrations of galactitol. The results showed that the activity of its galactitol dehydrogenase and the yield of tagatose were significantly improved. After 24 hours of cultivation, the yield of tagatose reached a maximum of 3160 mg/L (20 g/L galactitol, 24 h), and the conversion rate reached 6.6 × 10-3 L/h.
3 Applications of tagatose
3.1 Flavor enhancement of tagatose
Tagatose has a good synergistic sweetening effect on strong sweeteners [1]. A small amount of tagatose can significantly enhance the sweetness. When used in combination with a strong sweetener, it can replace a significant amount of the strong sweetener, and the amount can even be below the sweet taste threshold. 0∙1 to 50g/kg of tagatose can achieve a good synergistic sweetening effect, especially at a dosage of 0∙5 to 20g/kg. The sweetness of tagatose can be greatly enhanced by combining it with a powerful sweetener, and its taste, flavor and aftertaste can be significantly improved.
Tagatose has a good synergistic effect on many powerful sweeteners, including cyclamate, saccharin, aspartame, acesulfame-K, licorice sweetener, stevia, monk fruit extract, thaumatin, alitame, neotame and sucralose. Depending on the type of intense sweetener, the sweetness of the final product and the sensory requirements (mouthfeel, aftertaste and flavor), the mass ratio of tagatose to sweetener is usually 1:1 to 1000:1, and preferably between 4:1 and 200:1 [2].
Sensory analysis shows that in lemonade and cola drink systems, the addition of a small amount of tagatose can significantly improve the mouthfeel of the product, reduce the bitter aftertaste, metallic aftertaste and astringent taste caused by strong sweeteners (such as acesulfame, saccharin, etc.), and make the sweet taste of the product stimulate faster, with a fresher and more refreshing mouthfeel. In addition, it can increase the content of soluble solids in the beverage system, making the beverage taste more complete, which is exactly what the intense sweeteners lack. Therefore, overall, tagatose makes the taste and flavor of low-energy light beverages closer to that of full-energy traditional beverages sweetened with sucrose.

For low-fat milk drinks sweetened with intense sweeteners (including chocolate, yogurt and fruit flavors), the addition of tagatose can significantly improve the taste, reduce the bitter aftertaste caused by intense sweeteners, and achieve the best sweetness and aftertaste. Especially for chocolate milk drinks, tagatose can significantly enhance their rich and mellow creamy flavor.
In addition, tagatose is also a good flavor enhancer for confectionery and chocolate products. Sensory evaluation has shown that adding tagatose to chocolate sweetened with intense sweeteners can significantly enhance the sweetness and aftertaste, while reducing the bitterness; at the same time, the mouthfeel is also significantly improved and the creamy flavor is significantly enhanced [1].
3.2 Application of tagatose in foods
When using tagatose in cereal foods, it is important to pay sufficient attention to several important physical properties of tagatose, including its high melting point, low glass transition temperature, non-hygroscopic crystals, high solubility, easy crystallization, and pH stability. In particular, it should be noted that tagatose has good Maillard reaction characteristics. Lower temperatures are conducive to enhancing the flavor, but high temperatures and long processing times can lead to an excessively dark color and a bitter aftertaste.
In the production of ready-to-eat cereals, the cooking process is a key step, which can be carried out using either the traditional batch steam cooking process or the extrusion process [1]. Depending on the cooking process used, the starch gelatinization degree, flavour, tissue structure and nutritional properties of the product will differ. The traditional steam cooking process is usually carried out at high temperatures and pressures, while the extrusion process requires lower temperatures and shorter times. When the processing temperature during the extrusion process is relatively low (e.g. 130°C) and the processing time is short, tagatose can be used as the sole sweetener in low-energy ready-to-eat cereals.
Tagatose can also be sprayed onto the surface of cereals to increase the sweetness of the product, and it can be used to make various flavored icing or sugar-coated cereals. Due to its low viscosity, rapid crystallization and low moisture absorption, the shelf life of the icing coating is also longer. Dissolve tagatose in water to form an 83° Brix aqueous solution, heat to 97°C until completely dissolved, then cool the solution to 70°C and spray it onto the surface of the cereal. Finally, dry at 80°C for 15 minutes, and the tagatose crystals will form a uniform white sugar crystal frost coating.

Tagatose can also be used to coat in a non-crystalline form, creating a shiny sugar-coated surface and allowing other additives (such as nuts) to adhere to the surface of the cereal. However, tagatose must be used in combination with non-crystalline sweeteners such as fructooligosaccharides, dextrans, lactitol, maltitol and isomalt to create a stable sugar-coated surface. The use of tagatose gives the sugar coating a better sweetening profile, increases its crispness and prevents it from caking.
Tagatose is also very suitable for use in confectionery and chocolate. It can be used as the sole sweetener in sugar-free chocolate without major changes to the process. A proportion of cocoa butter is mixed with the other ingredients except the fat, then finely ground and conched. Lecithin and flavourings are added, the temperature is adjusted, the mixture is poured into moulds, cooled and the finished product is ready. Taffy can also be used in combination with other sweeteners such as isomalt to make high-quality, low-energy sugar-free sweets such as toffee.
Reference:
[1]http://www∙tagatose∙dk
[2]Andersen H ,Vigh M L ∙Synergistic combination of sweeten- ers including D-tagatose[P ]∙ US Patent ,6432464B1∙2002 -08-13
[3]Levin G V.Tagatose ,the New GRAS sweetener and health product[J]∙ Journal of Medicinal Food ,2002,5(1):1~19
[4]Donner T W ,Wilber J F ,Ostrowski D ∙ D-Tagatose ,a novel hexose: acute effectson carbohydrate tolerance in subjects with and without type 2diabetes[J]∙ Diabetes ,Obesity and Metabolism ,1999,1:285~291
[5]Zehner L R ,Levin G V ,Saunders J P et al ∙ D-tagatose as anti -hyperglycemic agent[P]∙ US Patent ,5447917.1995 -09-05
[6]Bertelsen H ,Jesen B B ,Buemann B ∙ D-tagatose:a novel low -calorie bulk sweetener with prebiotic properties[J]∙ World Review of Nutrition and Dietetics ,1999,85:98~ 109
[7]Levin G V-Use -of tagatose to enhance key blood factors [P].US Patent ,6015793.2000-01-18
[8]Levin G V ∙ Increased fertility and improved fetal develop- ment drug[P]∙ US Patent ,6225452B1.2001-05-01
[9]Valeri F ,Boess F ,Wolf A et al ∙ Fructose and tagatose pro- tect against oxidative cell injury by iron chelation[J]∙ Free Radical Biology & Medicine ,1997,22:257~268
[10]Beadle J R ,Saunders J P ,Wajda J et al.J ∙ Process for manufacturing tagatose[P]∙ US Patent ,5002612.1991-03 -26
[11]Kim P ,Yoon S H ,Seo M J et al ∙Improvement of tagatose conversion rate by genetic evolution of thermostable galactose isomerase[J]∙ Biotechnol Appl Biochem ,2001,34:99~ 102
[12]Roh H J ,Kim P ,Park Y C et al ∙Bioconversion of D-galac- tose into D-tagatose by expression of L-arabinose isomerase [J]∙ Biotechnol Appl Biochem ,2000,31:1~4
[13]Kim B C ,Lee Y H ,Lee H S et al ∙Cloning ,expression and characterization of L-arabinose isomerase from Thermotoga neapolitana:bioconversion of D-galactose to D-tagatose using the enzyme[J]∙ FEMS Microbiology Letters ,2002,212: 121~126
[14]Ibrahim,Spradlin J E ∙ Process for manufacturing D- tagatose ∙ US Patent ,6057135.2000-05-02
[15]Manzoni M ,Rollini M ,Bergomi S ∙Biotransformation of D- galactitol to tagatose by acetic acid bacteria[J]∙ Process Bio- chemistry ,2001,36:971~977


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese