The Method to Synthesis of D Tagatose Powder?
Humans cannot do without sugar in their three meals a day. Eating the right amount of sugar not only meets the needs of the body's functions, but also brings a sense of happiness. However, with the improvement of living standards, people consume too much sugar, which has led to an increase in the prevalence of diseases such as obesity, diabetes, tooth decay, and heart disease [1]. In recent years, traditional sugars with high absorption and high calorie content (such as sucrose, white sugar, glucose, etc.) have gradually been replaced by rare sugars with low calorie and low absorption (such as xylitol, erythritol, D-allulose, etc.). The International Society of Rare Sugars (ISRS) defines rare sugars as a class of monosaccharides and their derivatives that exist in nature but in very small quantities [3]. Rare sugars not only have a sweet taste but are also low in calories. More importantly, they have physiological functions that are beneficial to human health and have great development prospects.
Rare sugar D-tagatose, with the molecular formula C6H12O6 and molecular weight 180.16, has the structural formula shown in Figure 1. It is an isomer of D-galactose, a diastereoisomer of D-sorbitol at the C-3 position, and a diastereoisomer of D-fructose at the C-4 position. It white crystalline granules or white powder, easily soluble in water, slightly soluble in ethanol. It is a naturally occurring low-calorie functional sweetener with a sweetness that is 92% that of sucrose[4] and a caloric value of 1.5 kcal/g[5]. D-tagatose has been approved by the US Food and Drug Administration (FDA) as a safe (Generally Recognized as Safe, GRAS) ingredients [5–7]. In 2014, China's National Health and Family Planning Commission approved D-tagatose as a new food ingredient [6]. D-tagatose not only has the effect of preventing dental caries and obesity and lowering blood sugar, but also has a beneficial effect on intestinal health [8].
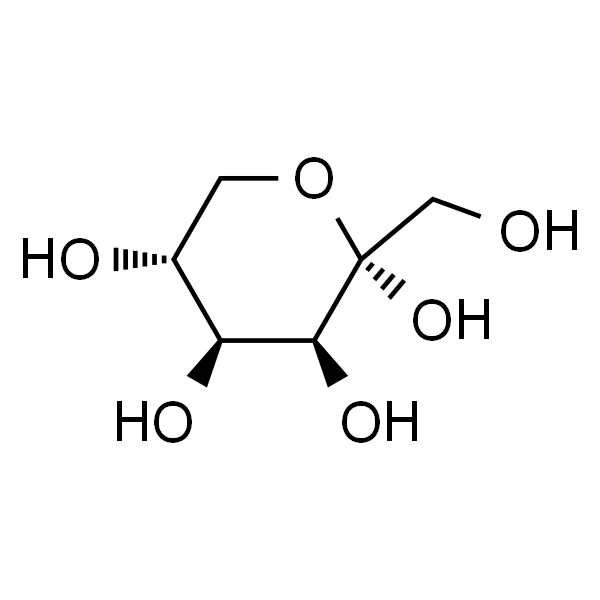
This article briefly describes the physiological functions and applications of D-tagatose, introduces the main biological enzymes required for the biosynthesis of D-tagatose, summarizes the research progress of D-tagatose biosynthesis in recent years, and provides an outlook on the biosynthesis of D-tagatose.
1 Physiological functions and applications of D-tagatose
1.1 Low-calorie sweetener that can undergo the Maillard reaction and be used in foods
D-tagatose is a low-calorie sweetener. It is 92% as sweet as sucrose, but only 37.5% as high in calories (4 kcal/g) [5]. It can react with proteins in food to form a Maillard reaction, which improves the colour and flavour of the food. It is therefore used in baked goods, drinks and confectionery.
1.2 Prevents obesity, lowers blood glucose, and assists in the treatment of type 2 diabetes
D-tagatose is a low-calorie functional sweetener that can be used in food to replace traditional sweeteners such as sucrose. It can alleviate obesity and lower blood glucose [9]. In the field of medicine and healthcare, D-tagatose can be used to prepare drugs for the treatment of type 2 diabetes and obesity [10-12].
1.3 Excellent prebiotic, beneficial to intestinal health
D-tagatose can be fermented by intestinal flora in the colon, stimulating the growth of beneficial intestinal bacteria and inhibiting the growth of pathogenic bacteria in the intestine [13]. In addition, the fermentation of D-tagatose can produce short-chain fatty acids such as butyric acid, which are beneficial to intestinal health. These acids can promote the growth and reproduction of colon epithelial cells and inhibit the occurrence of colon cancer [14].
1.4 Anti-caries, beneficial to protecting dental health
Because D-tagatose cannot be utilized by microorganisms in the mouth, it helps to reduce the production of acidic substances in the mouth and reduce tooth decay, thereby effectively preventing the occurrence of dental diseases such as gingivitis, tooth decay, and bad breath [13].

1.5 Used as a substrate to produce other rare sugar alcohols
According to the strategy of biotransformation to produce hexose, that is, the Izumoring strategy [15], starting from D-tagatose, rare sugar alcohols with important physiological functions such as D-sorbitol, D-tagatose and galactitol can be obtained through suitable enzymatic catalysis (Figure 2).
2 Production method of D-tagatose
2.1 Natural extraction method
D-Tagatose in nature is mainly found in the gums of tropical evergreen trees, mosses, lichens, hot cocoa, cheese and yogurt, and the content is very small [13, 16, 17]. Directly extracting D-tagatose from these substances requires a large amount of raw materials, which makes the cost very high and makes it difficult to achieve industrial production of D-tagatose.
2.2 Chemical synthesis method
D-Tagatose can be obtained from D-galactose by chemical synthesis. The chemical catalyst used is a alkali metal salt, which catalyzes the isomerization reaction of D-galactose with a metal hydroxide to form a metal hydroxide-D-tagatose complex. After acid neutralization, the complex releases D-tagatose [13, 14, 18]. However, the chemical synthesis method for producing D-tagatose is relatively complex, and it is easy to produce by-products, which reduces the purity of the target product D-tagatose and makes it inconvenient to separate and purify later. In addition, the use of chemical reagents will cause an environmental burden and is not in line with the concept of green production [19].
2.3 Biosynthetic method
There are two main ways to synthesize D-tagatose biologically: one is to use a single enzymatic reaction to synthesize D-tagatose, and the other is to use a multi-enzymatic reaction to synthesize D-tagatose. According to the Izumoring strategy (Figure 2), the appropriate single aldose isomerase, D-tagatose 3-epimerase and reductase can be selected to catalyze the conversion of D-galactose, D-sorbitol and galactitol to D-tagatose, respectively. However, the relatively high prices of D-galactose, D-sorbitol and galactitol make it difficult to apply them in industrial production, limiting the industrial production of D-tagatose. Currently, some researchers have chosen low-cost substrates such as lactose, maltodextrin and milk whey powder as the starting materials, and used a multi-enzyme catalytic reaction to synthesize D-tagatose, with some research results achieved. The biosynthetic method of producing D-tagatose has the advantages of high production efficiency, high purity of the product, mild reaction conditions, and low cost, making it the preferred method for industrial production of D-tagatose [20].
3 Synthesis of D-tagatose by single enzymatic reaction
3.1 L-arabinose isomerase catalyzes the synthesis of D-galactose to D-tagatose
The single-enzyme method of biosynthesizing rare sugars can fully exploit the physicochemical properties of the enzyme and is applied in the production of rare sugars. It has the advantages of being simple and efficient, high utilization of the enzyme catalyst, and high production efficiency. L-arabinose isomerase (L-AI) is currently the most studied enzyme for the biosynthesis of D-tagatose, and can catalyze D-galactose to D-tagatose.
This enzyme has a wide range of microbial sources, including Acidothermus cellulolytics ATCC43068[21], Bacillus subtilis str. 168[22], Lactobacillus sake i 23K[23] , Lactobacillus fermentum CGMCC2921[24] , Bacillus thermoglucosidasius KCTC 1828[25] , Alicyclobacillus hesperidum URH17-3-68[26] , Bacillus coagulans NL01[27] , Pseudoalteromonas haloplanktis ATCC 14393[28], Geobacillus stearothermophilus [4] , Clostridium hylemonae DSM 1505 3[29], Lactobacillus brevis MF 465792[30], Enterococcus faecium DBFIQ E36[31], Bifidobacterium adolescentis CICC 6178[32], Klebsiella pneumoniae DSM 681[33], etc.
The enzymatic properties of L-arabinose isomerase from the above microorganisms are shown in Table 1. The optimum reaction temperature is between 40-75 ℃, the optimum reaction pH is between 5.0-8.0, and various metal ions are activators of the enzyme, such as Mn2+, Co2+, and Mg2+. Most L-AIs have substrate specificity for L-arabinose and D-galactose, while a small number have substrate specificity only for L-arabinose and no substrate specificity for D-galactose, such as L-AIs from Bacillus subtilis str. 168[22] and Pseudoalteromonas haloplanktis ATCC 14393[28]. specificity, such as L-AI from Bacillus subtilis str. 168[22] and Pseudoalteromonas haloplanktis ATCC 14393[28]. In addition, L-AI enzymes from Acidothermus cellulolytics ATCC43068[21], Lactobacillus sakei 23K[23], Lactobacillus fermentum CGMCC2921[24], Bifidobacterium adolescentis CICC 6178[32], etc. exhibit strong substrate specificity for D-galactose.
The L-AI from Bacillus coagulans NL01 was heterologously expressed in an E. coli expression system and catalyzed by whole cells at 60 °C and pH 7.5. When the concentration of the substrate D-galactose was 150 g/L and 250 g/L, the conversion rates of the resulting D-tagatose were 32% and 27%, respectively, and the conversion times were 32 and 48 h, respectively [27]. The D-tagatose conversion rate was 56.7% when 100 mmol/L D-galactose (containing 6 mmol/L Mn2+) was catalyzed by purified L-AI enzyme from Bifidobacterium adolescentis CICC 6178 at 55 °C and pH 6.5 for 10 h [32]. L-AI from Klebsiella pneumoniae DSM 681 was heterogeneously expressed in an E. coli expression system. The substrate was 100 g/L D-galactose (containing 1 mmol/L Mn2+). The whole-cell catalytic reaction was carried out at 50 °C and pH 8.0 for 30 min, and the conversion rate of D-tagatose was 33.5% [33].
Spore surface display technology is a method that relies on the anchoring effect of the spore coat protein to display the target enzyme on the spore surface by fusing the target enzyme with the spore coat protein, thereby immobilizing the enzyme. Immobilized enzymes can maintain catalytic activity in extreme environments and overcome the barrier of substrate and product permeation through the membrane [10]. It is a beneficial attempt at enzyme immobilization. In 2014, LIU et al. [16] used the spore surface display technology to display the L-AI enzyme from Lactobacillus fermentum CGMCC2921 on the surface of Bacillus subtilis 168 spores. The obtained recombinant L-AI spores showed relatively high catalytic activity and strong thermal stability. After being stored at 80 °C for 30 min, they still retained 87% of their enzyme activity.
Using this recombinant L-AI spore as a biocatalyst, 100 g/L D-galactose was used as a substrate, and the reaction was carried out at 70 °C for 24 h, with a conversion rate of D-tagatose of about 75%. In 2018, GUO et al. [10] also used spore surface surface display technology to display the L-AI enzyme derived from Lactobacillus brevis PC16 on the surface of Bacillus subtilis DB403 spores. Recombinant L-AI spores were used as a biocatalyst. Using 125 g/L D-galactose (containing 1 mmol/L Mn2+) as a substrate, the reaction was carried out at 6 7 °C and pH 6.5 for 28 h. The conversion rate of D-tagatose was 79.7%, and the recombinant L-AI spores had good reusability. After 5 cycles, the specific activity was still 87%, and the conversion rate of D-tagatose was 40.7%. Spore surface display technology has the disadvantage of low spore yield, making it difficult to apply industrially. Table 2 summarizes the above-mentioned literature reports on the catalytic synthesis of D-tagatose from D-galactose.
Due to the limitations of thermodynamic equilibrium, isomerase-catalyzed reactions are characterized by low conversion rates, which reduces production efficiency and is not conducive to the separation and purification of products. Although increasing the reaction temperature can shift the reaction equilibrium to the product side, but excessively high temperatures not only reduce enzyme activity, but also easily lead to browning of the sugar, affecting product quality, especially under alkaline conditions. Therefore, the development of an enzyme catalyst with low reaction temperature, acidic reaction pH, high catalytic activity and strong heat resistance will be beneficial for industrial applications.
3.2 D-Tagatose 3-epimerase catalyzes the production of D-tagatose from D-sorbitol
D- tagatose 3-epimerase (D-tagatose 3-epimerase, DTE) or D-psicose 3-epimerase (D-psicose 3-epimerase, DPE) are commonly used enzymes for the biosynthesis of D-psicose. They have a wide range of substrate specificities. For example the DPE enzymes from Agrobacterium tumefaciens[34] and Arthrobacter globiformis[35] and the DTE enzyme from Caballeronia fortuita[36] can all convert between D-sorbitol and D- Tagatose, in which the ratio of D-Tagatose to D-Sorbitol in the equilibrium is 30.7:69.3 when biocatalyzed by DTE enzyme from Caballeronia fortuita (Figure 3). However , there has been little research on the catalysis of D-tagatose production from D-sorbitol due to the high cost of D-sorbitol, which makes industrial production of D-tagatose from D-sorbitol uneconomical.
3. 3 Galactitol dehydrogenase catalyzes the production of D-tagatose from galactitol
Galactitol 2-dehydrogenase (GDH) can oxidize various polyalcohols and polyols to the corresponding ketones and ketoses, respectively, in the presence of the coenzyme NAD+. JA GTAP, etc. [37] heterogeneously expressed the GDH enzyme from Rhizobium leguminosarum bv. viciae 3841 in an E. coli expression system.
The GDH enzyme protein was purified using His-tag affinity chromatography. Sodium dodecyl sulfate-polyacrylamide gel gel electrophoresis was used to determine the molecular weight of the enzyme to be 28 kDa, and gel filtration chromatography was used to determine the molecular weight of the enzyme to be 114 kDa, indicating that the enzyme is a homotetramer. Analysis of enzymatic properties showed that the optimum temperature is 35 °C and the optimum reaction pH is 9.5. When the substrate is galactitol, the kinetic parameters Km are 8.8 mmol/L, Kcat are 835 min-1, and Kcat/Km are 94.9 min-1mmol·L-1, indicating that the enzyme has good substrate specificity for galactitol. The G DH enzyme catalyzes the reaction of galactitol for 30 min, and the conversion rate of D-tagatose is as high as 72%. It is verified by measuring the optical rotation that the oxidation product is D-tagatose.
Although high D-tagatose conversion rate can be obtained by catalyzing the production of D-tagatose from galactitol using galactitol dehydrogenase, but this oxidation reaction requires the addition of the coenzyme NAD+, and the substrate galactitol is expensive, so it is not economical as a raw material for industrial production.
4 Catalyzing the synthesis of D-tagatose from inexpensive substrates by multi-enzyme catalysis
4. 1 Catalyzing the production of D-tagatose from lactose
Lactose is a disaccharide composed of one molecule of D-glucose and one molecule of D-galactose. Because its price is much lower than that of D-galactose, D-sorbitol and galactitol, it is the preferred substrate for the production of D-tagatose. Zhang et al. [38] constructed a Lactiplantibacillus plantarum engineer strain, knocking out the galactokinase gene to block D-galactose metabolism; it also expresses β-galactosidase (β-g galactosidase, β-GAL) and L-arabinose isomerase, which catalyzes D-galactose to D-tagatose, thereby achieving the direct biosynthesis of D-tagatose from lactose in one pot. Using This engineered strain was used in a resting cell reaction at 65 °C and pH 7.5 for 56 h with a substrate of 175 g/L lactose, and the conversion rate of D-tagatose was 33%.
4. 2. Catalytic production of D-tagatose from whey powder
Dairy industry waste is used as a cheap raw material to produce rare sugar products [39, 40]. In 2022, ZHANG et al. [41] reported the conversion of a lactose-rich dairy by-product, Cheese Whey Powder (CWP), into three low-calorie sweeteners, D-tagatose, D-arabitol and galactitol, using continuous whole-cell catalysis and fermentation (Fig. 4). First , an engineered E. coli strain co-expressing β-galactosidase and L-arabinose isomerase was used to hydrolyze the lactose in CWP to D-galactose and glucose and to isomerize D-galactose to D-tagatose. Subsequently , D-glucose and the remaining D-galactose are fermented by Metschnikowia pulcherrima E1 to D-arabitol and galactitol. Finally , 68.35 g/L D-tagatose, 60.12 g/L D-arabitol and 28.26 g/L galactitol were obtained using 428.57 g/L CWP (containing 300 g/L lactose). This report also achieved the full utilization of the intermediate metabolites D-glucose and residual D-galactose, producing a series of valuable products from industrial by-products.
4.3 Catalytic production of D-tagatose from maltodextrin
20 22 years, DAI et al. [42] constructed a system composed of α-glucan phosphorylase (α-glucan phospho-lyase, αGP), phosphoglucomutase (PGM), glucose 6-phosphate isomerase (PGI), D-tagatose 1,6-bisphosphate aldolase (GatZ), and phosphoglycolate phosphatase (PGP). phosphatase (PGP) composed of a whole-cell biocatalyst of Escherichia coli. The CRISPR-Cas9 technology was further used to knock out the gene that causes the metabolism of the intermediate product (Figure 5), in order to increase the accumulation of the intermediate product. The resulting engineered E. coli strain was used as a biocatalyst to obtain 3.383 g/L D-tagatose with a conversion rate of 33.83 g/L using 10 g/L maltodextrin as a substrate for 3 h.
Multienzyme promoted reactions have great potential in terms of biosynthesis and conversion. Compared with single-enzyme-promoted reactions, multi-enzyme-promoted reactions can achieve more complex reactions, produce high value-added products from low-cost substrates, avoid the separation of intermediates, reduce the inhibition of intermediates, and even change the reaction balance [5]. However, due to the unbalanced ratio of various enzymes, the unbalanced metabolic flux of intermediates, and the different optimal reaction conditions of various enzymes, the conversion rate of D-tagatose is not high. In the later stage , it is possible to use techniques such as synthetic biology, metabolic engineering, and protein engineering to optimize enzyme synthesis and expression, improve enzyme performance, increase the synergy between various enzyme molecules, and improve the conversion rate of D-tagatose.
5 D -tagatose separation, purification and crystallization
D-tagatose separation and purification is a crucial step that affects the later crystallization of D-tagatose and product quality. In 2008, Huang Wenxia et al. [43] reported the use of Ca2+ ion exchange resin to separate D-galactose and D-tagatose, and the The purity of the D-tagatose obtained was 98%, and the recovery rate was 83%; the D-tagatose solution obtained was then subjected to anion and cation exchange resins for desalination and decolorization, with a desalination rate of 93% and a D-tagatose recovery rate of 87%. Subsequently , D-tagatose was crystallized by adding ethanol. Su Qi et al. used simulated moving bed chromatography to separate D-tagatose and D-galactose and found that when the valve switching time was 6.43 min, the purity of the D-tagatose obtained by separation reached 100%, and the recovery rate reached 99.93% [44]. In recent years, simulated moving bed chromatography has been widely used in the production of rare sugars due to its advantages of high separation efficiency, high solvent utilization and low energy consumption.
At present , there have been few reports on the crystallization of D-tagatose. The biosynthesis of D-tagatose is prone to the production of other heterosaccharides (such as D-glucose, D-fructose, etc.), and they often cannot be completely removed during industrial separation, which affects the nucleation and growth of D-tagatose crystals, as well as the morphology, particle size distribution and purity of D-tagatose crystals. In 2022 , WANG et al. [45] studied the effect of three impurity sugars (D-maltose, D-fructose, and D-glucose) on the nucleation rate of D-tagatose crystals and found that the adsorption of impurity sugars on the surface of D-tagatose crystals hinders the growth of D-tagatose crystals (Figure 6). WANG et al. also studied the effect of impurity sugars on the growth rate of D-tagatose crystals through single crystal growth experiments, and used molecular dynamics simulations to reveal the crystal nucleation and growth mechanism of D-tagatose at the molecular scale. There are currently few reports on the industrial crystallization process of D-tagatose.
6 Summary and outlook
As a functional natural sweetener, D-tagatose not only has important application value in the food industry, but also plays a vital role in the pharmaceutical and healthcare industries. Although D-tagatose has been approved as a new food ingredient in China, large-scale production has not yet been realized for the following reasons: (1) an enzyme catalyst with high production intensity, strong thermal stability and high conversion rate has not yet been obtained; (2) insufficient development of food-grade host bacteria; (3) high substrate cost; (4) high difficulty in product separation and purification.
In view of the above reasons, it is recommended to focus on the following research: (1) use protein engineering, enzyme engineering and other technologies to modify the molecular structure of enzymes to obtain enzyme molecules with high catalytic activity, high conversion rate and high thermal stability; (2) develop food-grade host bacteria with GRAS certification as biocatalytic carriers, including Bacillus subtilis, yeast yeasts, lactic acid bacteria, etc.; (3) give full play to the potential of multi-enzyme catalysis in biosynthesis and transformation, balance the expression levels of various enzyme molecules and the flux of intermediate metabolism, increase the synergistic effect between various enzyme molecules, and use low-cost substrates to mass-produce D-tagatose; (4) optimize the separation, purification and crystallization processes of D-tagatose. Through the above efforts, a simple, efficient and innovative process route for the industrial production of D-tagatose has been established.
Reference:
[1] SURAPUREDDI S, RAVINDHRANATH K, KUMAR G , et al. High resolution and high throughput analytical methods for D -tagatose and process related impurities using capillary electrophoresis[J] . Analytical Biochemistry, 2020, 609: 11398 1.
[2] BAPTISTA S, ROMANÍA, OLIVEIRA C, et al. Galactose to tagatose isomerization by the L-arabinose isomerase from Bacillus subtilis: a biorefinery approach for gelidium sesquipedale valorisation[J] . LWT-Food Science and Technology, 15, 2021,151: 112199.
[3] BEERENS K, DESMET T, SOETAERT W. Enzymes for the biocatalytic production of rare sugars[J] . Journal of Industrial Microbiology and Biotechnology, 2012, 39(6): 823-834.
[4] LAKSMI F, ARAI S, TSURUMARU H, et al. Improved substrate specificity for D-galactose of L-arabinose isomerase for industrial application[J] . Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 2018, 1866(11): 1084-1091.
[5] DAI Y, LI M, JIANG B, et al. Whole-cell biosynthesis of D-tagatose from maltodextrin by engineered Escherichia coli with multi-enzyme co-expression system[J] . Enzyme and Microbial Technology, 2021, 145: 109747.
[6]WANG J. Production of sweetener D-tagatose and its application in food[J] . China Condiment, 2016 , 41(01): 140-143.
[7] ROY S, CHIKKERUR J, ROY S , et al. Tagatose as a potential nutraceutical: production, properties, biological roles, and applications[J] . Journal of Food Science, 2018, 83(11): 2699-2709.
[8] LAKSMI F, ARAI S, ARAKAWA T, et al. Expression and characterization of L-arabinose isomerase from Geobacillus stearothermophilus for improved activity under acidic condition[J] . Protein Expression and Purification, 2020, 175: 105692.
[9] BOBER JR, NAIR NU. Galactose to tagatose isomerization at moderate temperatures with high conversion and productivity[J] . Nature Communications, 2019, 10(1): 4548.
[10] GUO Q, AN Y, YUN J, et al. Enhanced D-tagatose production by spore surface-displayed L-arabinose isomerase from isolated Lactobacillus brevis PC16 and biotransformation[J] . Bioresource Technology, 2018, 247: 940 -946.
[11] DE SOUSA M, MELO V, HISSA D, et al. One-step immobilization and stabilization of a recombinant Enterococcus faecium DBFIQ E36 L- arabinose isomerase for D-tagatose synthesis[J] . Applied Biochemistry and Biotechnology, 2019, 188(2): 310 -325.
[12] DE SOUZA T, OLIVEIRA R, BEZERRA S, et al. Alternative heterologous expression of L-arabinose isomerase from Enterococcus faecium DBFIQ E36 by residual whey lactose induction[J] . Molecular Biotechnology, 2021, 63(4): 289 -304.
[13] DAI Y. Biosynthesis of D-tagatose from maltodextrin by multi-enzyme catalytic system[D] . Jiangnan University, 2021.
[14] GUO Q. The production of D-tagatose from lactose using β-galactosidase and L-arabinose isomerase[D] . Shandong University, 2018.
[15] IZUMORI K. Izumoring: a strategy for bioproduction of all hexoses[J] . Journal of Biotechnology, 2006, 124 (4): 717-722.
[16] LIU Y, LI S, XU H, et al. Efficient production of D-tagatose using a food-grade surface display system[J] . Journal of Agricultural and Food Chemistry, 2014, 62(28): 6756-6762.
[17] ZHENG Z, XIE J, LIU P, et al. Elegant and efficient biotransformation for dual production of D-tagatose and bioethanol from cheese whey powder[J] . Journal of Agricultural and Food Chemistry, 2019, 67(3): 829 -835.
[18] WANG Z, WANG M, LYU X, et al. Recycling preparation of high-purity tagatose from galactose using one-pot boronate affinity adsorbent - based adsorption-assisted isomerization and simultaneous purification[J] . Chemical Engineering Journal, 2022, 446: 137089.
[19] BORTONE N, FIDALEO M. Stabilization of immobilized L-arabinose isomerase for the production of D-tagatose from D-galactose[J] . Biotechnology Progress, 2020, 36(6): e3033 .
[20] DE SOUSA M, SILVA GURGEL B, PESSELA B , et al. Preparation of CLEAs and magnetic CLEAs of a recombinant L -arabinose isomerase for D-tagatose synthesis[J] . Enzyme and Microbial Technology, 2020, 138: 109566.
[21] CHENG L, MU W, ZHANG T, et al. An L-arabinose isomerase from Acidothermus cellulolytics ATCC 43068: cloning, expression, purification, and characterization[J] . Applied Microbiology and Biotechnology, 2010, 86(4): 1089 -1097.
[22] KIM J, PRABHU P, JEYA M, et al. Characterization of an L-arabinose isomerase from Bacillus subtilis[J] . Applied Microbiology and Biotechnology, 2010, 85(6): 1839-1847.
[23] RHIMI M, ILHAMMAMI R, BAJIC G, et al. The acid tolerant L-arabinose isomerase from the food grade Lactobacillus sakei 23K is an attractive D-tagatose producer[J] . Bioresource Technology, 2010, 101(23): 9171 -9177.
[24] XU Z, QING Y, LI S , et al. A novel L-arabinose isomerase from Lactobacillus fermentum CGMCC2921 for D-tagatose production: gene cloning, purification and characterization[J] . Journal of Molecular Catalysis B: Enzymatic, 2011, 70(1): 1 -7.
[25] SEO M. Characterization of an L-arabinose isomerase from Bacillus thermoglucosidasius for D-tagatose production[J] . Bioscience Biotechnology and Biochemistry, 2013, 77(2): 385-388.
[26] FAN C, LIU K, ZHANG T, et al. Biochemical characterization of a thermostable L-arabinose isomerase from a thermoacidophilic bacterium, Alicyclobacillus hesperidum URH17-3-68[J] . Journal of Molecular Catalysis B: Enzymatic , 2014, 102: 120-126.
[27] MEI W, WANG L, ZANG Y, et al. Characterization of an L-arabinose isomerase from Bacillus coagulans NL01 and its application for D - tagatose production[J] . BMC Biotechnology, 2016, 16(1): 55.
[28] XU W, FAN C, ZHANG T, et al. Cloning, expression, and characterization of a novel L-arabinose isomerase from the psychrotolerant bacterium Pseudoalteromonas haloplanktis[J] . Molecular Biotechnology, 2016, 58(11): 695-706.
[29] NGUYEN T, HONG M, CHANG P, et al. Biochemical properties of L-arabinose isomerase from Clostridium hylemonae to produce D- tagatose as a functional sweetener[J] . PLoS One, 2018,13(4): e0196099 .
[30] DU M, ZHAO D, CHENG S, et al. Towards efficient enzymatic conversion of D-galactose to D-tagatose: purification and characterization of L-arabinose isomerase from Lactobacillus brevis[J] . Bioprocess and Biosystems Engineering, 2019, 42(1): 107 -116.
[31] MANZO R, ANTUNES A, DE SOUSA M , et al. Biochemical characterization of heat-tolerant recombinant L-arabinose isomerase from Enterococcus faecium DBFIQ E36 strain with feasible applications in D-tagatose production[J] . Molecular Biotechnology, 2019, 61(6): 385 - 399.
[32] ZHANG G, AN Y, PARVEZ A , et al. Exploring a highly D-galactose specific L-arabinose isomerase from Bifidobacterium adolescentis for D-tagatose production[J] . Frontiers in Bioengineering and Biotechnology, 2020, 8: 377.
[33] SHIN K, SEO M, KIM S , et al. Characterization of L-arabinose isomerase from Klebsiella pneumoniae and its application in the production of D-tagatose from D-galactose[J] . Applied Sciences, 2022, 12(9): 4696.
[34] KIM H, HYUN E, KIM Y, et al. Characterization of an Agrobacterium tumefaciens D-psicose 3-epimerase that converts D-fructose to D- psicose[J] . Applied and Environmental Microbiology, 2006, 72(2): 981 -985.
[35] YOSHIHARA A , KOZAKAI T, SHINTANI T, et al. Purification and characterization of D-allulose 3-epimerase derived from Arthrobacter globiformis M30, a GRAS microorganism[J] . Journal of Bioscience and Bioengineering, 2017, 123(2): 170 -176.
[36] LI S, CHEN Z, ZHANG W, et al. Characterization of a D-tagatose 3-epimerase from Caballeronia fortuita and its application in rare sugar production[J] . International Journal of Biological Macromolecules, 2019,138: 536 -545.
[37] JAGTAP S, SINGH R, KANG Y, et al. Cloning and characterization of a galactitol 2-dehydrogenase from Rhizobium legumenosarum and its application in D-tagatose production[J] . Enzyme and Microbial Technology, 2014, 58 -59: 44-51.
[38] ZHANG S, GUO T, XIN Y, et al. Biotechnological production of D-tagatose from lactose using metabolically engineering Lactiplantibacillus plantarum[J] . LWT - Food Science and Technology, 2021, 142: 110995.
[39] RAI S, KAUR H, KAULDHAR B , et al. Dual-enzyme metal hybrid crystal for direct transformation of whey lactose into a high-value rare sugar D-tagatose: synthesis, characterization, and a sustainable process[J] . ACS Biomaterials Science and Engineering, 2020, 6(12): 6661 - 6670.
[40] ZHANG G, ZABED H, YUN J, et al. Two-stage biosynthesis of D-tagatose from milk whey powder by an engineered Escherichia coli strain expressing L-arabinose isomerase from Lactobacillus plantarum[J] . Bioresource Technology, 2020, 305: 123010.
[41] ZHANG G, ZABED H, AN Y, et al. Biocatalytic conversion of a lactose-rich dairy waste into D-tagatose, D-arabitol and galactitol using sequential whole cell and fermentation technologies[J] . Bioresource Technology, 2022, 358: 127422.
[42] DAI Y, LI C, ZHENG L , et al. Enhanced biosynthesis of D-tagatose from maltodextrin through modular pathway engineering of recombinant Escherichia coli[J] . Biochemical Engineering Journal, 2022, 178: 108303.
[43] HUANG W, MU W, JIANG B. Study on separation and purification of D -tagatose[J] . Food and Fermentation Industries, 2008 , 34(06):168-171.
[44] SU Q, LI H, ZHA X, et al. Simulation study on separation of D-tagatose and D-galactose in simulated moving bed[J] . Contemporary Chemical Industry, 2014 , 43(07): 1379-1381+1385 .
[45] WANG D, WANG Y, LI Y, et al. Uncovering the role of impurity sugars on the crystallization of D-tagatose crystal: experiments and molecular dynamics simulations[J] . Food chemistry, 2022, 397: 133762.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




