What Are the Uses of D Tagatose?
D tagatose is a monosaccharide with special health benefits that was discovered in recent years. It was determined to be Generally Recognized as Safe (GRAS) by the US Food and Drug Administration (FDA) in 2001. Studies have shown that D-tagatose not only has similar taste and physical properties to sucrose1), and can be used as a sweetener to replace sucrose, but also has a complementary therapeutic effect on the treatment of diseases in specific populations (2), so it is currently attracting a lot of attention from the market and consumers. In view of this, it is of great significance to make full use of the superior properties of D-tagatose and apply it to the fields of food, medicine, cosmetics, etc.
1 Structure and physical and chemical properties of D-tagatose
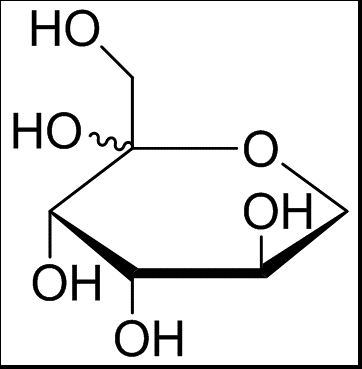
D tagatose is a rare natural hexopyranose, an isomer of D-galactose, and a reverse D-fructose diastereoisomer at the C-4 position (as shown in Figure 1(3)). According to the Lobry de Bruyn-Al- berda van Ekenstein transformation, the D-talose and D- tagatose in the figure can interconvert with each other due to the α-carbon atom of the sugar molecule providing active hydrogen to give a carbonyl group, resulting in an enol form, in an alkaline solution. D-Tagatose is a white powdery crystal with no odor. Its molecular formula is CgH₁₂O₆, molecular weight 180.16, melting point 132°C–135°C, and 1% aqueous solution [α]20-6±1° (c=5,H₂O);D-Tagatose is acid-resistant and can exist stably at pH 3~7. It is also heat-resistant and alkali-resistant. It is prone to Maillard browning and can undergo caramelization at lower temperatures.
2 D-Tagatose Functional properties
2.1 Inhibits high blood sugar
D-Tagatose is a low-energy sugar with a sweetness that is about 92% that of sucrose, and it is not well absorbed by the body (4). Donner Thomas et al. (2) used 6 healthy people and 6 non-insulin-dependent diabetes mellitus patients as research subjects, and measured the changes in blood glucose and insulin levels after they were given 75 g of glucose or 75 g of D-tagatose orally over a period of 3 hours, or 75 g of tagatose orally for 30 minutes followed by 75 g of glucose.
The results showed that oral administration of D-tagatose alone did not cause changes in blood glucose and insulin levels in healthy people and type 2 diabetes patients. On the contrary, it suppressed the increase in blood glucose caused by glucose intake in diabetic patients, but had no significant effect on the insulin sensitivity of diabetic patients in type 2 diabetes patients. Similarly, giving type 2 diabetes patients 75g of sucrose, or 75g of D-tagatose 30 minutes before taking 75g of sucrose, can also suppress the rise in blood sugar caused by sucrose. Therefore, D-tagatose is considered to have a supplementary effect on the treatment of type 2 diabetes, which may be because D-tagatose weakens the absorption of sucrose in the stomach and intestines, while effectively preventing glucose spikes in other sugar products and preventing an increase in glucose levels in the blood.
2.2 Improves intestinal flora and promotes the growth of beneficial bacteria
D-tagatose is fermented by microorganisms in the human gastrointestinal tract, mainly producing acetic acid, butyric acid, and hexanoic acid, with butyric acid being the main component. Butyric acid can promote the growth of beneficial bacteria such as lactobacilli and is a good source of colon epithelial cells. It also has a certain effect in inhibiting colon cancer and intestinal pathogenic bacteria. Laerke et al. (5) collected and analyzed the feces of 16 healthy people (10 g D-tagatose was given three times a day for 14 days), and found that the concentration of lactic acid bacteria in the stool on the 14th day after ingestion was significantly higher than that without ingestion, while the concentration of coliform bacteria in the stool on the 14th day after ingestion was reduced.
2.3 Not easily broken down and utilized by oral bacteria, and does not cause tooth decay
In 2002, the US FDA confirmed that D-tagatose is not broken down by oral bacteria and does not cause tooth decay. D-tagatose can also effectively eliminate bad breath and prevent diseases such as gingivitis.
3. Method of producing D-tagatose
D-tagatose is found in many foods, mainly dairy products such as sterilized milk powder, cheese, and yogurt[6]. Currently, D-tagatose is produced by isomerizing galactose using biological and chemical methods.
3.1 Biological method
Lzumori et al. [78] used Mycobacterium smegmatis and Arthrobacter globiformis, and Muniruzzaman et al. [9] used Enterobacter applomerans to convert the expensive galactitol to tagatose, which is a method of using galactitol to biosynthesize D-tagatose, i.e., the galactitol biosynthesis method. In addition, the enzymatic synthesis method is also used in industrial production. Studies have shown that the enzyme L-galactose isomerase has catalytic activity in the conversion process, converting L-galactose to L-ribulose. Since L-galactose isomerase is a relatively specific (group specific) enzyme, it can convert aldose to ketose. Therefore, while catalyzing L-tagatose, it can also convert other aldose (such as D-galactose, DL-fructose and D-xylose) to the corresponding hexose form. If lactose permeate is used as the raw material for enzymatic preparation of tagatose, the process flow is:
lactose permeate → ultrafiltration → reverse osmosis → microfiltration → hydrolysis → concentration → fermentation → isomerization → concentration → crystallization → finished product.
3.1.1 Removal of proteins, salts and high-molecular substances
High-molecular substances such as proteins are removed from the lactose permeate to prevent bacterial contamination of the subsequent process steps. Salts, for example, inhibit the crystallization of tagatose during the crystallization stage and should therefore also be removed.
3.1.2 Hydrolysis
After the removal of high molecular weight substances such as proteins from the lactose permeate, the immobilized lactase enzyme is used to continuously hydrolyze the lactose to glucose and galactose. The hydrolysis conditions are a temperature of 40°C to 60°C and a pH of 4 to 6.
3.1.3 Concentration
The lactose hydrolysate is concentrated to a solution containing 2% to 20% galactose and 2% to 20% glucose, with the ratio of the two being 1:1, and the rest being protein, fat, salt and undigested lactose.
3.1.4 Fermentation
Lactose is hydrolyzed to form a mixture of galactose and glucose. After selective fermentation by yeast or bacteria, glucose is fermented to ethanol, while galactose is not fermented and enters the next step of isomerization, or the galactose is separated out, evaporated, concentrated, and crystallized to obtain pure galactose powder.
3.1.5 Enzyme isomerization
The fermentation broth is centrifuged or microfiltered to remove microbial cells, and distilled to remove ethanol to obtain a galactose concentrate. L-Tagatose is obtained by isomerization with L-galactose aldehyde isomerase.
3.1.6 Purification
The mixture obtained by isomerization is separated by cation exchange column chromatography, and the finished product is obtained by concentration and crystallization.
3.2 Chemical method
Beadle et al. (10) used a chemical method, a two-step method, to produce D-tagatose from galactose. Galactose is first isomerized to the insoluble metal hydroxide tagatose complex under the catalysis of a soluble alkali metal salt (e.g., Ca(OH)₂), and the final product of tagatose is obtained by neutralizing the complex with acid.
3.2.1 Isomerization
Isomerization is the formation of a metal hydroxide-tagatose complex, an insoluble intermediate complex, which is important in the chemical production of tagatose. This is because: (1) the formation of an insoluble intermediate complex facilitates the positive direction; (2) galactose is unstable under alkaline conditions and is prone to side reactions and degradation reactions, and the formation of the complex intermediate can inhibit the occurrence of side reactions; (3) the insoluble complex intermediate precipitates and can be effectively separated from other by-products.
3.2.2 Acid neutralization
The purpose of acid neutralization is to generate insoluble metal salts to separate tagatose from metal complex intermediates.
3.2.3 Purification
D-tagatose is separated from the reaction system by crystallization, while the filtrate still contains tagatose. Therefore, the filtrate is reacted with an alkaline metal hydroxide to form a metal hydroxide-tagatose complex to extract tagatose.
Currently, Maryland Biospherics has patented a chemical method for producing tagatose products. Of course, there are also some problems with the chemical production of D-tagatose, such as the use of strongly alkaline metal hydroxides and high pH, which are not conducive to food processing.
4 D-tagatose in human biochemical processes
4.1 Human digestion and absorption
D-tagatose is mainly digested and absorbed in the intestines, but there is still some debate in the academic community about the exact amount of digestion and absorption. Bär (4) used L-rhamnose as a reference to study the human body's absorption of D-tagatose. Since the rate of passive diffusion of substances through the intestinal mucosa depends on the molecular weight and lipophilicity, and L-rhamnose is a slightly lower molecular weight and slightly more lipophilic compound, so D-tagatose is slightly more absorbed in the intestine than L-rhamnose (14% to 24%). Therefore, Bär estimates that the body's absorption of D-tagatose will not exceed 20%.
In contrast to Bär's report of low D-tagatose absorption, Norman et al. [11 found that D-tagatose absorption in patients with ileostomies could reach 80%. Jenkins et al. (12 ) previously found that L-rhamnose absorption in patients with this operation was close to 50%. Although ileostomy can only provide a limited assessment of incomplete digestion and absorption of monosaccharides, the conclusions of these studies are contrary to those of Bär. Similarly, Buemann et al. (13) found in a double-blind crossover trial that only 1.5% of the 30 g D-tagatose ingested orally was excreted in the urine after 8 individuals had ingested 30 g D-tagatose and 30 g D-fructose alone.
4.2 Human biotransformation
D-tagatose can be phosphorylated and converted to 1-phospho-D-tagatose in the human body. Raushel & Cleland reported [14] that under conditions of ATP and K+, bovine liver fructokinase can catalyze the phosphorylation of D-tagatose to produce 1-phospho-D-tagatose. Since fructokinase is only found in the liver and kidneys, 1-phospho-D-tagatose is also mainly produced in the liver and kidneys, and is formed less in intestinal mucosa and islet cells. Buemann et al. [13] found through a double-blind crossover trial that serum inorganic phosphorus was temporarily reduced, which may be directly related to the formation of 1-phospho-D-tagatose.
1-Phospho-D-tagatose can continue to be broken down by aldolase in the liver to form D-glyceraldehyde and dihydroxypropanone phosphate, which leads to the gluconeogenic conversion of D-tagatose to glucose. Rognstad (15.16) used mouse liver cells and D-tagatose in an in vitro culture to produce glucose, in which the gluconeogenic rate of 20 mmol/L D-tagatose was twice that of 20 mmol/L D-galactose and half that of fructose. “C-fructose and ¹⁴C-D-tagatose both form lC glucose via the gluconeogenic pathway, indicating that they have the same gluconeogenic pathway.
Although the gluconeogenic pathway of D-tagatose has not yet been established, it is likely to be metabolized via the galactose-phosphoric acid pathway in the liver of mammals. For example, an isomerase catalyzes the conversion of 1-phospho-D-tagatose to 1-phospho-galactose. Isotope tracing has found that increasing fructose to D-tagatose inhibits the conversion of D-tagatose to glucose, and increasing D-tagatose to fructose does not affect fructose gluconeogenesis. This may be due to the fact that the structure of D-tagatose is more prone to gluconeogenesis than fructose.
5 D-tagatose applications
5.1 Application in food
As people's living standards continue to improve, more and more people are beginning to pay attention to their health and eat low-energy foods. D-tagatose, as a low-energy sugar, can give full play to its advantages in foods. Marzur (17) reported that in addition to being low in energy, D-tagatose generally does not cause diarrhea during digestion and absorption in the human body, which is the case with other polyhydroxy compound sugar substitutes. In a 3-month feeding experiment with mice, it was found that the body weight of mice fed 15% (w/w) sucrose was twice that of mice fed 15% (w/w) D-tagatose. Because D-tagatose is similar to sucrose in taste and physical properties, and has physical and chemical properties such as heat resistance, acid resistance and alkali resistance, it is widely used as a perfect sweetener in the food industry. It is mainly used in the production of chocolate, baked goods, ice cream, hard and soft candies, chewing gum, soft drinks, condiments and other products.
5.1.1 Application in the beverage industry
In the beverage industry, the main sweeteners added are saccharin, cyclamate, aspartame, acesulfame, stevia and sucralose. These are all strong sweeteners, and sometimes, in consideration of the taste, their The result is a bitter aftertaste, metallic taste and astringent taste. However, Tagatose can effectively reduce these undesirable factors. D-Tagatose has a synergistic sweetening effect, and just a small amount can significantly enhance the sweetness. For example, adding D-tagatose to low-fat milk drinks (chocolate and yogurt flavors) can not only reduce undesirable flavors, but also make the sweetness of the product more stimulating, the flavor more mellow and rich; adding D-tagatose to lemon juice drinks can make the product taste fresher and cleaner; it can also increase the soluble solids content of the beverage system.

Therefore, in 2001, the US Food and Drug Administration officially approved the use of D-tagatose as a sweetener in the food and beverage industry. It is now widely used in the US as a sucrose substitute in health drinks, yogurt, fruit juice and other products.
5.1.2 Application in cereal foods
The high melting point, heat resistance, acid and alkali resistance, and low-temperature caramelization of tagatose are important during the processing of cereals. This is because tagatose has low viscosity and is easy to crystallize. Therefore, applying icing made from tagatose to the surface of cereals not only increases the sweetness of the product, but also extends its shelf life.
5.2 Applications in medicine, cosmetics and other fields
In addition to its excellent properties as a sweetener in food and beverages, D-tagatose can also be used as an excipient in pharmaceuticals and as a humectant and stabilizer in cosmetics. At present, in toothpaste and mouthwash, D-tagatose is mainly used as a wetting agent, followed by a stabilizer, but it can also effectively improve the taste of toothpaste and mouthwash. Nowadays, many toothpastes use D-sorbitol or glycerin, or a combination of the two, as a wetting agent.

However, the sweetness of the mixture of D-sorbitol and glycerin is half that of sucrose, and this sweetness is not as desired when brushing teeth. Therefore, saccharin, a high-intensity sweetener, is added to many toothpastes to make the product taste as good as it should. However, this compound, while providing the required wettability and sweetness, also has a metallic taste, a bitter aftertaste and a saccharin aftertaste. D-tagatose, like sucrose, is twice as sweet as D-sorbitol, and its addition not only maintains the sweetness in toothpaste, but also prevents tooth decay. Lu6 By measuring the water activity and moisture content of D-tagatose and D-sorbitol and comparing their wettability, it can be seen from the desorption curves of D-tagatose and D-sorbitol curves, when D-tagatose is dissolved in water above 0.62, D-tagatose and D-sorbitol are considered to have the same wettability in terms of water activity. When toothpaste is added with 20% to 25% (w/w) D-tagatose, not only does the sweetness reach a satisfactory level, but it also maintains good wettability and stability.
At present, although the mechanism of action of D-tagatose as a new monosaccharide in the human body needs to be further explored, its excellent physical and chemical functional properties are undeniable. It is expected that in the next few years, D-tagatose will become a best-selling natural sweetener on the international market, and will be widely used in food, medicine, cosmetics and other fields. Its market prospects are very broad.
Reference:
〔1〕Sang-Hyun Yoon,Pil Kim,Deok-Kun Oh.Properties of L- arabinose isomerase from escherichia colias biocatalyst for tagatose production[J].World Journal of Microbiology& Biotechnology,2003,19:47—51.
〔2〕Donner Thomas,Wilber John,Ostrowski Debra.D-tagato- se:a novel therapeutic adjunct for non-insulin-dependent diabetes [J].Diabetics,1996,45(supplement):125A.
〔3〕Hans Beyer,Wolfgang Watler,Tang Weicheng, Organic Chemistry Textbook [M], Beijing: Higher Education Press, 1989, 306-307.
〔4〕Bär A.D-tagatose:dossier prepared by bioresco on behalf of MD foods amba,videbaek,denmark [J].Regulatory Toxicology and Pharmacology,1999,29(2):83-93.
〔5〕Laerke H.N,Jensen B.B,Hφjsgaard S.The influence of D-tagatose on bacterial composition and fermentation capa- city of faecal samples from human volunteers [J.J.Nutr., 2000,130:1772-1779.
〔6〕Lu,Y.Humectancies of D-tagatose and D-sorbitol [J].In- ternational Journal of Cosmetic Science,2001,23(3):175— 181.
〔7〕Izumori K,Tsuzaki K.Production of D-tagatose from D galactitol by mycobacterium smegmatis [J].J.Ferment. Technol.,1988,66:225-227.
〔8〕Izumori K,Miyoshi T,Tokuda S,et al.Production of D tagatose from ducitol by arthrobacter globiformis [J].Appl. Environ.Microbiol,1984,46:1055—1057.
〔9〕Muniruzaman S,Tokunaga H,Izumori K.Isolation of en- terobacter agglomerans strain 22le from soil,a potent D-ta- gatose producer from galactitol [J].J.Ferment.Bioeng, 1994,78:145-148.
〔10〕Beadle J.R,Saunders J.P,Wajada T.J.Process for manu- facturing tagatose [P].World Patent.12263,1992.
〔11〕Normen L,Laerke H.N,Langkilde A.M,et al.Small bo- wel absorption of D-tagatose and related effects on car- bohydrate digestibility [J].Am.J.Clin.Nutr.,2001,73: 105-110.
〔12〕Jenkins A.P,Menzies I.S,Nukajam W.S,etal.The effect of ingested lactulose on absorption of L-rhamnose,D-xylo- se,and 3-0-methyl-D-gluose in subjects with ileostomies [J]. Scand.J.Gastroenterol,1994,29:820—825.
〔13〕Buemann B,Toubro S,Holst J,et al.D-Tagatose,a ste- reoisomer of D-fructose,increased blood uric acid concen- tration [M].Metabolism,1999.
〔14〕Raushel F.M.Cleland W.W.Bovine liver fructokinase: purificartion and kinetic properties [J].Biochemistry,1977, 16:2169--2175.
〔15〕Rognstad R.Gluconeogenesis from D-tagatose by isolated rat and hamster liver cells[J].FEBS Lett.,1975,52:292— 294.
〔16〕Rognstad R.Pathway of gluconeogenesis from tagatose in rat hepatocytes [J].Arch.Biochem.Biophys.,1982,218: 488-491.
〔17〕Marzur A.W.Functional sugar substitutes with reduced ca- lories [P].EP 341062,1989.
-
Prev
What Is the Use of Tagatose Powder in the Food Field?
-
Next
The Method to Synthesis of D Tagatose Powder?


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




