What Is the Extraction Method of Lutein from Marigold Flower?
Tagetes erecta (marigold) is native to Mexico. It is also known as calendula because it is rich in lutein, a natural carotenoid that accounts for more than 90% of its content. As further research is carried out into the pharmacological functions of lutein, it is found to have a variety of active functions, such as preventing and treating atherosclerosis, boosting the body's immune system, preventing cataracts and protecting vision. Nowadays, there is a deviation in people's understanding of the toxicity of synthetic pigments. The world's control of the types and quantities of synthetic pigments used is increasing year by year. Due to their widespread use, people's demand for natural pigments is increasing year by year. People's understanding of lutein is gradually deepening, and the focus is shifting from simply synthesizing pigments to researching how to extract natural pigments and use them to uncover more functions.

Lutein not only has natural pigments, but also antioxidant activity, and is becoming increasingly popular with consumers. Both domestically and abroad, more and more of its functions are being discovered. In China, regions such as Yunnan, Guizhou and Sichuan have widely planted marigolds as a way to revitalize rural areas. However, because research on lutein in China started later than in foreign countries, some countries are more advanced in the chemical preparation technology of high-purity lutein and have applied for many patents on chemical preparation, making it more difficult for China to improve the extraction and preparation of marigolds. China's lutein extraction products are mainly crude extracts with low added value. Based on the above problems, the preparation of high-purity lutein has become an urgent task. Moreover, the eight special isomers in lutein can only be extracted and separated from plants so far, and none of them can be synthesized by chemical methods. Therefore, the extracted lutein has certain practical significance [1].
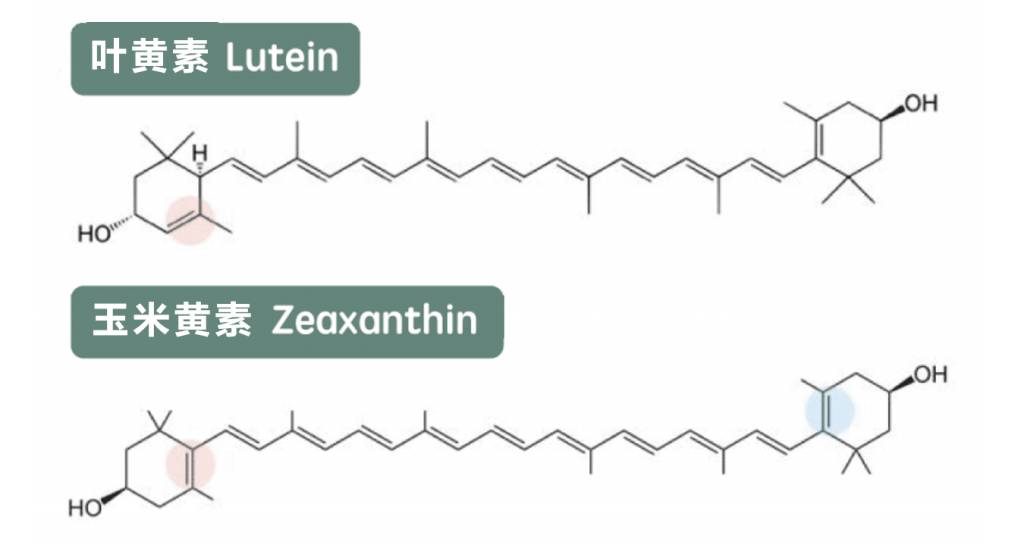
1 Practical value
1.1 Ornamental value
Marigolds are annual herbaceous plants with a plant height of 70–100 cm. They are widely used in the food processing industry because of the strong yellow pigment in their flowers. Currently, this yellow pigment is mainly exported to neighboring countries, and there is a certain production prospect. It is in short supply in the international market. Marigolds planted in a continuous field also have the function of beautifying the environment as a characteristic agricultural product for rural revitalization, and have a high ornamental value [2].
1.2 Coloring function
Lutein has a strong coloring power, which can increase the hatching of eggs and the breeding rate of poultry. As people tend to judge the quality of poultry based on the nutritional value of meat, lutein is also a natural pigment with no side effects, which can be used to color egg yolks, poultry and chicken feed. Because its safety and nutritional functions meet market demand, it is widely used in feed coloring in various countries [3].
1.3 Anti-cancer function
Lutein is a carotenoid with specific physiological activity that can inhibit the growth of human tumors [4] and protect against many types of cancer, such as skin cancer, breast cancer, and colon cancer. There is a correlation between breast cancer incidence and lutein intake, as experiments have shown that the breast cancer incidence in the low-lutein intake group is 2.08 to 2.20 times that of the high-lutein intake group [5-6].
1.4 Antioxidant function
Lutein is an antioxidant with good antioxidant activity, which can effectively resist the damage of free radicals to the human body in cells. The antioxidant lutein can prevent skin problems caused by too much sunlight; lutein, antioxidant vitamins, zeaxanthin and free radical scavengers can prevent cataracts. In addition, zeaxanthin can also resist the damage of age-related macular degeneration and the oxidation of the retina [7].
1.5 Skin care effect
The natural antioxidant lutein can effectively prevent skin damage caused by sunlight and protect against the adverse effects of ultraviolet light on the human body. Once the skin is exposed to ultraviolet light, the absorption of light energy by lutein helps the immune system maintain a normal response and provides skin care. This finding provides a reference for the research of other skin care products [8].
2 Extraction method
At present, the common extraction methods for natural lutein include: CO2 supercritical extraction, organic solvent extraction, microwave extraction, etc. The common drawback of these extraction processes is their low extraction efficiency. Some lutein is attached to the plant cell walls and is difficult to dissolve during extraction, which makes the process time-consuming and affects the yield. Therefore, in the above extraction process, it is often necessary to use ultrasound for further processing. Therefore, the thermal effect and mechanical action of ultrasound are used to accelerate the breaking of cell walls, so that lutein dissolves in the cell walls, thereby reducing the extraction time and improving the yield of lutein [9].
2.1 Supercritical CO2 extraction
Jin Limei et al. used supercritical CO2 extraction technology to extract marigold seed oil. An orthogonal design experiment was used to study factors such as temperature, pressure, extraction time and CO2 flow during the extraction process to determine the optimal extraction rate of seed oil. The conclusion was that: CO2 at a temperature of 40 °C, flow rate of 20–40 kg·h-1, extraction time of 3 h, and pressure of 35 MPa. The resulting seed oil is odorless, orange-yellow in color, with an iodine value of 50.17 g/100 g and an acid value of 46.93 mg KOH/g oil [10]. Song Dawei et al. studied the factors affecting the extraction of xanthophylls, and conducted single-factor experiments on the factors affecting the extraction of xanthophylls by supercritical CO2 fluid. The final optimal factors were determined by response surface: extraction time 180 min, extraction temperature 60 °C, and extraction pressure 48.6 MPa. The final result is that the maximum yield of lutein (mg/g) is 8.44, the verified value is 8.41, and the relative error compared to the predicted value is only 0.0036. Therefore, the response surface method can be used as a method for extracting marigold yellow pigment [11].

Yang Zhonglin et al. used supercritical CO2 extraction to extract lutein, and used single factor and orthogonal experiments to determine the influencing factors. The influence sizes were: time > temperature > pressure > CO2 flow rate; the optimized process conditions were: extraction time 4 h, extraction pressure 24 MPa, temperature 54 °C, CO2 flow rate 12 L · h-1, separation I temperature 42 ℃, pressure 11 MPa, separation II temperature 38 ℃, pressure same as storage tank. Under these conditions, using lutein as the raw material with a yield of 825 mg · (100 g)−1, the yield was 95.6% [12].
2.2 Ultrasonic method
Kuang Yang et al. calculated the lutein yield using an orthogonal design method. The effects of factors such as the ultrasonic extraction time, liquid-to-material ratio, particle size, and ultrasonic power on the extraction yield were determined in the experiment, so as to obtain the optimal process conditions for marigolds. Under the conditions of marigold particle size 150-180 μm (80-100 mesh), the optimal process is ultrasonic power 150 W, ultrasonic time 40 min, and liquid-to-solid ratio 1:15 (g:mL) [13].
2.3 Organic solvent extraction method
With the development of extraction processes, organic solvent extraction is commonly used to extract plant lutein because lutein is widely distributed in the cells of plants and is difficult to dissolve in organic solvents, resulting in a low yield of lutein. The use of ultrasonic waves can change this situation and greatly increase the extraction yield. Chen Bin et al. used acetone as the solvent to extract under the conditions of a material-to-liquid ratio of 1:50 (g:mL) by the general extraction method, and finally obtained an absorbance of 1.3325. The absorbance of the extract obtained using the ultrasonic-assisted method reached 2.2076. Factors affecting the size are: ultrasonic power > liquid-to-material ratio > ultrasonic time > ultrasonic temperature. The optimal conditions for the experiment were: temperature 50 °C, liquid-to-material ratio 1:30 (g:mL), ultrasonic power 400 W, time 40 min. The best experimental result was: maximum absorption wavelength 442 nm, absorbance 3.673. When not saponified, the product has a high lutein ester content after extraction. The saponification time is controlled at 9 h for the best results, with the lutein content reaching up to 96% [14].
Wang Xia et al. extracted lutein esters from baked goods by selecting an appropriate extraction solvent, and studied the saponification time, KOH concentration, and extraction effect of different extraction solvents. The best saponification results were obtained using 2 g of sample (batter/dough), 10 mL of absolute ethanol, 0.2 g of BHT, 10 mL of 60 g/100 mL KOH solution, and 3 h of shaking at room temperature. The best extraction solvent is Vcyclohexane: Vhexane: Vethyl acetate = 1: 2: 2, with a yield of 94.31% to 103.83%. The relative standard deviation of the experimental precision is less than 5%. The results show that this method can be used to extract lutein esters from baked goods [15].
2.4 Ultrasonic-assisted method
Ye Zhaowei et al. studied the optimal process conditions for lutein in marigolds by using microwave, water bath heating and ultrasonic methods to study the experimental conditions and finally determine the optimal process conditions. The results showed that the extraction yield of lutein by ultrasonic method was higher than that by water bath heating method and microwave method, and the lutein content could be as high as 21.9 mg/g. Therefore, the yield of lutein extraction can be improved using an ultrasonic-assisted method [16]. Yang Yunshang et al. determined that the final extraction method is to use petroleum ether-ethanol containing 40% ethanol as the extraction agent, with a material-to-liquid ratio of 1:10 (g:mL), an ultrasonic frequency of 100 kHz, conditions are: add 4 mL of 15% sodium hydroxide ethanol solution to 1 g of marigold, saponification time 3 h, saponification temperature 65 ° C, ultrasonic power 500 W. Under these extraction conditions, the extraction result was 0.872 mg/g [17].
2.5 Supercritical fluid extraction
Supercritical fluid extraction is currently a common method used to extract active ingredients from plants and animals. This extraction method does not destroy the active ingredients of plants and animals and is non-toxic. Many current supercritical fluid extraction methods have been scaled up from the initial experimental scale to mass production. Li Dajing et al. fermented dried flowers and then supercritical CO2 was produced by controlling the pressure at about 20–40 MPa, supercritical extraction was carried out for 1–10 h, and the extract was obtained by vacuum distillation. After saponification, the sample purity was 18%–22%, and the color value was 212–321 [18].
2.6 Microwave-assisted extraction process
The results showed that the extraction solvent was ethyl acetate, and the extraction conditions were optimized to give the following results: microwave power of 560 W, extraction time of 20 s, and material-to-liquid ratio (g:mL) of 1:20 [19]. Fan Jianfeng et al. studied the synergistic effect of microwave and surfactant to optimize the experimental conditions. The final results were: ethyl acetate was selected as the extraction agent, surfactant Tween-20 with a mass fraction of 0.03% was selected as the co-solvent, the microwave power was 400 W, the extraction time was 2 min, and the material-to-liquid ratio (g:mL) was 1:60. The final extraction data under this process showed 3.209 mg/g [20]. Zhang Lingling et al. optimized the extraction process conditions for the three lutein lipids using organic solvent extraction, ultrasonic-assisted extraction, and microwave extraction, respectively. Using different methods, they concluded that the microwave-assisted extraction method (liquid-to-material ratio 75:2 (g:mL), irradiation time 15 min, extraction temperature 30 ℃, extraction power 400 W). The optimal process for extracting lutein lipids is microwave-assisted extraction because it has low extraction costs, high lutein lipid content, and is convenient and quick to operate [21].
2.7 Response surface methodology
Zhang Weihong used marigolds as the raw material to study the extraction of carotenoids from marigolds. The optimal experimental process was obtained by response surface methodology, and a quadratic regression model was established. The results showed that after the carotenoids were extracted by ultrasonic method, the model fitting was good. The optimal extraction results are: temperature 39.5 °C, marigold: extraction agent = 1:20 (g:mL), solvent Vpetroleum ether: Vethyl acetate = 2:3, ultrasonic time 35 min, ultrasonic power 450 W. Through optimization and the actual carotenoid extraction rate was 26.78 mg/g, and the predicted carotenoid extraction value was 27.45 mg/g. The high degree of agreement between the verified and predicted values indicates that the response surface method has a certain degree of reliability [22].
Zhang Rui and others have experimentally demonstrated that response surface analysis can be used as an effective extraction method to optimize experimental conditions. After optimizing the experimental conditions using the response surface method, it was concluded that the optimal process conditions for extracting carotenoids from marigolds were a solid-liquid ratio of 1:25 (g:mL), a temperature controlled at 45 °C, and a time of 4.5 h. After optimizing the experiment three times and verifying the data, the absorbance value of the actual carotenoid extract from marigolds was 0.824. The predicted value basically matched the average of the verification experiments [23].

Wang Dianbei's research found that the optimal experimental conditions were: a temperature of 32.09 °C, an extraction time of 54 min, and a liquid-to-material ratio of 16:1 (mL:g). Under these experimental conditions, the absorbance of the marigold lutein extract was 2.375. The final preferred process results are: extraction time 55 min, extraction temperature 32 ℃, liquid-to-material ratio 16:1 (mL:g), and the final absorbance value is 2.356. Therefore, the response surface method was used to study the extraction conditions of lutein from marigold leaves, which can obtain accurate and reliable experimental parameters and provide a theoretical basis for further in-depth extraction of lutein [24].
3 Outlook
China is rich in marigold resources. In recent years, with the increasingly widespread application of lutein in various fields and the further exploration of pharmacological activity, the application of resources with multiple activities in various fields will be a research hotspot in the future. Lutein has broad market prospects and will play an important role in various fields [6]. Therefore, it is necessary to invest in the realization of mass production, optimize the best product process conditions, and further research more domestically produced lutein products and lutein-rich health foods in the future. Focusing on the extraction and development of lutein products from marigold, improving the extraction process technology, reducing the cost of extraction and development, increasing the yield of lutein, upgrading the product form, and improving the product profit margin, all have a certain practical significance for promoting the development of China's national economy and improving people's health.

References
[1] Shi Gaofeng, Li Ganggang, Li Na, et al. Extraction process research of lutein esters in marigolds [J]. Food Science and Technology, 2010, 35(9): 254-257.
[2] Chen Zhiwen. Discussion on the development of marigold industry in Qiubei County [J]. Yunnan Agriculture, 2017(8): 70-71.
[3] Zhang Hui, Li Tao, Xu Gongshi. A natural food colorant with a promising future: lutein [J]. China Food Additives, 2004(5): 45-48.
[4] Meng Xianghe, Mao Zhonggui, Pan Qiuyue. Health-promoting function of lutein [J]. China Food Additives, 2003(1): 17-20.
[5] Guo Zhiyou, Gao Cuiling, Song Ru, et al. Function and application of lutein [J]. Hebei Agricultural Science, 2010, 14(2): 52-53.
[6] Chen Cheng, Cheng Xi, Huang Conglin. Research progress on extraction methods and functions of lutein [J]. Hebei Forestry Science and Technology, 2016(3): 71-75.
[7] Lin Dengui, Zeng Li, Wang Peng, et al. Research status and development trend of marigold [J]. Shanghai Agricultural Science Bulletin, 2014, 30( 6) : 145-149.
[8] Guo Wei. Optimization of marigold lutein extraction process and separation and purification [D]. Harbin: Harbin Engineering University, 2006.
[9] Yang Yunshang, Zhang Haixia, Li Chunlei, et al. Study on the ultrasonic extraction process of marigold lutein [J]. Food Research and Development, 2007(1): 97-99.
[10] Jin Limei, Yang Pengfei, Wei Chunhong, et al. Process research on supercritical CO2 extraction of marigold seed oil [J]. Chemical Engineer, 2005(10): 41-45.
[11] Song Dawei, Jia Jian, Zhang Liping. Optimization of process parameters for supercritical extraction of marigold yellow pigment [J]. Processing of Agricultural Products, 2010(8): 36-38.
[12] Yang Zhonglin. Extraction and antioxidant research of lutein from marigold [D]. Wuhan: Hubei University of Technology, 2009.
[13] Kong Yang, Ma Yangmin, Li Yanjun, et al. Process optimization of lutein extraction from marigold by ultrasonic method [J]. China Brewing, 2009, 28(10): 72-74.
[14] Chen Bin, Jin Daxiong. Study on the process of ultrasonic-assisted extraction of lutein from marigold [J]. Guangdong Chemical Industry, 2012, 39(8): 66-68.
[15] Wang Xia, Zhu Jinjin, Cao Yanping. Extraction and detection method of lutein esters in baked foods [J]. Food Industry Science and Technology, 2022, 43( 1) : 304-310.
[16] Ye Zhaowei, Li Xun, Liu Zhuming, et al. Comparison of three extraction methods for lutein from marigold [J]. Hubei Agricultural Sciences, 2014, 53( 4) : 874-876.
[17] Ding Yan, Liu Hongcheng, Ma Wei, et al. Study on the conditions of ultrasonic-assisted saponification extraction of marigold lutein [J]. Journal of Southwest Agricultural University, 2013, 26(5): 2057-2061.
[18] Li Dajing, Liu Chunquan. Research progress on the extraction and analysis methods of marigold lutein [J]. Food Science, 2005, 26(9): 582-586.
[19] Chenggong, Huang Wenshu, Su Yazhou, et al. Study on the process conditions of microwave-assisted extraction of marigold pigments [J]. Chinese Food and Nutrition, 2008(12): 43-46.
[20] Fan Jianfeng, Hao Yufei. Study on the process of microwave-surfactant synergistic extraction of marigold lutein [J]. Modern Chemical Industry, 2008, 28 (supplement 2): 398-400 +402.
[21] Zhang Lingling, Shi Yanfang, Guo Youhong. Study on the extraction process of lutein from medicinal marigold [J]. Strait Pharmacy, 2018, 30( 7) : 33-36.
[22] Zhang Weihong, Li Pengcheng, Xu Sumei, et al. Response surface optimization of ultrasonic-mixed solvent extraction process conditions for carotenoids in marigolds [J]. Food Science and Technology, 2014, 39( 3) : 164-168+ 172.
[23] Zhang Rui, Xing Jun, Zhang Juan, et al. Optimization of the extraction process of carotenoids from marigolds using response surface methodology [J]. Food Industry Science and Technology, 2012, 33(22): 319-322.
[24] Wang Dianbei, Zhang Shenghong, Li Jianhua. Optimization of the extraction process of lutein from marigold using response surface methodology [J]. Northern Gardening, 2012(22): 111-114.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






