Reduced CoQ10 Raw Material: Powering Next-Gen Supplement Innovation
Since its discovery in 1957, Coenzyme Q10 has been hailed as the “source of cellular vitality.” As the only endogenous lipid in the human body with redox functions, it plays an irreplaceable role in cellular energy metabolism and antioxidant defense. However, traditional CoQ10 raw materials face two core challenges in practical applications: low bioavailability and poor stability, severely limiting its innovative use in the health supplement sector.
Technological Breakthrough: The Superior Properties of Reduced Coenzyme Q10
Compared to traditional oxidized CoQ10, reduced Coenzyme Q10 represents a significant technological advancement. Its unique advantages include:
★ Direct Bioavailability
Exists in an active form, enabling physiological functions without requiring conversion within the body
★ Enhanced Antioxidant Efficacy
Significantly improved free radical scavenging capacity
★ Superior Stability
Effectively overcomes photothermal instability issues
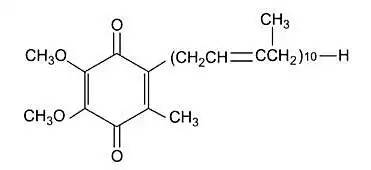
Green Spring Technology Solution: Redefining Raw Material Standards
Based on in-depth research into CoQ10 properties, Green Spring Technology has launched a new generation of reduced CoQ10 raw material with the following core advantages:
① Superior Bioavailability
Patented bioconversion technology ensures the raw material exists in a highly active reduced state, significantly improving absorption efficiency compared to traditional sources.
② Exceptional Stability
Innovative encapsulation processes effectively address photodegradation issues, guaranteeing sustained activity throughout the product's shelf life.
③ Broad Formulation Adaptability
Enhanced hydrophilic-lipophilic balance enables excellent dispersion and compatibility in both water-based and oil-based systems.
Core Value Creation for Customers
Green Spring Technology's reduced CoQ10 raw material provides multi-faceted solutions for health supplement enterprises:
◆ Enables development of innovative products with compelling efficacy claims
◆ Elevates product technical sophistication to build differentiated competitive advantages
◆ Provides robust scientific backing for product efficacy claims
◆ Suitable for developing various dosage forms including softgels, tablets, and powders
1 Green Spring Technology's Highly Stable Reduced Coenzyme Q10 Raw Material Pioneers a New Era of Formulation Innovation
As a key coenzyme in cellular energy metabolism, CoQ10's unique benzoquinone structure plays an irreplaceable role in electron transfer and energy conversion. However, traditional CoQ10 raw materials face multiple challenges in practical applications: their lipophilic nature causes dispersion difficulties in aqueous systems, while light sensitivity compromises product stability. These factors have limited innovative applications in the health supplement sector.
1.1 Technological Breakthrough: Transitioning from Oxidized to Reduced Form
Green Spring Technology leverages advanced bioconversion technology to successfully develop reduced CoQ10 raw materials. Compared to traditional oxidized forms, reduced CoQ10 offers significant biological advantages:
▲ Directly exists in its active form, performing physiological functions without requiring in vivo conversion
▲ Higher bioavailability and antioxidant activity
▲ Enhanced stability, effectively overcoming photodegradation issues
1.2 The Ideal Choice for Formulation Innovation
Based on in-depth research into the physicochemical properties of CoQ10, Green Spring Technology optimizes the raw material through patented techniques:
■ Employing specialized encapsulation techniques to enhance dispersibility in aqueous systems
■ Utilizing microencapsulation technology to significantly improve stability against environmental factors like light and heat
■ Preserving high-efficiency mobility within the mitochondrial electron transport chain

1.3 Scientific Value and Market Prospects
It is noteworthy that certain commonly used medications may impact endogenous CoQ10 synthesis in the human body. Green Spring Technology's reduced CoQ10 raw material offers a premium choice for developing targeted health supplements, particularly suited for:
● Formulation systems requiring high-efficiency antioxidant protection
● Product development supporting cellular energy metabolism
● Innovative solutions to enhance the efficacy of traditional supplements
Green Spring Technology remains committed to innovative R&D in raw material technology, providing the health supplement industry with more premium raw material solutions to help companies create more market-competitive innovative products.
2 Green Spring Technology Achieves Breakthrough in Bioavailability with Reduced CoQ10 Technology
Based on in-depth research into CoQ10 metabolic pathways, Green Spring Technology innovatively launched reduced CoQ10 raw material. This product exists directly in its active form, enabling direct cellular utilization without requiring conversion within the body, thereby significantly enhancing bioavailability.
Additionally, the raw material employs patented stabilization technology to ensure high activity across various formulation systems, providing reliable technical assurance for product innovation.
2.1 Precisely Meeting Diverse Health Needs
Given that CoQ10 synthesis in the body involves a complex process requiring multiple vitamins and trace elements, and is easily affected by factors like age and medication, efficient supplementation is crucial. Green Spring Technology's reduced CoQ10 raw material is particularly suitable for:
◆ Anti-aging health products for middle-aged and elderly populations
◆ Energy supplements for athletes
◆ Preventive products for cardiovascular and cerebrovascular health
2.2 Technology-Driven Product Innovation
Green Spring Technology leverages its comprehensive quality system and professional technical service team to provide customers with high-quality raw materials and customized application solutions. This empowers health supplement companies to overcome technical bottlenecks and develop more market-competitive innovative products.

3 Green Spring Technology Launches High-Potency Reduced CoQ10 Raw Material to Advance Health Industry Upgrades
Green Spring Technology introduces a high-potency reduced CoQ10 raw material produced using advanced biotechnology. Leveraging years of technical expertise in CoQ10, the company's innovative process successfully addresses industry challenges such as low bioavailability and poor stability in traditional raw materials, offering health supplement manufacturers a more competitive ingredient option.
3.1 Technological Breakthrough: Three Core Advantages Setting Industry Standards
3.1.1 Exceptional Bioavailability
Green Spring Technology's reduced CoQ10 features a unique active structure whose molecular form perfectly matches naturally occurring CoQ10 in the human body. Research indicates this raw material can be directly utilized by cells without requiring conversion within the body, significantly improving absorption efficiency compared to traditional oxidized CoQ10.
3.1.2 Superior Antioxidant Performance
Leveraging its reduced molecular structure, this ingredient demonstrates exceptional efficacy in scavenging free radicals and countering oxidative stress. Experimental data confirms its antioxidant activity significantly surpasses that of standard CoQ10, providing enhanced antioxidant support for supplement formulations.
3.1.3 Exceptional Formulation Adaptability
Utilizing Green Spring Technology's patented stabilization technology, the ingredient maintains excellent activity stability across various dosage forms. Its favorable compatibility also enables synergistic use with multiple functional ingredients, opening greater possibilities for product innovation.
3.2 Application Value: Comprehensive Empowerment for Product Innovation
This ingredient demonstrates unique value across multiple application areas:
In energy-boosting products, the ingredient supports ATP synthesis by participating in mitochondrial energy metabolism, delivering core efficacy for anti-fatigue and vitality-enhancing supplements.
In metabolic health applications, its potent antioxidant mechanisms provide scientific rationale for product formulation upgrades, addressing modern consumers' urgent health management needs.
Furthermore, its potential applications in cardiovascular health are gaining significant attention, offering novel technical pathways for developing circulatory system health products through multiple action mechanisms.
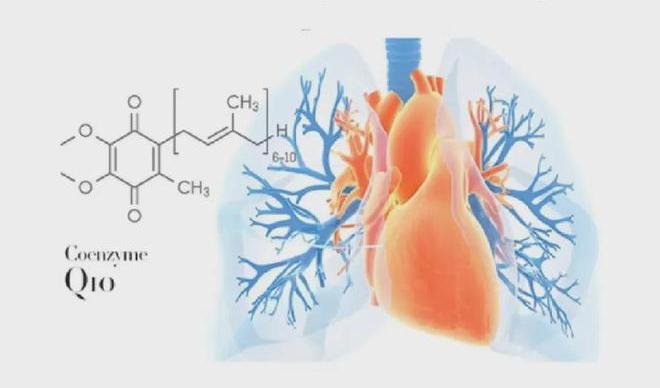
3.3 Quality Assurance: Stringent Standards Ensure Product Safety
Green Spring Technology's Quality Director stated: “We have established a comprehensive quality control system spanning from raw materials to finished products, ensuring every batch meets pharmaceutical-grade quality standards. Concurrently, we provide complete regulatory support documentation to facilitate our clients' rapid product launches.”
As consumer expectations for health product efficacy continue to rise, the innovative advancement of CoQ10 raw materials has become a key driver propelling the health supplement industry forward. Green Spring Technology's high-potency reduced CoQ10 raw material delivers breakthrough formulation solutions for the industry through its exceptional bioavailability and stability performance.
We recognize that raw material innovation is merely the first step toward product success. Green Spring Technology looks forward to deepening collaborations with health food enterprises to jointly explore innovative applications of reduced CoQ10 across diverse scenarios. Whether upgrading existing products or developing entirely new product lines, we provide comprehensive technical support from raw material selection to formulation optimization.
For detailed information on innovative application solutions for reduced CoQ10 raw materials, or to request samples and technical support, please contact us at helen@greenspringbio.com or WhatsApp: +86 13649243917.
References:
[1] Baggio E, Gandini R, PlancherAC, et al. Italian multicenter study on the safety and efficacy of coenzymeQ10 as adjunctive therapy in heart failure.CoQ10 Drug Surveillance Investigators[J]. Mol Aspects Med, 1994;15 Suppl:s287-94.
[2] Yang YK, Wang LP, Chen L, et al. CoenzymeQ10 treatment of cardiovascular disorders of ageing including heart failure, hypertension and endothelial dysfunction [J]. Clin Chim Acta,2015Oct 23;450:83-9.
[3] Mortensen SA, Rosenfeldt F, Kumar A, et al. The effect of coenzymeQ10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial [J]. JACC Heart Fail, 2014 Dec;2(6):641-9.
[4] Garrido-Maraver J, Cordero MD, Oropesa-Avila M, et al. Clinical applications of coenzymeQ10[J]. Front Biosci (Landmark Ed),2014 Jan
1;19:619-33.
[5] Acosta MJ, Vazquez Fonseca L, Desbats MA, et al. Coenzyme Q biosynthesis in health and disease[J]. Biochim Biophys Acta, 2016 Aug;1857(8):1079-85.
[6] Niklowitz P, Onur S, Fischer A, et al.CoenzymeQ10 serum concentration and redox status in European adults: influence of age, sex, and lipoprotein concentration[J].J Clin Biochem Nutr,2016 May;58(3):240-5.
[7] Berthold HK, Naini A, Di Mauro S, et al. Effect of ezetimibe and/or simvastatin on coenzymeQ10 levels in plasma: a randomised trial [J]. Drug Saf, 2006.
[8] Yubero D, Montero R, Armstrong J, et al. Molecular diagnosis of coenzymeQ10 deficiency [J]. Expert Rev Mol Diagn, 2015;15(8):1049-59.
[9] Bhagavan HN, Chopra RK. Potential role of ubiquinone (coenzymeQ10) in pediatric cardiomyopathy [J]. Clin Nutr,24 (2005) 331–338.
-
Prev
Beyond Supply: We Empower Your Brand with Fully-Compliant, Auditable Coenzyme Q10 Ingredient
-
Next
Green Spring's Fermentation-Based CoQ10 Powder Overcomes Bioavailability Limits


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese



