What Are the Benefits of Phycocyanin?
Phycocyanin (abbreviated as PC, absorption spectrum 615-640 nm) is a light-trapping pigment protein commonly found in cyanobacteria for photosynthesis[1] . Phycocyanin has many pharmacological activities, such as anti-inflammatory, anti-tumor, anti-allergy, maintenance of homeostasis, and toxicity reduction and potentiation, etc.[2] , and pharmacological experiments have proved that it has a significant role in regulating the metabolism of free radicals in the organism. Free radicals are involved in the development of many diseases, including inflammation, atherosclerosis, cancer, damage caused by reperfusion, and other dysfunctions induced by oxidative stress [3]. In this paper, the antioxidant activity and pharmacological effects of phycocyanin are summarized, and the relationship between the pharmacological activity of phycocyanin and its antioxidant mechanism is inferred, which will provide a reference for the pharmacological study of phycocyanin.
1 Structure and Function of Phycocyanin
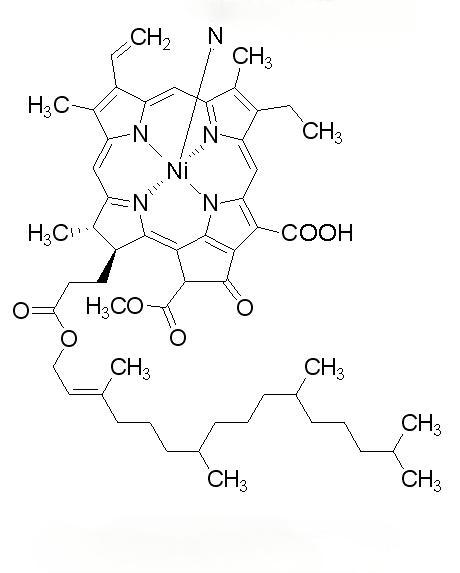
Algae trap light using the rod-like structure of their own phototrapping pigment complex, which contains thousands of phototrapping pigment molecules that help algae to absorb very low levels of sunlight. The C-Phycocyanin (C-PC) and Allophycocyanin (APC) of Spirulina obtusususus gallbladder are noncovalently crosslinked by a connexin protein, and the energy is transferred from the CPC to the APC, and then to the photosynthetic reaction center to promote photosynthesis [4]. The antioxidant effect of phycocyanin is related to its unique structure (Figure 1). In phycocyanin, there is a phenomenon of excitation energy transfer between the light-trapping pigments, tryptophan residues and chromophores [5], which has the ability to transition from the ground state to the excited state, and has the ability to transfer electrons, which involves the redox process.
In addition, Stocker et al.[7] showed that bilirubin has the effect of eliminating peroxyl radicals, and the mechanism is that bilirubin can bind peroxyl radicals to one hydrogen atom of tetrapyrrole molecule at C-10 position, so that the free radicals can form a resonance stabilization with the central carbon atom, and then eventually extend to the whole bilirubin molecule. The open tetrapyrrole chain in the chromophore (phycocyanin) of phycocyanin (Fig. 2A) is very similar to that of bilirubin (Fig. 2B), and may contribute to its antioxidant activity.
Romay[10] firstly reported the antioxidant property of alginate, and evaluated the potential of alginate as an antioxidant in vitro and in vivo, and the experimental results showed that alginate could effectively eliminate hydroxyl and alkyl radicals, which indicated that alginate had the potential to be used as an antioxidant in vitro and in vivo. Halliwell[11] found that alginate also had an inhibitory effect on hepatic microsomal lipid peroxidation, and the amount of superoxide dismutase (SOD) was 3 times higher than that in this experiment, but increasing the amount of SOD did not change the antioxidant activity of alginate. Halliwell [11] found that alginate also inhibited lipid peroxidation in liver microsomes. In this experiment, the antioxidant capacity of superoxide dismutase (SOD) was 3 times higher than that of phycocyanin, but increasing the amount of SOD did not change the antioxidant capacity of phycocyanin, which indicated that they have different antioxidant mechanisms.
In addition, the antioxidant mechanism of alginate is similar to that of commonly used antioxidants, such as tocopherol and ascorbic acid, and it can inhibit hemolysis of erythrocytes induced by 2, 2-azobis(2-squintylpropane) dihydrochloride.12 Hirata et al.[13] studied the antioxidant effects of alginate on the hydrophobic system in the liposomal modes of methyl linoleate and phosphatidylcholine. They showed that the antioxidant activity of phycocyanin (a component of phycocyanin) per mole was higher than that of α-tocopherol in the same molar amount. Moreover, phycocyanin extracted from spray-dried Spirulina showed similar antioxidant activity to that of fresh Spirulina. Since some of the de-coordinated proteins of phycocyanin are denatured during the drying process, these results suggest that phycocyanin contributes significantly to the antioxidant capacity of phycocyanin. These results suggest that phycocyanin contributes significantly to the antioxidant activity of phycocyanin. This study shows that phycocyanin has good antioxidant activity, and that the dried phycocyanin is of great commercial value because of its good stability.
2 Antioxidant Activity of Phycocyanin and Related Pharmacological Activity
2.1 Antioxidant Activity and Anti-Inflammatory Effect of Phycocyanin
Firstly, alginin can inhibit the oxidative luminescence of luminal under alkaline conditions [14], which acts on the respiratory burst of phagocytes and reduces free radicals (-OH, H2O2, RO-) and excess peroxides, thus achieving an inhibitory effect. Evidence[15] suggests that reactive oxygen species, such as superoxide anion, hydrogen peroxide and hydroxyl radical, can cascade arachidonic acid, resulting in mast cell degranulation and release of histamine, 5-hydroxytryptamine, tumor necrosis factor and other inflammatory mediators. Phycocyanin is able to scavenge peroxide, hydroxyl and alkyl radicals. Spillert et al.[16] investigated the inhibitory effect of phycocyanin on hydrogen peroxide-induced inflammation in an in vitro model in order to understand the potential scavenging ability of phycocyanin on H2O2 and -OH. The results showed that alginate reduced the edema induced by glucose oxidase in mouse paws. Thus, the scavenging effect of alginate on -OH gives it an anti-inflammatory effect.
In the same dose range, alginate showed anti-inflammatory activity in carrageenan-induced hind paw edema in rats and in cotton ball granuloma in rats[17] . Alginin significantly reduced arachidonic acid-mediated ear edema in mice and carrageenan-induced foot and plantar edema in rats, which was attributed to scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism. Gonzalez and Fretland et al. [18-19] applied phycocyanin to an animal model of acetic acid-induced ulcerative colitis, and analyzed the colonic tissues and measured the activity of myeloperoxidase. The results showed that the administration of phycocyanin significantly reduced the infiltration of eosinophils into the colonic mucosa of a damaged animal model of colitis, and significantly lowered the production of free radicals and a variety of reactive substances in the disease-inducing myeloperoxidase activity. The results showed that phycocyanin was effective in reducing the incidence of acetic acid-induced colitis in rats, and its anti-inflammatory mechanism was related to its antioxidant activity.
2.2 The Antioxidant Activity of Phycocyanin and the Role of Liver Protection
Bhat et al [20] investigated the pharmacological activity of phycocyanin against hepatotoxicity induced by R-(+)-longleaf menthone and CCl4 in rats. The results showed that phycocyanin significantly reduced the hepatotoxicity induced by the production of large amounts of free radicals by the two compounds. 2002, Remirez et al.[21] investigated the effect of phycocyanin on parameters related to oxidative stress in hepatic blight cells. In 2002, Remirez et al. [21] investigated the effect of alginate on parameters related to oxidative stress in hepatic blight cells, and the results showed that alginate significantly reduced the phagocytosis and related respiratory burst activity of blight cells, which was attributed to the fact that alginate reduced the tumor necrosis factor TNF-α produced by oxidative stress and nitric oxide produced by the hyperthyroid state. Therefore, we believe that the hepatoprotective effect of alginate is mainly attributed to its inhibition of the production of reactive metabolites in the oxidative reaction and its effective elimination of free radicals. In addition, alginate can also inhibit some cytochrome P450-mediated reactions, for example, inhibit the production of reactive metabolites of oxidative reactions involved in P450. Bhat et al.[22] also proved that alginate can inhibit CCl4-induced hepatic lipid peroxidation in rats.
2.3 The Antioxidant Effect of Phycocyanin and the Elimination of Cataracts
Ou et al[23] found that phycocyanin inhibited D-galactose-induced apoptosis of human lens epithelial cells through the mitochondrial and unfolded protein response pathways. Since apoptosis of lens epithelial cells is an important cause of cataract formation, prevention of LEC apoptosis may be a therapeutic strategy for cataracts.Kumari et al.[24] also investigated the modulating effect of phycocyanin on sodium selenite-induced cataracts in rats. The experimental results showed that phycocyanin could reduce oxidative stress by regulating the levels of antioxidant enzymes in vivo and ex vivo, thus reducing the incidence of cataract induced by sodium selenite.
2.4 Antioxidant Activity and Vasoprotective Effects of Phycocyanin
Ross[25] suggested in 1999 that atherosclerosis is an inflammatory disease characterized by chronic inflammatory reaction. Riss et al.[26] demonstrated that phycocyanin in Spirulina can inhibit the production of reactive radicals and cyclooxygenase-2, increase the level of antioxidant enzymes in vivo, effectively improve the inflammatory damage caused by oxidative stress in atherosclerotic animals, and regulate the effect of blood lipids. Since the formation of atherosclerotic lesions is a result of inflammatory-fibro-proliferative response of arteries to endothelial injury, Chu et al.[27] investigated the inhibitory effect of Spirulina obtusususifera alginate on the hyperproliferation of vascular smooth muscle cells, endothelial proliferation and luminal stenosis after vascular injury through in vivo and ex vivo experiments.
It has been demonstrated that phycocyanin can reduce oxidative inflammatory damage in blood vessels and inhibit luminal narrowing by inhibiting the G1/S cycle and suppressing the excessive proliferation of VSMCs and the formation of new endothelial membranes, thus proving that phycocyanin antioxidant activity has a good preventive and health care effect on vascular health. Strasky et al[28] investigated the activation effect of alginate on heme oxygenase-1, an enzyme that enables the breakdown of heme to produce a potent antioxidant bilirubin. Strasky et al. [28] studied the activation of heme oxygenase-1 by phycocyanin, an enzyme that metabolizes heme to produce potent antioxidant bilirubin. Experimental results showed that phycocyanin in Spirulina reduced oxidative stress and activated HMOX1 in endothelial cells, resulting in increased expression of HMOX1 in atherosclerotic lesions in ApoE-deficient mice. This also provides a new rationale for algin to reduce atherosclerosis by increasing the expression of heme oxygenase-1 to increase the antioxidant effect.
2.5 Antioxidant Activity and Neuroprotective Effects of Phycocyanin
Decreased antioxidant capacity and increased nitrogen-oxygen reactive radicals are strongly associated with aging of human organs and neurodegenerative diseases[29-31] . Injections of SOD into certain animal models have been found to inhibit the inflammatory response in these models and to increase the expression of certain molecules of immune function in isolated immune cells as well as in animal and human bodies[32] . Many clinical trials have reported that cytokine expression is significantly increased in the cerebrospinal fluid and brain tissue of patients with brain injury or infarction [33-34]. The antioxidant property of alginate may act on cytokines to repair brain damage and inhibit cell necrosis in a timely manner.
Rimbau et al.[35] found that alginate could reduce erythrocyanine-induced epileptic responses in the hippocampus of rats and had a protective effect on neurons. The reason why erythrocyanine causes epilepsy is that it generates a large number of oxygen-active free radicals, and alginate can protect neuronal damage by reducing the free radicals. This experiment suggests that alginate can be used to treat oxidative stress-induced neuronal damage in neurodegenerative diseases, such as Alzheimer's disease and Parkinson's syndrome. In addition, Rimbau et al.[36] found that algin protects cerebellar granule cell death in potassium- and serum-deficient cultured rats in vitro by reducing oxygen free radicals, and Marin et al.[37] demonstrated that algin protects SH-SY5Y neuronal cells from oxidative damage, and reduces Ca2+/phosphate-induced functional impairment of rat retinal transient ischemia and rat mitochondrial mitochondria.
2.6 Antioxidant Activity of Phycocyanin and Kidney and Lung Organs
Shukkur et al[38] found that phycocyanin prevented oxidative stress-mediated cellular damage triggered by oxalic acid in canine kidney cells, reduced oxalic acid-induced reactive oxygen species (ROS) and lipid peroxidation (LPO), and had a significant protective effect on mitochondrial membrane permeability.39 Zheng et al[40] found that Spirulina algal cyanobacterial proteins and phycocyanin could prevent diabetic nephropathy by inhibiting oxidative stress, Zeng et al.[39] found that Spirulina cyanobacteria and phycocyanin could prevent diabetic nephropathy by inhibiting oxygenation stress.
Oral administration of alginate for 10 weeks prevented proteinuria and mesangial dilatation, normalized the expression of tumor growth factor-β and fibronectin, and reduced the expression of NADPH oxidase, a marker of oxidative stress, in type 2 diabetic mice (db/db mice). In addition, Gonzalez et al.[40] demonstrated that alginate in combination with kanamycin reduced renal tubular vascular congestion and inflammatory infiltration in mice, and decreased the nephrotoxicity of kanamycin, an aminoglycoside antibiotic. SOD activity, decreased hydroxyproline (HYP), malondialdehyde (MDA) and plasma MDA in lung tissue, reduced the degree of alveolitis and fibrosis in paraquat-intoxicated rats, and had a significant inhibitory effect on paraquat-induced alveolitis and pulmonary fibrosis in rats.
2.7 Antioxidant Activity and Tumor Prevention of Phycocyanin
The results of o-toluene red bleaching assay[42] showed that algin cofactor had a stronger scavenging effect on peroxynitrite anion than phycocyanin, and that phycocyanin significantly inhibited DNA single-strand breaks caused by peroxynitrite anion in a dose-dependent manner, which may provide a basis for the prevention of cellular carcinogenesis. -Gupta et al. [43] investigated the protective effect of phycocyanin on skin oncogenes in mice exposed to 12-O-Tetradecanoylphorbol-13-acetate (TPA). The use of phycocyanin inhibited the TPA-induced expression of key tumorigenic factors, such as ornithine decarboxylase, cyclooxygenase-2, interleukin 6, and phosphorylated signal transducer and activator of transcription 3, in a dose-dependent manner in TPA-induced mice. Thangam et al [44] investigated the antioxidant properties of phycocyanin and its ability to inhibit cancer cell growth. Fluorescence and phase contrast microscopy showed that phycocyanin inhibited the growth of HT-29 (colon cancer) and A549 (lung cancer) cells, and caused DNA arrest and cleavage of cancer cells at G(0)/G(1) phase.
Fernandez et al[45] studied the effect of alginate on cisplatin-induced nephrotoxicity, and the results showed that alginate could prevent cisplatin-induced decrease in glutathione reductase, reduce the content of hydrogen peroxide, maintain the level of urea nitrogen in the blood, and inhibit oxidative stress, and had a good effect on cisplatin-induced nephrotoxicity. It has a good inhibitory effect on oxidative stress and inhibits the nephrotoxicity caused by cisplatin. In addition, many years of cancer research have proved that the appropriate combination of drugs can effectively improve the safety and efficacy of a single drug in the treatment program.46 In 1998, Xin Huawen et al.[47] studied the in vitro potentiation of methotrexate and cisplatin by alginate. The results showed that the combination of alginate and methotrexate significantly enhanced the cytotoxicity of the latter, and the potentiation effect increased with the increase of methotrexate concentration, and there was a highly significant difference in the cell viability of cells without alginate and those with alginate, which was similar to that of the existing potentiator verapamil (a calcium channel inhibitor), but with less toxicity.
Miroslav et al[48] showed that the combination of 10% conventional dose of topotecan with phycocyanin was more effective than conventional dose of topotecan alone, activating large amounts of cysteine aspartate proteinase-9 (caspase-9) and cysteine aspartate proteinase-3 (caspase-3). The effect of topotecan was increased while the occurrence of side effects was reduced. Saini et al.[49] also demonstrated in the same year that the combination of piroxicam, a traditional NSAID, and phycocyanin increased the effect by more than 70% compared with that of single use, and the expression of cyclooxygenase 2 (COX-2) and the level of prostaglandin E2 (PGE-2) were greatly reduced, and the inhibition of DNA breakage was also demonstrated. It is not difficult to see that the antioxidant effect of alginate plays a role in improving immunity and protecting damaged organs, and the combination of alginate as a photosensitizer with existing clinical anticancer drugs will play an important role in increasing the efficacy of chemotherapy.
3 Summary and Outlook
The antioxidant activity of recombinant alloxyalanine in our laboratory[50-51] showed that recombinant alloxyalanine has the ability to scavenge free radicals, but its scavenging effect on different types of free radicals varies greatly. Since the structural composition of albumin is similar to that of alloxan, the cofactor proteins of albumin also have similar antioxidant activities[52] . Subsequently, it was further verified that the scavenging effect of phycocyanobilin (PCB) on DPPH radicals showed a certain quantitative effect relationship[53] ; the latest results of Pleonsil et al.[54] showed that the antioxidant activity of natural phycocyanobilin was greater than that of recombinant phycocyanobilin. The antioxidant activity of phycocyanin is partly attributed to phycocyanin[13] , and cofactor proteins should have different antioxidant effects from phycocyanin[52] , which indirectly confirms that phycocyanin scavenges free radicals at the level of both de-cofactorized proteins and phycocyanin.
Nowadays, it is possible to obtain phycocyanins of high purity by means of sophisticated chemical and biological engineering techniques. However, the activity varies depending on the raw material and the extraction process. The good antioxidant activity of algal cyanoproteins can be the basis for applications for nutraceuticals and drug candidates, the success of which depends on good quality control.

While traditional open culture of algal cyanobacteria requires a lot of conditioning to achieve constant quality, industrial recombinant expression is more controllable, but it is difficult to bioengineer the assembly of phycocyanin into the protein subunits of polymers, and further research is needed to determine the activity and quality of the resulting algal cyanobacterial proteins. In addition, the metabolites and derivatives of phycocyanin are complex. To determine whether the active effect of phycocyanin is due to its metabolites as a whole or through oral or injectable routes of administration is the next goal of our research. It is mentioned in the paper that alginate can play an antioxidant role in different disease organs, how alginate enters the cell and how it works is the key to research. The large-scale cultivation of Spirulina has laid the foundation for the development and utilization of algal cyanoproteins, and its functional food has explored its application. Therefore, in the future, in-depth research on the activity and mechanism of algal cyanobacterial protein and clinical trials will open up more space for its application.
References:
[1] Eriksen N T. Production of phycocyanin - a pigment with applications in biology, biotechnology, foods and medicine [J]. Appl Microbiol Biotechnol, 2008, 80(1): 1-14.
[2] WANG Tingjian, LIN Fan, QIN Song. Algal bile proteins and their applications in medicine[J]. Plant Physiology Letters, 2006, 42(2): 303-307.
[3] Kehrer J P. Free radicals as mediators of tissue injury and disease [J]. Crit Rev Toxicol, 1993, 23: 21-48.
[4] WANG Guangze, ZHOU Baicheng, ZENG Chengkui. Construction of an energy transfer model for C-phycocyanin and iso-phycocyanin in Spirulina obtususus[J]. Science Bulletin, 1996, 40(8): 741-743.
[5] WANG Guangze, ZENG Chengkui. Study on the energy transfer between different groups in the C-phycocyanin molecule of Spirulina obtususus[J]. Oceans and Lakes, 1998, 29(5): 471-475.
[6] Grossman A R, Schaefer M R, Chiang G G. Thephycobilisome, alight-harvesting complexes ponstveto environmental conditions[J]. Microbiological Reviews, 1993, 57(3): 725-749.
[7] Stocker R, Yamamoto Y, Mcdonagh A F, et al. Bilirubin is an antioxidant of possible physiological importance [J]. Science, 1987, 235: 1043-1046.
[8] Gossauer A, Hirsch W. Syntheses of bile-pigments. 4. total synthesis of racemic phycocyanobilin (phycobiliverdin) and of ahomophycobiliverdin [J ]. Annalen Der Chemie- Justus Liebig, 1976, 9: 1496-1513.
[9] Bonnett R, Davies J E, Hursthouse M B. Structure of bilirubin [J]. Nature, 1976, 262: 327-328.
[10] Romay C, Armesto J, Remirez D, et al. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae [J]. Inflamm Res, 1998, 47(1): 36-41.
[11] Halliwell B. How to characterize a biological antioxidant [J]. Freerad Res Comm, 1990, 9: 1-32.
[12] Romay C, Gonzalez R. Phycocyanin is an antioxidant protective of human erythrocytes against lysis by peroxyl radicals [J]. J Pharm Pharmacol, 2000, 52: 367-368.
[13] Hirata T, Tanaka M, Ooike M, et al. Antioxidant activities of Phycocyanobilin prepared from Spirulina platensis [J]. ApplPhycol, 2000, 435-439.
[14] Romay C, Ledon N, Gonzalez R. Phycocyanin extract reduces leukotrienes B4 levels in arachidonic acid-induced mouse ear inflammation test [J]. J Phar Pharmacol, 1999, 51: 641-642.
[15] Lissi E A, Pizarro M, Romay C, et al. Kinetics of phycocyanin bilin groups destruction by peroxyl radicals [J]. Free Radic Biol Med, 2000, 28: 1051-1055.
[16] Spillert C R, Pelosi M A, Parmer L P, et al. A peroxide-induced inflammation model for drug-testing [J]. Agents and Actions, 1987, 21: 297-298.
[17] Romay C, Ledon N, Gonzalez R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation [J]. Inflammation Research, 1998, 47 (8): 334-338.
[18] Gonzalez R, Rodriguez S, Romay C, et al. Anti-inflammatory activity of Phycocyanin extract in acetic acid-induced colitis in rats [J]. Pharmacological Research, 1999, 39(1): 55-59.
[19] Fretland D J, Djuric S W, Gaginella T S. Eicosanoids and inflammatory bowel disease: regulation and prospects for therapy [J]. Prost Leukotr Ess Fatty Acids, 1990, 41(4): 215-233.
[20] Vadiraja B B, Gaikwad N W, Madyastha K M. Hepatoprotective effect of C-Phycocyanin: Protection for carbon tetrachloride and R-(+)-pulegone- mediated hepatotoxicity in rats [J]. Biochem and Biophys Res Com, 1998, 249(2): 428-431.
[21] Remirez D, Fernandez V, Tapia G, et al. Influence of C-phycocyanin on hepatocellular parameters related to liver oxidative stress and Kupffer cell functioning [J]. Inflammation Research, 2002, 51(7): 351-356.
[22] Bhat V B, Madyastha K M. C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro [J]. Biochem Biophys Res Comun, 2000, 275(1): 20-25.
[23] Ou Y, Yuan Z J, Li K P, et al. Phycocyanin may suppress D-galactose-induced human lens epithelial cell apoptosis through mitochondrial and unfolded protein response pathways [J].Toxicology Letters, 2012, 215(1): 25-30.
[24] Kumari R P, Sivakumar J, Thankappan B, et al. C-Phycocyanin Modulates Selenite-Induced Cataractogenesis in Rats [J]. Biological Trace Element Research, 2013, 151(1): 59-67.
[25] Ross R. Atherosclerosis an inflammatory disease [J]. New Engl J Med, 1999, 340(2): 115.
[26] Riss J, Decorde K, Sutra T, et al. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters [J]. J Agric Food Chem, 2007, 55(19):7962-7967.
[27] Chu Xianming, Leng Min, Li Bing, et al. Mechanism of inhibition of vascular smooth muscle cell hyperproliferation and luminal stenosis by phycocyanin[J]. Chinese Journal of Pharmacology, 2012, 28(10): 1383-1388.
[28] Strasky Z, Zemankova L, Nemeckova I, et al. Spirulina platensis and phycocyanobilin activate atheroprotective heme oxygenase-1: a possible implication for atherogenesis [J]. Food & Function, 2013, 4(11): 1586-1594.
[29] Harman D. Aging: to theory based on free radical and radiation chemistry [J]. J Gerontol, 1956, 11(3): 289-300.
[30] Leibovitz B E, Siegel B V. Aspects of free radical reactions in biological systems: aging [J]. J Gerontol, 1980, 35(1): 45-56.
[31] Ames B N, Shigenaga M K, Hagen T M. Oxidants, antioxidants, and the degenerative diseases of aging [J]. Proc Nati Acad Sci USA, 1993, 90(17): 7915-7922.
[32] Han S N, Meydani S N. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans [J]. J Infect Dis, 2000, 182: 74-80.
[33] Lynch M A. Age-related impairment in long-term potentiation in hippocampus: A role for the cytokine, interleukin-1 beta? [J]. Prog Neurobiol, 1998, 56: 571-589.
[34] Knoblach S M, Fan L, Faden A I. Early neuronal expression of tumor necrosis factor-quadrature after experimental brain injury contributes to neurological impairment [J]. J Neuroimmunol, 1999, 95: 115-125.
[35] Rimbau V, Camins A, Romay C, et al. Protective effect of C-phycocyanin against kainic acid-induced neuronal damage in rat hippocampus [J]. Neuroscience Letters, 1999, 276: 75-78.
[36] Rimbau V, Camins A, Pubill D, et al. C-phycocyanin protects cerebellar granule cells from low potassium/ serum deprivation-induced apoptosis [J]. Naunyn Schmiedebergs Arch Pharmacol, 2001, 364(2): 96-104.
[37] Marin-Prida J, Penton-Rol G, Rodrigues F P, et al. C-Phycocyanin protects SH-SY5Y cells from oxidative injury, rat retina from transient ischemia and rat brain mitochondria from Ca2+/phosphate-induced impairment [J]. Brain Research Bulletin, 2012, 89(5-6): 159-167.
[38] Farooq Shukkur M, Boppana Nithin B, Asokan Devarajan, et al. C-Phycocyanin confers protection against Oxalate-Mediated oxidative stress and mitochondrial dysfunctions in MDCK cells [J]. Plos One, 2014, 9(4).
[39] Zheng J, Inoguchi T, Sasaki S, et al. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress [J]. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 2013, 304(2): 110-120.
[40] Gonzalez Nunez, Rodriguez Salgueiro, Barco Herrera, et al. Phycocyanin accelerates recovery of renal tissue damaged by kanamycin overdosein rodents [J]. Acta Microscopica, 2012, 21(3): 147-154.
[41] Sun Y X, Zhang J, Yan Y J, et al. The protective effect of C-phycocyanin on paraquat-induced acute lung injury in rats [J]. Environmental Toxicology and Pharmacology, 2011, 32(2): 168-174.
[42] Bhat V B, Madyastha K M. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: protection against oxidative damage to DNA [J]. Biochemical and Biophysical Research Communications, March 2011
2001, 285, 262-266.
[43] Gupta N K, Gupta K P. Effects of C-Phycocyanin on the representative genes of tumor development in mouse skin exposed to 12-O-tetradecanoyl-phorbol -13- acetate [J]. Environmental Toxicology and Pharmacology, 2012, 34(3): 941-948.
[44] Thangam R, Suresh V, Princy W A, et al. C-Phycocyanin from Oscillatoria tenuis exhibited an antioxidant and in vitro antiproliferative activity through induction of apoptosis and G(0)/G(1) cell cycle arrest [J]. Food Chemistry, 2013, 140(1-2): 262-272.
[45] Fernandez-Rojas, Noel Medina-Campos, Omar Hernandez- Pando, et al. C-Phycocyanin prevents cisplatin- induced nephrotoxicity through inhibition of oxidative stress[J]. Food & Function, 2014, 5(3): 480-490.
[46] Jiang M. Study of regular combinations of drugs and their pharmacological significance[J]. Journal of Chinese Medicine Literature, 2006, 47(4): 243.
[47] XIN Huawen, WANG Runbang, OU Yang Sha Huai, et al. In vitro potentiation of methotrexate and cisplatin by alginate[J]. Journal of Hubei Medical University, 1998, 19(1), 22-24.
[48] Gantar M, Dhandayuthapani S, Rathinavelu A. Phycocyanin Induce Apoptosis and Enhances the Effect of Topotecan on Prostate Cell Line LNCaP [J]. Journal of Medicinal Food, 2012, 15(12): 1091-1095.
[49] Saini M K, Vaiphei K, Sanyal S N. Chemoprevention of DMH-induced rat colon carcinoma initiation by combination administration of piroxicam and C- phycocyanin [J]. Mol Cell Biochem, 2012, 361(1-2): 217-228.
[50] Ge B S, Qin S, Han L. Antioxidant properties of recombinant allophycocyanin expressed in Escherichia coli [J]. Journal of Photochemistry and Photobiology B-Biology, 2006, 84(3): 175-180.
[51] HAN Lu, GE Baosheng, QIN Song, et al. Antioxidant activity of several recombinant algal cyanoproteins[J]. Antioxidant activity of several recombinant algal cyanoproteins[J]. Marine Science, 2007, 31(8): 71-74.
[52] ZHOU Zhan, CHEN Xiu, ZHANG Yuzhong, et al. Influence of algal bile proteins on their antioxidant activities[J]. Marine Science, 2003, 27(5): 77-80.
[53] Chen YJ, Liu SF, Qin S, et al. Interaction of antioxidant-active algal blue pigments with bovine serum albumin[J]. Interaction of antioxidant active phycocyanin with bovine serum albumin[J]. Journal of Pharmaceutical Analysis, 2011, 31(1): 87-89.
[54] Pleonsil Pornthip, Soogarun Suphan, Suwanwong Yaneenart. Antioxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes [J]. International Journal of Biological Macromolecules, 2013, 60: 393-398.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






