Study on Rare Ginsenoside Rg1 Rb1
Ginseng (Panax ginseng C. A. Meyer) is a perennial herb in the family Araliaceae and is a traditional precious Chinese medicine. Ginsenosides are one of the main active ingredients of ginseng. They belong to the group of triterpenoid glycosides and are formed by the condensation of a sugar and a glycoside precursor. Studies have shown that ginsenosides have a variety of pharmacological effects [1-3]. After oral administration, most protopanaxadiol-type ginsenosides are hydrolyzed by intestinal flora to ginsenoside C-K, Rg3, Rh2 and PPD [4-5], and protopanaxatriol-type ginsenosides are mainly degraded to ginsenoside F1 and 20(S)-PPT[6- 7]. The metabolite C-K of protopanaxadiol-type ginsenosides has significant antitumor activity in vitro and in vivo, and its activity is enhanced compared to the precursor[8]. Rg1, Re and 20(S)-PPT have a strong anti-tumor cell metastasis effect after oral administration, while only 20(S)-PPT has an anti-tumor effect when administered intravenously, indicating that the anti-tumor metastasis effect of Rg1 and Re after oral administration is produced by their metabolite 20(S)-PPT [9].
A large number of studies have shown that ginsenoside is deglucosylated by intestinal bacteria to produce rare ginsenosides with two or single glycosidic bonds, which have stronger biological activity [10].

Biotransformation can change the chemical structure of ginsenosides, effectively improve the in vivo utilization of ginsenosides, optimize the clinical efficacy of the drug, and reduce adverse reactions [11]. The biotransformation of ginsenosides includes changes in glycosylation, hydroxylation, and double bonds. The glycosylation of ginsenosides mainly occurs at C-3, C-6 and C-21, including glycoside hydrolysis and glycosylation; some transformations also occur on the C-3, C-12 and C-17 side chains, connecting methyl groups and hydroxyl groups; the modification of double bonds mainly involves the addition or oxidation of the C-24/C-25 double bond. This article reviews the development of microbial biotransformation of rare ginsenosides in recent years, the structural changes of the parent nucleus and the application of its derivatives, with the hope of providing a theoretical basis for the structural research and application of ginsenosides and their derivatives.
1 Structure and application of rare ginsenosides
1.1 Structure of rare ginsenosides
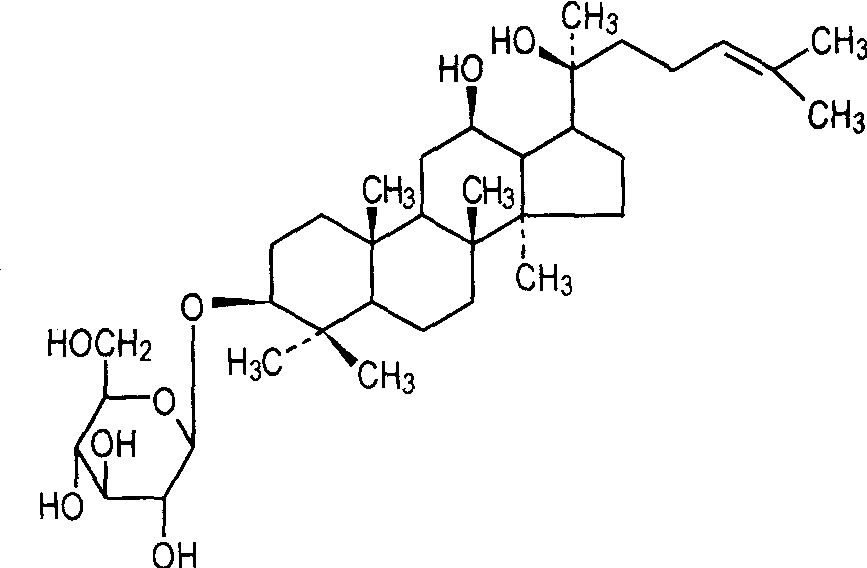
Ginsenosides are divided into tetracyclic triterpenoids and pentacyclic triterpenoids according to the structure of the aglycone. Protopanaxadiol-type ginsenosides (Fig. 1a) and protopanaxatriol-type ginsenosides (Fig. 1b) are both dammarane-type tetracyclic triterpenoid ginsenosides, Oxytirone type saponins (Figure 1c) are a type of tetracyclic triterpene saponin with a side chain containing a furan ring; oleanane type saponins with oleanolic acid as the parent nucleus belong to the pentacyclic triterpene saponins (Figure 1).
The name “rare ginsenosides” is derived from the fact that they are naturally present in low or almost non-existent quantities. Rare ginsenosides are mainly damascan-type saponins, with no more than three sugar moieties attached to the C-3, C-6 and C-20 positions of the aglycone, and with no more than two sugar moieties attached to a single position. Rare ginsenosides are mainly obtained by deglycosylation or dehydration of ginsenosides. At present, the main rare ginsenosides identified are Rg3, Rg1, Rh1, Rh2, Rh3, RT3, F1, F2, C-K, PPD, PPT, etc., as shown in Table 1.
1.2 Application of rare ginseng saponins
Rare ginseng saponins are mainly found in red ginseng and black ginseng, and are the basis of the physiological activity of red ginseng and black ginseng, which is different from that of ginseng. Ginseng powder and ginseng tablets sold on the market only grind the raw materials (ginseng, American ginseng, etc.) from which ginseng saponins are extracted into powder, or add excipients and then press into tablets. Ginseng saponin tablets and capsules only extract the total ginsenosides from the medicinal herb, without further separation or transformation. This directly extracted ginsenoside needs to be broken down by specific enzymes before it can be absorbed by the body. However, these enzymes are either rare or absent in the human body. This results in low bioavailability and weak pharmacological effects in the human body.
At present, most of the rare ginsenoside preparations in China contain the monomeric components Rh2 or Rg3. Preparations containing only one type of ginsenoside monomer are shown in Table 2.
Common rare ginsenosides such as ginsenoside C-K, Rg3 and Rh2 have been used in clinical applications to improve the body's immune health or in combination with other drugs for adjuvant therapy. Meanwhile, the application research of rare ginsenosides is still an important part of ginsenoside development. Using 20(R)-ginsenoside as a raw material, the researchers synthesized a new ginsenoside glycinate derivative through an esterification reaction between the C-3 position and tert-butoxycarbonyl glycine. and determined that the anti-tumor activity of this derivative is more than 100 times that of 20(R)-ginsenoside (IC50 >30 mmol/L). The in vivo inhibitory effect of A11 is more significant than that of 20(R)-ginsenoside (P<0.01). At the same time, A11 dose-dependently inhibits the proliferation, migration and invasion of HeLa cells and promotes apoptosis [12]. Ginsenoside Rg4 is a rare ginsenoside found in ginseng leaves and black ginseng. Rg4 inhibits inflammation and exhibits protective effects against CLP-induced sepsis [13]. Rg5 forms a stable complex with human purinergic receptor 12 (P2RY12) through allosteric interactions, reducing its activity via residues E188 and R265, resulting in a decrease in the release of interleukin (IL)-6, IL-1β and tumor necrosis factor-α in the blood plasma, improving the inflammatory response while inhibiting the formation of venous thrombi [14]. Ginsenoside Rk1 has anti-tumor [15], regulates blood sugar [16-17], protects the nervous system [18-19], and when used in combination with ginsenoside Rk5, promotes osteoblast differentiation and growth by increasing alkaline phosphatase activity, thereby treating osteoporosis [20]. As research progresses, more biological activities of rare ginsenosides and their derivatives will be discovered.

2 Research status of the biotransformation of rare ginsenosides
The main methods of ginsenoside transformation are chemical transformation and biotransformation. Chemical transformation mainly uses reactions such as acid-base hydrolysis of glycosidic bonds, oxidative addition and acetylation to change substituents. Biotransformation uses microbial cells to modify the structure of exogenous substrates, and uses one or more enzymes produced during metabolism to catalyze reactions on specific parts (groups) of the substrate, thereby increasing the content and medicinal properties of the active ingredients [21]. Bioconversion methods include bacterial transformation, intestinal flora transformation, fungal transformation and in vitro enzyme catalysis, which make up for the shortcomings of chemical transformation methods, such as drastic reaction conditions, environmental pollution and the generation of by-products. They are widely used in research and production. The main reaction types of biotransformation include glycoside hydrolysis reactions and redox reactions [22], among which glycoside hydrolysis reactions are the most widely used [23]. In recent years, the addition reaction at the C-24 and C-25 positions has also been gradually applied in practical production.
2.1 Glycoside hydrolysis of rare ginseng saponins
2.1.1 Microbial glycoside hydrolysis of ginseng saponins
The metabolic pathway of ginseng saponins in vivo is an important part of the research on the structure-activity relationship of ginseng saponins. Stepwise deglycosylation is the main metabolic pathway of ginseng saponins in vivo. The rare ginseng saponins and aglycones produced by metabolism have significant pharmacological effects in the body. The main transformation products of ginsenoside Rd were found to be F2, Rg3, C-K, Rh2 and PPD; the main transformation products of ginsenoside F2 were C-K and PPD; the main transformation products of ginsenoside Rg3 are Rh2 and PPD[24]; and C-K and Rh2 can be converted to PPD[25]. Through the analysis of the transformation products of protopanaxatriol-type saponins Re, Rg1, Rh1, Rf, F1 and R1 in the human intestinal flora, their metabolites and transformation pathways were determined i.e., the conversion pathway of ginsenoside Re is Re →Rg1/Rg2 →Rh1/F1 →PPT; the conversion pathway of ginsenoside Rg1 is Rg1 →Rh1/F1 →PPT; the conversion pathway of ginsenoside Rf is Rf→Rh1 →PPT; and the metabolic pathway of notoginsenoside R1 is R1 →Rg1/Rg2 →Rh 1 →PPT[26].
The metabolic pathway of ginsenoside Rb1 by intestinal bacteria in vitro is a process of deglucosylation, and the hydrolysis of ginsenoside Rb1 by intestinal bacteria in vitro is also a stepwise deglucosylation process[27]. The same is true for ginsenoside metabolism by intestinal bacteria in vivo. GUO et al. [28] used broad-spectrum antibiotics to construct a model of germ-free rats and verified the conversion of notoginseng saponins (1.535 g/kg) by the intestinal microbiota in rats. The results showed that the four metabolites ginsenoside F1, Rh2, C-K and PPT were detected in the plasma of the germ-free rats, but not in the plasma of the germ-positive rats. Therefore, it is inferred that the basic law of ginsenoside metabolism in vivo is tetraglycoside → trisaccharide → disaccharide → monosaccharide → aglycone.
There are many types of enzymes present in microorganisms, which have the potential to convert glycosides [29]. At the same time, the sugars removed after fermentation can be used as a carbon source for fungal growth and reproduction, thereby metabolizing to produce more enzymes [30]. Therefore, different microorganisms can be used as biocatalysts to play an important role in the biotransformation of natural products. Scholars at home and abroad have used bacteria and fungi to conduct biotransformation research on ginsenosides (Figure 2).
Studies have shown that some fungi and bacteria such as Aspergillus niger, Fusarium sp., Penicillium sp., Cordyceps sinensis and Armillaria mellea, Bifidobacterium breve, Bacillus sp., Lactococcus lactis and Lactobacillus plantarum sub sp, Terrabacter sp., Cellulosimicrobium cellulans sp., and Thermotoga thermarum can metabolize the sugar groups on ginsenoside C-3 or C-20 to produce rare ginsenosides or aglycones (Table 3). Aspergillus niger can metabolize ginsenoside Rb1 and Rb3 to ginsenosides Rd, F2 and C-K, respectively [31, 38]; it can also hydrolyze the 3-O-Glc of ginsenosides Rb2 and Rc first, and then hydrolyze the 20-O-Ara to produce a single hydrolysis pathway: Rb2 → C-O → C-Y → C-K, Rc → C-Mc1 → C-Mc → C-K, and mainly through the pathway of Rb3 → C-Mx1 → C-Mx → C-K to hydrolyze the 3-O-Glc of Rb3, and slowly hydrolyzes Rb3 20-O-Xly via the pathway Rb3 → Rd → F2 → CK [38]. Penicillium hydrolyzes ginsenosides and ginsenoside monomers via the pathway Rb1 → Rd → F2 → C-K, producing rare ginsenosides [32-33,52]; Fusarium converts ginsenoside total saponins to rare ginsenoside C-K through fermentation culture [47]; Bifidobacterium fermentum can convert ginsenoside F2 to ginsenoside C-K [39]; Bacillus subtilis hydrolyzes the C-3 and C-20 positions of the ginsenoside Rb1 at the C-3 and C-20 positions of the aglycone, respectively, to generate ginsenosides Rd, Rg3[39] and Gyp-xⅦ, F2[40]. The glucosidase genes cloned from Lactococcus lactis[36] and Thermus thermophilus[42-43] were expressed in Escherichia coli to produce glucosidases that can gradually hydrolyze the external and internal glucose parts of the C-20 and C-3 positions of ginsenoside Rb1. The reaction is as follows: Rb1 → Rd → Rg3 (S) and F2 → C-K.
In recent years, edible fungi have also been used in the study of the glycosidase hydrolysis of ginseng saponins. For example, Cordyceps militaris can convert ginseng saponin Rb1 to the rare ginseng saponin F2, with the conversion pathway being Rb1→Rd→F2 [44]. The honey ring fungus can convert ginsenoside Rb2 into the rare ginsenosides C-Y and C-K, with the conversion pathway being Rb2→C-Y→C-K [45]. This is the first time in the literature that a basidiomycete has been used to convert ginsenoside Rb2 into the rare ginsenoside C-K.
Microbial transformation has its own unique advantages. The reaction conditions are mild, and compared with physical and chemical transformation methods, it does not require high temperatures and pressures, which reduces costs. During the reaction, almost no organic reagents are used, which can ensure the activity of ginsenosides to the greatest extent. The enzymes secreted by microorganisms can decompose and consume impurities such as sugars and proteins, increase the concentration of ginsenosides, and increase the conversion rate. The microbial conversion method has high raw material utilization efficiency and high conversion efficiency, which not only saves raw materials but also increases production [46].
2.1.2 Enzyme hydrolysis of the sugar group of ginsenosides
The specificity of microbial conversion is lower than that of direct enzyme conversion, so the activity of glycosidase needs to be adjusted to limit the production of by-products and improve the specificity of the conversion.
Saponins have structural differences and functional diversity. Ginseng saponin structure includes 1 to 4 glycosidic bonds. Common sugar moieties include β-glucose, L-arabinose, D-xylose and L-rhamnose, which require the synergistic action of different enzymes to achieve bioconversion. Common enzymes include glucosidase, mannosidase, arabinosidase, and xylosidase, which are mainly isolated and purified from microorganisms or animals and plants. In the study of enzymatic conversion of ginsenosides, researchers first used enzymes produced by natural microorganisms to hydrolyze the glycosidic bonds of ginsenosides. For example, using Monascus purpureus, which can produce β-glucosidase extracellularly, to ferment ginsenosides achieved a 2.3-fold increase in the mass fraction of Rg3 in ginsenosides [57]. As research progressed, researchers extracted relatively pure β-glucosidase from fungi, animals and plants to hydrolyze ginsenosides. Jin et al. [58] used β-glucosidase to convert the ginsenosides Rb1, Rb2, Rc and Rd were converted into the rare saponin Rh2 using β-glucosidase. However, the enzymes isolated from microorganisms and animals and plants are mostly mixed enzymes, which are difficult to convert in a targeted manner and have many side reaction products, which increases the difficulty of isolating and purifying the conversion products.
With the development of molecular biology and genetic engineering technology, the use of genetic recombination technology to construct genetically engineered bacteria to carry out the glycosylation of ginseng saponins is an effective way to improve the conversion efficiency. Escherichia coli and yeast cells, as mature expression hosts, are often used as the preferred expression vectors for recombinant biological enzyme proteins. The introduction of the glycosyltransferase gene into the vector cell efficiently produces ginsenoside Rh2 [59]. In recent years, the xylosidase gene xln-DT from a thermophilic bacterium has been recombined into the pET-20b plasmid downstream of the pelB signal peptide using genetic splicing technology. The recombinant plasmid pelB-xln-DT was transformed into the E. coli expression host E. coli BL21 (DE3), and the enzyme was used as a catalyst to hydrolyze the glycosidic bond of notoginseng saponin R1. and successfully produced ginsenoside Rg1[44]. Jeon et al. used recombinant glucosidase (MT619) to convert ginsenoside Re, and the conversion pathway was Re →Rg1 →F1 →MT1[60]. MT1 is a new PPT-type ginsenoside. The gene encoding β-glucosidase from two strains of Bacillus was cloned and highly expressed in E. coli BL21 (DE3). Using BL21 (DE3) crude extract to hydrolyze ginsenoside Rb1 to produce ginsenoside F2[40]; the recombinant enzyme expressed in tandem in E. coli is of higher purity and can convert PPD-type ginsenosides to the rare ginsenoside Rh2(S)[61].
Currently, most studies use a single enzyme to convert ginsenosides. Therefore, future studies can use genetic recombination technology to express enzymes with different functions in the same host. Through the synergistic effect of different enzymes, the goal of producing rare ginsenosides and their derivatives that meet specific needs can be achieved, thereby improving the utilization rate of ginseng resources.
2.2 Microbial modification of the aglycone structure of ginsenosides
Research on the structural modification of ginsenoside aglycones has mainly focused on PPD-type ginsenosides, and derivatives such as 25-OH-PPD and 25-OCH3-PPD have been obtained. Derivatization methods are usually chemical and physical methods, and there have been few studies on changing the aglycone structure by biological methods. The main methods that have been reported are hydration of the double bond at the C24-C25 position, in rats. The main metabolic pathway of 20S-dammar-24-en-2α , 3β , 12β , 20-tetrol (GP) observed is the oxidation of the 24, 25-double bond to form the 24, 25-epoxide, followed by hydrolysis and rearrangement to form the 20, 24-epoxide (Figure 3) [62]. Researchers have used the co-cultivation of ginseng saponins and Cordyceps militaris to convert ginsenoside Rg1 to 25-OH-(S/R)-Rh1 and panaxoside R1 to 25-OH-20(S/R)-R2. The content of 25-OH derivatives in the conversion products is much higher than that of ginsenoside Rh1 and panaxoside R2 making it easy to isolate and purify the derivative [63-64]. Using Mucor spinosus in co-cultivation with 20(S)-protopanaxatriol, six derivatives were obtained. The results of the study proved that ginsenoside derivatives produced by dehydrogenation at the C-12 position followed by further hydroxylation at the C-7 or C-11 position and carbonylation at the C-15 position, as well as double bond rearrangement at the C-26 position, have significant inhibitory activity against cancer cells [65].
According to domestic and foreign research, it is speculated that this may be the most effective biocatalytic system for the regionally selective hydroxylation of ginsenosides. Experimental studies have found that these derivatives exert different effects in vivo and in vitro through different mechanisms, and their biological activity is higher than that of common ginsenosides [66]. For example, Rb1 undergoes a dehydrogenation reaction to form dihydroginsenoside Dg-Rb1, which can repair damaged neurons without affecting systemic parameters, and the effective dose of Dg-Rb1 is 10 times lower than that of Rb1 [67]. The octyl ester derivative Rh2-O of ginsenoside Rh2 has higher anticancer activity than Rh2 by activating the intrinsic apoptosis pathway [68]. Acetyl derivatives of PPD have significant antiproliferative and apoptotic induction activities, and some derivatives have higher anticancer activity than PPD [69]. The pyrazine ring was introduced into 25-OH-PPD to improve its antitumor activity. 2-Pyrazine-PPD can significantly inhibit the proliferation of gastric cancer cells and has little toxicity to normal cells (human gastric epithelial cell line GES-1) [70]. The derivative 25-OH-PPD of the aglycone PPD has better anticancer activity than existing drugs on the market such as ginsenoside Rg3, paclitaxel and aminolevulinic acid [71].
Therefore, while maintaining the original active structure of ginsenoside, the aglycone structure of ginsenoside can be modified by introducing polar groups and pharmacophore groups to enhance the water solubility, targeting and efficacy of ginsenoside, and improve the bioavailability of ginsenoside.
2.3 Glycosylation of ginsenosides
Starting from 2,3-oxidosqualene, the biosynthesis of ginsenosides can be divided into three steps: 2,3-oxidosqualene cyclase (OSC) cyclizes 2,3-oxidosqualene; hydroxylation and glycosylation reactions are then carried out by cytochrome (Cyt) and glycosyltransferase (GT), ultimately producing ginsenosides [72]. The UGT enzyme is one of the most critical enzymes in the synthesis of rare ginsenosides in living organisms [73] and is also the final step in the biosynthesis of ginsenosides. The UGT gene was cloned from Lactobacillus rhamnosus and expressed in E. coli BL21 (DE3). The recombinant UGT protein can convert Rh2 into two new ginsenosides, glucosylated ginsenoside Rh2 and diglucosylated ginsenoside Rh2 [74].

The triterpenoid aglycon is catalyzed by UGT to form ginsenosides with different structures. The protopanaxadiol-type aglycon is glycosylated at the C-3 and C-20 positions, and the protopanaxatriol-type aglycon is glycosylated at the C-6 and C-20 positions [80]. The rare ginsenoside glycosylation pathway is shown in Figure 4. The glycosyltransferase 73C5 (UGT 73C5) was isolated from Arabidopsis thaliana and added stepwise to PPD. This glycosyltransferase can selectively transfer glucose to the C-3 hydroxyl group of PPD to synthesize ginsenoside Rh2, achieving the largest reported yield of ginsenoside Rh2 (3.2 mg/mL) [75]. The glycosyltransferase 74AE2 (UGT 74AE2) catalyzes the transfer of glucose to the C-3 hydroxyl groups of PPD and C-K to form Rh2 and F2, respectively [76]. The glycosyltransferase 94Q2 (UGT 94Q2) can transfer glucose to Rh2 and F2 to form Rg3 and Rd, respectively [77]. UGT51 is one of the glycosyltransferases in Saccharomyces cerevisiae S288c [78], which can transfer glucose to the C-3 hydroxyl group of PPD to form Rh2 [79]. Future research on UGTs may provide insights into the regulatory mechanism of ginsenoside glycosylation and offer new ways to modify ginsenoside production.
As the direct factor catalyzing the formation of ginsenosides, UGTs play a vital role in the production of ginsenosides by biocatalysis. Most plant-derived UGTs have low catalytic activity, while recombinant microbial UGTs have special biocatalytic properties, such as substrate promiscuity and high expression levels in microbial substrate cells, which make them effective tools for the production of ginsenosides. Therefore, it is necessary to improve the catalytic activity of UGTs in order to increase the yield and production of the target rare ginsenosides.
3 Conclusion and outlook
Studies have shown that rare ginsenosides have higher medicinal value. At the same time, the low water solubility and low content of rare ginsenosides have limited their application. The hydrolysis or glycosylation of ginsenosides by intracellular or extracellular enzymes of different microorganisms effectively converts polysacchride ginsenosides and inactive aglycones into rare ginsenosides, thereby improving the bioavailability of ginsenosides.
Studies have found that changes in some functional groups can change the molecular structure and properties, thereby affecting the binding of drugs to receptors and affecting efficacy. For example, alkyl groups can increase the lipid solubility of a compound, reduce dissociation, increase stability, and prolong the duration of action of the drug; sulfhydryl groups increase lipid solubility and facilitate drug absorption; amide bonds can easily form hydrogen bonds with biological macromolecules and bind to receptors, showing special structural specificity; and the breaking of a hydroxyl group to form a hydrogen bond increases the water solubility of the compound, thereby increasing the activity of the drug.

Based on the structure of ginsenosides, it is speculated that hydroxylation reactions can occur at the C-2, C-11, C-15, C-24, and C-30 positions can undergo hydroxylation reactions; the carbon-carbon double bonds at the C-12 and C-13 positions can undergo addition reactions and oxidation reactions; at the same time, the diol-type ginsenosides can be oxidized at the C-12 and C-24 positions to form the oryctol-type ginsenosides. Therefore, if enzymes that can modify the structure of ginsenosides can be found through the screening of different microorganisms and enzymes, and some groups can be added to or knocked out based on the existing active structure of rare ginsenosides, the pharmacological activity and targeting of ginsenosides can be improved, and the bioavailability of ginsenosides can be increased, providing a new direction for the structural modification of ginsenosides.
At the same time, with the continuous development of technology in the fields of food, medicine, the continuous development of technology in the fields of food, medicine, and biochemistry, researchers can use genetics, molecular cytology, and other interdisciplinary integration, as well as high-throughput screening and genomics technology to select or design enzymes or strains with high selectivity and conversion rates. Biological means can be used to achieve the industrial production of highly active rare ginseng saponins and their derivatives, further increasing the yield of rare ginseng saponins and their highly active derivatives. This is of great significance for making full use of ginseng resources for the benefit of the public.
Reference:
[1] Jin Hee Kim, Miseon Kim, Sun-Mi Yun, et al. Ginsenoside Rh2 induces apoptosis and inhibits epithelial-mesenchymal transition in HEC1A and Ishikawa endometrial cancer cells[J]. Biomedicine & Pharmacotherapy,2017,96. 871-876.
[2] Jiang Z, Yang Y , Yang Y , et al. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune[J]. Biomed. Pharm. 2017.96 .378-383.
[3] Yao W, Guan Y. Ginsenosides in cancer: A focus on the regulation of cell metabolism. Biomed Pharmacother. 2022 Oct 10;156:113756. doi: 10.1016/j.biopha.2022.113756.
[4] Yu H, Wang Y, Liu C, et al. Conversion of Ginsenoside Rb1 into Six Types of Highly Bioactive Ginsenoside Rg3 and Its Derivatives by FeCl3 Catalysis. Chem Pharm Bull (Tokyo). 2018;66(9):901-906.
[5] Zhang J, Ai Z, Hu Y, et al. Remarkable impact of commercial sterilizing on ginsenosides transformation in fresh ginseng pulp based on widely targeted metabolomics analysis. Food Chem X. 2022 Aug 9;15:100415.
[6] Hasegawa, Hideo, Sung, et al. Main Ginseng Saponin Metabolites Formed by Intestinal Bacteria. Planta Medica, 1998, 62(5), 453-457.
[7] Hasegawa, Hideo, Sung, et al. Role of Human Intestinal Prevotella oris in Hydrolyzing Ginseng Saponins. Planta Medica, 1997,63(5), 436-440.
[8]SHANGGUAN lihua, LIU guoquan. Research progress in the metabolism of ginseng components [J].Chinese Traditional and Herbal Drugs, 1999(11):865-870.
[9] Lee Byung-Hoon, Lee, Sang-Jun Hui, et al. In vitro Antigenotoxic Activity of Novel Ginseng Saponin Metabolites Formed by Intestinal Bacteria. Planta Medica, 1998, 64(6), 500 –503.
[10] Yoshimasa Yamaguchi, Masaya Higashi, Hideshi Kobayashi. Effects of ginsenosides on impaired performance caused byscopolamine in rats[J]. European Journal of Pharmacology, 1996, 312(2): 149-151.
[11] Kim WY, Kim JM, Han SB, et al. Steaming of ginseng at hightemperature enhances biological activity[J]. J Nat Prod, 2000, 63(12):1702-1704.
[12] Guo HY, Xing Y, Sun YQ, et al. Ginsengenin derivatives synthesized from 20(R)-panaxotriol: Synthesis, characterization, and antitumor activity targeting HIF-1 pathway. J Ginseng Res. 2022 Nov;46(6):738-749.
[13] Kim GO, Kim N, Song GY, et al. Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis. IntJ Mol Sci. 2022 16; 23(18):10836. doi: 10.3390/ijms231810836.
[14] Chen Z, Wang G, Xie X, et al. Ginsenoside Rg5 allosterically interacts with P2RY12 and ameliorates deep venous thrombosis by counteracting neutrophil NETosis and inflammatory response. Front Immunol. 2022 Aug 12;13:918476.
[15] LIU yannan. Preparation of ginsenoside Rg5 and its anti gastric and breast cancer activity[D]. Shaanxi: Northwest University, 2019.
[16] DENG J J, LIU Y, DUAN Z G, et al. Protopanaxadiol and protopanaxatriol-type saponins ameliorate glucose and lipid metabolism in type 2 diabetes mellitus in high-fat diet /streptozocin-induced mice[J]. Front Pharmacol ,2017, 8: 506 .
[17] MAENG Y S, MAHARJAN S, KIM J H, et al. Rk1 ,a ginsenoside ,is a new blocker of vascular leakage acting through actin structure remodeling[J], PLoS One, 2013, 8(7) : e68659 .
[18] RYOO N, RAHMAN M A, HWANG H, et al. Ginsenoside Rk1 is a novel inhibitor of NMDA receptors in cultured rat hippocampal neurons[J]. J Ginseng Res, 2020, 44 (3) :490-495.
[19] OH J M, LEE J, IM W T, et al. Ginsenoside Rk1 induces apoptosis in neuroblastoma cells through loss of mitochondrial membrane potential and activation of caspases[J]. Int J Mol Sci, 2019, 20( 5) : 1213 .
[20] SIDDIQI M H, SIDDIQI M Z, AHN S ,et al. Stimulative effect of ginsenosides Rg5: Rk1 on murine osteoblasticMC3T3-E1 cells[J]. Phytother Res, 2014, 28( 10) : 1447-1455 .
[21] Krishika Sambyal, Rahul Vikram Singh. Production aspects of testosterone by microbial biotransformation and future prospects[J].Steroids, 2020, 159(C).
[22]NAN bo ,YOU ying ,WANG yushan, et al.Research Progress on Microbial Transformation of Ginsenosides[J]. Food Research And Development, 2017, 38(14): 196-199
[23]ZHOU Zhong-liu, LI Chun-yan, CHEN Lin-hao1, et al. Biotransformation of Natural Saponins[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2019, 25(16): 173-192.
[24] M. J. H, Eun-Ah BAE, Min-Kyung , et al. Metabolism of 20(S)- and 20(R)-Ginsenoside Rg3 by Human Intestinal Bacteria and Its Relation to in Vitro Biological Activities[J]. Biol. Pharm Bull, 2002, 25(1): 58-63.
[25] HAN mingxin, LI fangtong, ZHANG yan, et al. Biotransformation of Rare Protopanaxadiol Saponinby Human Intestinal Microflora[J].Chemical Journal of Chinese Universities, 2019, 40(07): 1390-1396.
[26] ZHANG yan, LI fangtong, HAN mingxin, et al.Analysis of Metabolites of Protopanaxatriol Saponins in Human Intestinal Flora by RRLC-Q-TOF MS and UPLC-QQQ MS[J].Journal of Chinese Mass Spectrometry Society, 2020, 41(01): 66-75.
[27] TANG lan1 , FU lulu,, SHEN liting, et al.Degradation of total saponins of Panax notoginseng by intestinal flora of rats in vitro[J]. Chinese Traditional and Herbal Drugs, 2018, 49(02): 396-399.
[28] Guo Y P, Chen M Y, Shao L, et al. Quantification of Panax notoginseng saponins metabolites in rat plasma with in vivo gut microbiota-mediated biotransformation by HPLC-MS/MS[J]. Chinese Journal of Natural Medicines, 2019, 17(3): 231-240.
[29]CHEN sijian, WU dongxue, LIU suying, et al. Advances in chemical and biological transformation of ginsenoside[J] Chinese Traditional Patent Medicine,2022, 44(5): 1539-1545.
[30] WANG shanshan ,HU ping,YU shaowen.Progress in research of biotransformation of natural products [J]. Chinese Journal of New Drugs, 2016, 25(1): 71-75.
[31]GAO juan, ZHOU andong, YUAN ye, et al. Enzymatic Degradation of Ginsenoside Rb1 for Preparation of Compound K by Aspergillus niger sp. J7[J]. Current Biotechnology, 2016, 6(02): 98-104.
[32] Song X L, Wu H, Piao X C, et al. Microbial transformation of ginsenosides extracted from Panax ginseng adventitious roots in an airlift bioreactor[J]. Electronic Journal of Biotechnology, 2017, 26 : 20-26.
[33] Yan Q, Zhou W, Shi X L, et al. Biotransformation pathways of ginsenoside Rb1 to compound K by β-glucosidases in fungus Paecilomyces Bainier sp. 229[J], Process Biochemistry, 2010, 45(9): 1550-1556.
[34] Hu Y B, Wang N, Yan X C, et al. Ginsenoside Re impacts on biotransformation products of ginsenoside Rb1 by Cellulosimicrobium cellulans sp. 21 and its mechanisms[J], Process Biochemistry, 2019, 77: 57-62.
[35] Jitendra Upadhyaya, Min-Ji Kim, Young-Hoi Kim, et al. Enzymatic formation of compound-K from ginsenoside Rb1 by enzyme preparation from cultured mycelia of Armillaria mellea[J]. Journal of Ginseng Research, 2016, 40(2): 105-112.
[36] Li Ling, Lee Soo Jin, Yuan Qiu Ping, et al. Production of bioactive ginsenoside Rg3(S) and compound K using recombinant Lactococcus lactis[J]. Journal of Ginseng Research, 2017, 42(4): 412-418.
[37] Hyojin Lee, Seung Il Ahn, Byung Wook Yang, et al. Biotransformation of Ginsenosides by Eoyukjang-derived Lactic Acid Bacteria in Mountain-cultivated Ginseng[J]. Microbiology and Biotechnology Letters, 2019, 47(2): 201-210.
[38] Liu C Y, Zuo K Z, Yu H S, et al. Preparation of minor ginsenosides C-Mx and C-K from notoginseng leaf ginsenosides by a special ginsenosidase type-I[J]. Process Biochemistry, 2015, 50(12): 2158-2167.
[39] Zhang R, Huang X M, Yan H J, et al. Highly Selective Production of Compound K from Ginsenoside Rd by Hydrolyzing Glucose at C-3 Glycoside Using β-Glucosidase of Bifidobacterium breve ATCC 15700[J]. Journal of Microbiology and Biotechnology, 2019, 29(3): 410-418.
[40] Almando Geraldi, Ni matuzahrohabet, Fatimahab, al. Enzymatic biotransformation of ginsenoside Rb1 by recombinant β - glucosidase of bacterial isolates from Indonesia[J]. Biocatalysis and Agricultural Biotechnology. 2020, 23(C): 101449-101449.
[41] Li L, Lee S J ,Yuan Q P, et al. Production of bioactive ginsenoside Rg3(S) and compound K using recombinant Lactococcus lactis[J]. Journal of Ginseng Research, 2017, 42(4): 412-418.
[42] Pei J J, Xie J C, Yin R, et al. Enzymatic transformation of ginsenoside Rb1 to ginsenoside 20(S)-Rg3 by GH3 β-glucosidase from Thermotogathermarum DSM 5069 T[J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 113:104-109.
[43] Zhang S H, Xie J C, Zhao L G. Cloning, overexpression and characterization of a thermostable β-xylosidase from Thermotoga petrophila and cooperated transformation of ginsenoside extract to ginsenoside 20(S)-Rg3 with a β-glucosidase[J]. Bioorganic Chemistry, 2019, 85:159-167.
[44]114-120. [LI qi, TONG xinyi, JIANG yujie, et al. Construction of whole cell catalyst pelB-Xln-DT and its application in biotransformation of Panax notoginsenoside R1[J]. Journal of Forestry Engineering, 2020, 5(04): 114-120.
[45] Min-Ji Kim, Jitendra Upadh ya ya, Min-SunYoon, et al. Highly regioselective biotransformation of ginsenoside Rb2 into compound Y and compound K by β-glycosidase purified from Armillaria mellea mycelia[J]. Journal of Ginseng Research. 2017, 42(4) : 504-511.
[46]ZHONG yating.Screening of Ginseng Saponin Transforming Strain GsBt3 and Its Transformation into Total Saponins of Panax quinquefolium[D].Shanghai: Shanghai Normal University, 2012.
[47] YANG yuanchao, WANG yingping, YAN meixia, et al. Screening of plant pathogenic fungi by ginsenoside compound K production[J]. China Journal of Chinese Materia Medica, 2011, 36(12): 1596-1598.
[48] CHEN yang,ZHANG mmeiping, WANG yi, et al. Microbial transformed ginsenoside Rg3 from total saponins of Panax ginseng by Bacillus subtilis [J]. Lishizhen Medicine and Materia Medica Research, 2014, 25(11):2676-2678.
[49] JIN yan, JIN xiangmei, YIN chengriBiotransformation of major ginsenoside Re to minor ginsenoside Rh1by Sphingomonas sp .2-F2[J]. Agricultural Science Journal of Yanbian University, 2011, 33(02): 103-107.
[50] LIANG zhiqi, ZHANG jinglou, JING haizhu, et al. Microbiological Transformation of Ginsenoside Rg3 into Rh2[J]. Ginseng Research, 2018, 30(03): 6-10.
[51] Yan Q, Zhou W, Shi X L, et al. Biotransformation pathways of ginsenoside Rb1 to compound K by β-glucosidases in fungus Paecilomyces Bainier sp. 229[J]. Process Biochemistry, 2010, 45(9): 1550-1556.
[52] Ye L, Zhou C Q, Zhou W, et al. Biotransformation of ginsenoside Rb1 to ginsenoside Rd by highly substrate-tolerant Paecilomyces bainier 229-7[J]. Bioresource Technology, 2010, 101(20):7872-7876
[53] CHEN xiaochun, DAI zhu, FU rongzhao. Biocatalytic Synthesis of rare ginsenoside Rh2[J]. Jiangxi Chemical Industry, 2019(02): 55-57.
[54] Su J H, Xu J H, Lu W Y, et al. Enzymatic transformation of ginsenoside Rg3 to Rh2 using newly isolated Fusarium proliferatum ECU2042[J]. Journal of Molecular Catalysis B Enzymatic, 2006, 38(2):113-118.
[55] Su J H, Xu J H, Yu H L, et al. Properties of a novel β-glucosidasefrom Fusarium proliferatum ECU2042 that converts ginsenoside Rg3 into Rh2[J]. Journal of Molecular Catalysis B Enzymatic, 2009, 57(1-4):278-283.
[56] WU xiuli, WANG yan, ZHAO wenqian, et al. Fungal biotransformation of ginsenoside Rg3[J]. Acta Microbiologica Sinica, 2008(09): 1181-1185.
[57] CONG yueyi, SUN jia, YU en, et al. Study on transformation of ginsenoside Rg3 fermented by Monascus purpureus[J]. Chinese Traditional and Herbal Drugs, 2018,49(06): 1298-1303.
[58] Jin F .X. In the Second International Symposium on Natural Medicine and Microflora[M]. Tokyo, Japan 1998, Oct, 24-26:1-15.
[59] Zhuang Y, Yang G Y, Chen X H, et al. Biosynthesis of plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme[J]. Metabolic Engineering, 2017, 42 : 25-32.
[60] Byeong-Min Jeon, Jong-In Baek, Min-Sung Kim, et al. Characterization of a Novel Ginsenoside MT1 Produced by an Enzymatic Transrhamnosylation of Protopanaxatriol-Type Ginsenosides Re[J]. Biomolecules. 2020, 10(4): 525-525.
[61] Muhammad Zubair Siddiqi, Hipolito Amaral Ximenes, Bong-Kyu Song, et al. Enhanced production of ginsenoside Rh2(S) from PPD-type major ginsenosides using BglSk cloned from Saccharibacillus kuerlensis together with two glycosidase in series[J]. Saudi Journal of Biological Sciences. 2021, 04, 079.
[62] Chen H, Dong, Zhi F, et al. Discovery, synthesis, and structure-activity relationships of 20S-dammar-24-en-2 α, 3 β, 12β,20-tetrol (GP) derivatives as a new class of AMPK α2β1 γ 1 activators[J]. Bioorganic & medicinal chemistry, 2016, 24(12): 2688-96.
[63] Xin S, Jl A, Yu X A, et al. Highly regioselective biotransformation of ginsenoside Rg1 to 25-OH derivatives of 20(S/R)-Rh1 by Cordyceps Sinensis-ScienceDirect[J]. Bioorganic & Medicinal Chemistry Letters. 2020, 30( 21): 127-504.
[64] Liu J S, Yu X N, Qiu Z D, et al.Cordyceps sinensis-mediated biotransformation of notoginsenoside R1 into 25-OH-20(S/R)-R2 with elevated cardioprotective effect against DOXinduced cell injury[J]. RSC Advances, 2022, 12, 129-38.
[65] Chen G T, Ge H J, Song Y, et al. Biotransformation of 20(S)-protopanaxatriol by Mucor racemosus and the anti-cancer activities of some products[J]. Biotechnology Letters, 2015, 37(10): 2005-2009.
[66] Kim M Y, Cho J Y. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages[J]. Journal of Ginseng Research, 2013, Jul, 37(3): 293-9.
[67] akanaka M, Zhu P, Bo Z, et al. Intravenous infusion of dihydroginsenoside Rb1 prevents compressive spinal cord injury and ischemic brain damage through upregulation of VEGF and Bcl-XL.[J]. J Neurotrauma, 2007, 24(6): 1037-1054.
[68] Chen F, Zheng S L, Hu J N, et al. Octyl Ester of Ginsenoside Rh2 Induces Apoptosis and G1 Cell Cycle Arrest in Human HepG2Cells by Activating the Extrinsic Apoptotic Pathway and Modulating the Akt/p38 MAPK Signaling Pathway[J]. Journal of Agricultural &Food Chemistry, 2016, acs.jafc., 6b03519.
[69] Du G J, Dai Q, Williams S, et al. Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities[J]. Anticancer drugs, 2011, 22(1):35.
[70] Xu D, Tao L, Yan L, et al. 2-Pyrazine-PPD, a novel dammarane derivative ,showed anticancer activity by reactive oxygen speciesmediate apoptosis and endoplasmic reticulum stress in gastric cancer cells[J]. European Journal of Pharmacology, 2020, 881.
[71] Xu D, Yuan Y, Fan Z, et al. 4-XL-PPD, a novel ginsenoside derivative, as potential therapeutic agents for gastric cancer shows anticancer activity via inducing cell apoptosis medicated generation of reactive oxygen species and inhibiting migratory and invasive[J].Biomedicine & Pharmacotherapy, 2019, (118): 108.
[72] Li Y, Baldauf S, Lim E K, et al. Phylogenetic Analysis of the UDP-glycosyltransferase Multigene Family of Arabidopsis thaliana[J].Journal of Biological Chemistry, 2001, 276(6):4338.
[73] Christensen L P. Ginsenosides: Chemistry, biosynthesis, analysis and potential health effects (Chapter 1)[J]. Adv Food Nutr Res, 2008, 55(55):1-99.
[74] Wang D D, Yeon-Ju Kim, Nam Baek, et al. Glycosyltransformation of ginsenoside Rh2 into two novel ginsenosides using recombinant glycosyltransferase from Lactobacillus rhamnosus and its in vitro applications[J]. Journal of Ginseng Research. 2021 45(1): 48-57.
[75] Hu Y, Xue J, Min J, et al. Biocatalytic synthesis of ginsenoside Rh2 using Arabidopsis thaliana glucosyltransferase-catalyzed coupled reactions[J]. Journal of Biotechnology, 2020, 309: 107-112.
[76] Jung S C, Kim W, Park S C, et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd.[J]. Plant & cell physiology, 2014, 55(12): 2177-88.
[77] Khorolragchaa, Altanzul, Kim, et al. Grouping and characterization of putative glycosyltransferase genes from Panax ginseng Meyer[J]. Gene, 536(1): 186 – 192.
[78] Warnecke D, Erdmann R, Fahl A, et al. Cloning and functional expression of UGT genes encoding sterol glucosyltransferases from Saccharomyces cerevisiae, Candida albicans, Pichiapastorisand Dictyostelium discoideum[J]. J Biol Chem, 1999, 274(19): 13048-13059. [79] Zhuang Y, Yang G Y, Chen X H, et al. Biosynthesis of plant-de-rived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme [J]. Metab Eng, 2017, 42: 25-32.
[80] Zhao J N, Wang R F, Zhao S J, et al. Advance in glycosyltransferases, the important bioparts for production of diversified ginsenosides[J]. Chinese Journal of Natural Medicines, 2020, 18(9): 643-658.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






