Study on Ginseng Extract Ginsenoside for Osteoarthritis
Osteoarthritis (OA) is a degenerative disease caused by many factors, such as aging, obesity, genetics, strain, trauma, congenital joint abnormalities, and joint deformities. It is characterized by degenerative damage to articular cartilage and reactive hyperplasia of the joint edges and subchondral bone. Clinical manifestations include slowly developing joint pain, tenderness, stiffness, joint swelling, limited movement, and joint deformity [1]. At present, the conventional treatments for OA mainly include non-steroidal anti-inflammatory drugs, glucocorticoids, and physiotherapy. Patients with severe conditions often require joint replacement [2]. Therefore, finding a drug with few side effects and good efficacy has become an urgent issue in the treatment of OA. With the deepening of research on traditional Chinese medicine, ginseng has been found to have good prospects for preventing and treating articular cartilage damage and degeneration, and for participating in the repair of articular cartilage defects by cultured cartilage cells in vitro[3], providing a new way of thinking for the treatment of OA.
Ginseng (Panax ginseng C. A. Mey.) is the dried root and rhizome of a perennial herb in the family Araliaceae. It is a traditional precious medicinal herb that has been used in China for many years [4]. As one of the “Three Treasures of Northeast China”, ginseng has pharmacological effects such as anti-inflammatory, anti-oxidant, anti-depressant, anti-Alzheimer's disease, anti-atherosclerosis and anti-OA, and has a long history of use and extremely high medicinal value [5]. Ginsenosides, the main pharmacological active ingredients of ginseng, play a key role in disease treatment. Studies have shown that ginsenosides can improve joint pain and repair damaged cartilage by inhibiting inflammatory factors, reducing chondrocyte apoptosis and matrix damage, and promoting chondrocyte repair, thereby exerting a therapeutic effect on OA [6]. Although the pharmacological activity of ginseng has been well studied, there are many types of ginsenosides. It is not yet fully understood which ginsenosides may have a therapeutic effect on OA, whether the combined application of ginsenoside monomers is effective, and the specific therapeutic mechanisms of action, such as the targeted specific proteins and/or macromolecules. This article reviews the research and progress of ginsenosides in the treatment of OA in recent years, providing a basis for the clinical application of ginsenosides in the treatment of OA.

1 Ginsenosides and their classification
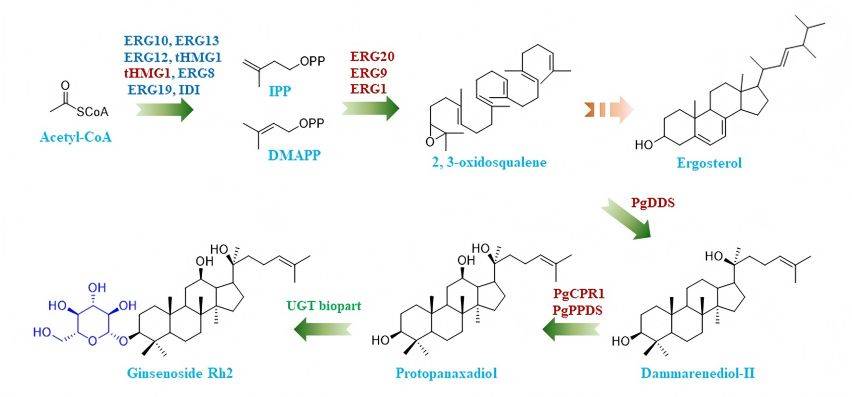
Ginsenosides (G), also known as steroid-like saponins, are unique components of ginseng and are generally denoted by Rx. Currently, more than 150 naturally occurring ginsenosides can be isolated from the roots, stems, leaves, flowers and fruits of the ginseng plant. These ginsenosides can be divided into four categories according to their skeleton type. They all have a common tetracyclic hydrophobic structure, but the sugar part is different. Different sugar molecules are attached to different regions of the four main chains to produce unique ginsenoside monomer molecules (Table 1), which determine different pharmacological activities [7-9].
Of these four ginsenosides, protopanaxadiol-type and protopanaxatriol-type ginsenosides account for more than 90% of total ginsenosides in ginseng and have stronger activity. They are the main active ingredients of ginsenosides and are also the focus of current research. Among these, G Rb1, G Rg 1, G Rg3, G Rd, G Re, G Rh1 and G Rh2 are the most frequently studied [10]. This study provides a detailed introduction to the research progress of ginsenoside monomers such as GRb1, GRg1, G Rg3 and G Ro, and monomers in combination and traditional Chinese medicine formulas for the treatment of OA.
2 OA risk factors and treatment methods
2.1 Factors contributing to the development of OA
As one of the most common forms of arthritis, OA is influenced by many factors, including individual factors such as age, gender and obesity. Studies have found that one-third of people over the age of 65 are affected by OA [11], and the incidence in women increases significantly after the age of 50 [12], mainly due to the decrease in estrogen after menopause, which leads to increased breakdown of articular cartilage in women [13]. In addition, sports injuries can lead to abnormalities in the joint structure, increasing the risk of cartilage loss and can directly lead to the development of OA [14]. Mechanical stress on the joints increases the expression of pro-inflammatory factors in joint cells, which is also partly responsible for the catabolic process of OA cartilage [15]. The development of OA is also closely related to factors such as metabolism [16, 17], genetics and immunity [18].
2.2 Treatment of OA
Currently, there are various technical methods for treating OA. In addition to physical therapy (including exercise therapy and patient education supplemented by orthopedic devices) [19], pain relief, disease progression delay, and function improvement can also be achieved through oral medication, intra-articular injections, and end-stage surgery [20].
Standard drug therapy includes pain and inflammation control drugs such as non-steroidal anti-inflammatory drugs, opioid analgesics and intra-articular corticosteroids, as well as symptomatic relief of arthritis such as oral glucosamine sulfate, chondroitin sulfate, dexamethasone, soybean and avocado unsaponifiables extracts, and intra-articular hyaluronic acid injections [21]. In addition, some studies have shown that Chinese herbal medicines such as Gan Cao Tang also have a certain effect in the treatment of OA [22]. However, drug therapy has little effect in severe cases, and surgery is the only option. The most common and most effective is joint replacement [23]. Of course, traditional Chinese medicine methods such as acupuncture [24], massage [25], physiotherapy, Chinese herbal plaster, Chinese herbal fumigation, Chinese herbal application [26], Chinese herbal ironing and targeted Chinese herbal medicine can also be used to slow the progression of OA [27].
3 Research progress on the therapeutic effects of ginsenosides on OA
3.1 Ginsenoside Rb1
Ginsenoside Rb1 (G Rb1) is mainly derived from Panax ginseng, a member of the genus Panax. The content in the stems and leaves of ginseng is much lower than that in the roots, rhizomes and root hairs. GRb1 is a triterpene saponin and is the most abundant of more than 40 individual ginsenosides. which can exert anti-inflammatory, anti-apoptotic and neuroprotective effects by inhibiting the expression of inflammatory cytokines, the Notch signaling pathway and the NF-KB signaling pathway [9].
GRb1 can exert a protective effect on articular cartilage by inhibiting the expression of inflammatory cytokines. Cheng et al. [28] constructed a cartilage OA model by stimulating with interleukin (IL)-1, and then administered a certain amount of GRb1 for index detection. It was found that GRb1 could inhibit the production of inflammatory factors such as matrix metalloproteinases (MMP)-13, cyclo-oxygenase-2 (COX-2), prostaglandin E2 (PGE2), inducible nitric oxide synthase ( iNOS) and nitric oxide (NO), and reduces the degradation of Collagen II and proteoglycan (ACAN) induced by IL-1β in human articular chondrocyte, which indicates that the mechanism of action of GRb1 may be similar to that of non-steroidal anti-inflammatory drugs, which can relieve the symptoms of OA by inhibiting the expression of COX-2 and PGE2.
This effect was also verified in an OA rat model. Ara-vinthan et al. [29] found that GRB1 may inhibit the levels of MMP13 and COX-2 mRNA and inhibit interferon (Interferon gamma, IFN-) monocyte chemoat-tractant protein-1 (MCP-1)/chemokine ligand-2 (CCL-2), IL-1P and IL-6 inflammatory cytokines/chemokines expression, thereby preventing cartilage degradation, and thus helping GRb1 exert anti-inflammatory effects on MIA-induced ovarian OA rats. Gao Zhi [30] established an osteoarthritis model by cutting the anterior cruciate ligament of the right knee joint of rats and treated the rats based on different doses of GRb1. The results showed that GRb1 significantly reduced the number of white blood cells and lymphocytes in the blood of model rats, and in a concentration-dependent manner, it reduced the levels of TNF-α and IL-1β in rat serum, and also reduced the expression of MMP-9, MMP-13 and AD-AMTS-5 expression, while increasing Collagen II and Aggrecan expression in cartilage tissue, indicating that G Rb1 can inhibit cartilage degradation by inhibiting inflammatory responses and the synthesis of MMPs in joint tissue, and has a good therapeutic effect on osteoarthritis induced by anterior cruciate ligament transection.
G Rb 1 has multiple mechanisms of action in the treatment of OA, possibly through the Notch signaling pathway and the NF-KB signaling pathway. For example, Wang Wei [31] found that GRb1 can reduce IL-1β-induced expression of type II collagen and MMP-13 in cells, while also reducing the expression levels of Notch1 and JAG1 after induction, indicating that the protective effect of GRb1 on chondrocytes may be achieved by inhibiting the expression of MMP-13 and activation of the Notch signaling pathway. In addition, Hossain et al. [32] constructed an OA rabbit model and found that GRb1 can prevent chondrocyte apoptosis, inhibit the production of reactive oxygen species (ROS) in chondrocytes, down-regulate the p38/MAPK and PI3K/AKT signal pathways to prevent the expression of MMPs and balance the expression of collagen type II and proteoglycans, and activate NF-KB signal transduction to exert a protective effect on chondrocytes, thereby being used to treat OA.

3.2 Ginsenoside Rg1
Ginsenoside Rg1 (G Rg1) is initially extracted from the root and stem of the Chinese medicinal herb ginseng. It is a triterpene dammarane ring derivative with thirty carbon atoms and a structure similar to that of glucocorticoid steroids. It can exert an anti-inflammatory effect similar to glucocorticoid drugs by binding to glucocorticoid receptors. Huang Yumin [33] constructed an OA model in rats through anterior cruciate ligament transection (ACLT) and MIA joint cavity injection, and found that G Rg1 can inhibit the activation of NF-KB by inhibiting the phosphorylation of IB, thereby reducing the synthesis of cartilage cell MMP-13 and PGE2 to exert a chondroprotective effect.
G Rg1 can also inhibit chondrocyte apoptosis and promote chondrocyte proliferation, exerting a protective effect on chondrocytes, and thus has potential value in the treatment of OA. Duan Chao et al. [3] used a 6-week modeling method of fixed straight tubular plaster to successfully establish a rabbit model of human OA similar to that seen in the clinic. The effect of GRg1 on the apoptosis of rabbit knee OA articular chondrocytes was observed, and it was found that GRg1 at a certain dose has a positive protective effect on cartilage tissue. It can effectively inhibit excessive apoptosis of chondrocytes and avoid progressive destruction of cartilage tissue by regulating the expression ratio of the key factors of chondrocyte apoptosis Bax/Bcl-2 and down-regulating the expression of Caspase-3. In addition, Huang et al. [34] found that GRg1-pretreated IL-1β-induced chondrocytes also promote Bcl-2 expression, inhibit Bax activity, and reduce CytC release and Caspase-3 activation. It also enhances TIMP-1 expression and inhibits MMP-13 synthesis, effectively inhibiting extracellular matrix degradation. It was also found that these effects of G Rg1 were mediated in part by enhanced PI3K/AKT signaling.
In addition, GRg1 can also inhibit the activity of inflammatory mediators and reduce articular cartilage damage. Cheng et al. [35] used an in vitro model of human chondrocytes and an in vivo model of rat OA to observe the effect of GRg1. found that GRg1 can inhibit IL-1-induced human chondrocyte MMP-13, COX-2 and PGE2 gene and protein expression, and prevent Col2A1 and ACAN degradation, slowing cartilage degeneration. This suggests that GRg1 has potential clinical benefits for OA treatment.
3.3 Ginsenoside Rg3
Ginsenoside Rg3 (Ginsenoside Rg3, G Rg3) is a tetraethylene triterpene glycoside monomer that can be obtained by the metabolism of Rb1, Rb2, Rb3, Rc and Rd. with hydroxyl groups at the 3b, 12b, and 20 pro-S positions and a 3-hydroxy group converted to a β-D-glucopyranosyl-β-D-glucopyranoside dammarane. It is a protopanaxatriol-type ginsenoside [36].
An in vitro study investigated the protective effect of G Rg3 on human OA chondrocytes. So et al. [37] stimulated chondrocytes with IL-1β, which showed an increase in MMP-1, MMP-3 and MMP-13 levels and a decrease in COL2A1 and ACAN expression. In cells treated with IL-1β and GRg3 together, the levels of MMP-1 and MMP-13 were lower than in cells treated with IL-1 alone, and the expression levels of COL2A1 and ACAN returned to the low values seen when the cells were cultured in the presence of IL-1β alone. In addition, the study found that IL-1β stimulation of chondrocytes alone leads to an increase in senescence-associated β-galactosidase (SA-β-Gal) positive cells, while the co-culture of IL-1β and GRg3 significantly inhibits the expression of this aging marker. In addition, chondrocytes cultured with GRg3 showed significantly higher proliferation and telomerase activity than control cells. The above studies show that GRg3 can protect cells from the effects of cartilage cell senescence in osteoarthritis.
GRg3 may also exert an effect on OA by regulating SIRT1-mediated anti-apoptotic and anti-inflammatory mechanisms. Ma et al. [38] used tumor necrosis factor (TNF)-α to stimulate TC28a2 human chondrocytes to induce chondrocyte damage, found that GRg3 reversed the suppression of SIRT1 expression by TNF-α and also activated the SIRT1/PGC-1a/SIRT3 pathway to inhibit the TNF-α-induced acetylation of acetylated cyclophilin D (CypD). This led to mitochondrial dysfunction and a reduction in reactive oxygen species (ROS) accumulation, thereby improving TNF-induced apoptosis. In addition, it was found that GRg3 reverses the activation of SIRT1/PGC-1a/SIRT3, mediates the inhibition of p38 MAPK, and thus downregulates the NF-KB translocation in TNF-α-treated cells. In summary, under TNF-α stimulation, GRg3 can reduce the production of IL-8 and MMP-9 in chondrocytes through the SIRT1/PGC-1a/SIRT3/p38 MAPK/NF-KB signaling pathway, thereby achieving the purpose of treating OA.
3.4 Ginsenoside Ro
Ginsenoside Ro (G Ro) is a type of ginsenoside with a triterpene structure. Some studies have shown that G Ro exerts an anti-inflammatory effect by directly inhibiting the TLR4 signaling pathway, indicating that G Ro can be used as a natural therapeutic compound for inflammation-related diseases [39].
Zhang et al. [40] first reported that G Ro can inhibit the apoptosis rate of IL-1β-induced rat chondrocytes and promote the expression of apoptosis proteins. It can also inhibit the expression of inflammatory factors such as COX-2, MMP-3 and MMP-9 to exert an anti-inflammatory effect. In addition, it was found that G Ro inhibited the activation of NF-KB phospho p65 stimulated by IL-1β in chondrocytes, indicating that GRo inhibited IL-1β-induced apoptosis and inflammation by inhibiting NF-KB.
3.5 Other ginsenoside monomers
Currently, there are relatively few studies on the treatment of OA with other ginsenosides. However, studies have shown that ginsenosides, including Rb2, Rh, Rc, Rd and Rf, can inhibit the expression of MMPs to exert anti-inflammatory effects, and have the potential to become a potential drug for the treatment of OA. However, further research is needed to investigate the targets involved in the mechanism of action [41]. Lee et al. [42] found that several ginsenosides, including ginsenosides Rd, Rf and F4, including several saponins, can inhibit the expression of MMP-13 in chondrocytes treated with IL-1P at non-cytotoxic concentrations (1–50 M). The most prominent inhibitors are ginsenosides F4 and Rg3. Further studies have found that GF4 can strongly inhibit the activation of p38 mitogen-activated protein kinase, thereby inhibiting the activity of MMP-13 [43].
In addition, studies have shown that GRb2 and GRg5 can inhibit apoptosis and matrix damage in OA rat articular chondrocytes, as well as inhibit IL-1β and TNF-α, thereby slowing the progression or severity of arthritis and preventing articular cartilage damage [44,45]. Zhang et al. [45] prepared an osteoarthritis rat model by transecting the ligament and removing the medial meniscus, and then administered different doses of GRg5 to observe the effect. It was found that 15 mg Rg5 can significantly prevent cartilage degeneration and prevent synovial collapse in the OA rat model. Treatment of rats with GRg5 increased the expression levels of Col2A1, ACAN and type II collagen, and reduced the levels of MMP-13, IL-1P, TNF-a, NO and iNOS, proving that GRg5 can prevent cartilage degradation, inhibit synovial inflammation in OA rats, and induce chondrocyte apoptosis, and can therefore be used in the treatment of osteoarthritis.
Studies have found that ginsenoside Rc can promote osteoblast differentiation and matrix mineralization by activating the classical Wnt pathway of p-catenin and Runx2, and upregulate the expression of bone markers to increase bone formation [43].
3.6 Synergistic treatment with multiple ginsenosides
The combined use of various ginsenosides to treat OA is also one of the current research directions. Some scholars have prepared a compound ginsenoside composed of ginsenoside Rd and ginsenoside Re as the active ingredients and used it as a drug for the treatment of OA [46]. Siddiqi et al. [47] isolated the Rg5:Rk1 mixture from fresh ginseng roots by repeated steaming and drying, studied its effect on the growth and differentiation of mouse MC3T3-E1 cells. It was found that Rg5:Rk1 stimulated MC3T3-E1 cell growth in a dose-dependent manner, up-regulated collagen synthesis, and had a proliferative and synthetic effect on the development of bone matrix. It also induced the differentiation and mineralization of MC3T3-E1 cells in vitro. while the effect of Rg5:Rk1 on MC3T3-E1 cell growth and differentiation is closely related to the expression of the BMP-2/Runx2 signaling pathway. Rg5:Rk1 may be useful in stimulating osteoblast differentiation and proper bone formation, and may become a potential drug for the treatment of OA.
In addition, studies have shown that traditional Chinese medicine compounds containing various ginsenosides also have certain prospects for the treatment of OA. Qinqi Rheu-matism Formula (QRF) is a clinical experience formula for the treatment of rheumatoid arthritis. It is composed of Radix Pseudostellariae (Kouziqi), Radix Gentianae Macrophyllae (Qinqiao) and Fructus Corni (Shanzhuyu). Su Jie et al. [48] speculated that it also has a certain curative effect on OA. Using network pharmacology methods and based on a systematic network database, a network of QRF targets for OA intervention was established. The active ingredients and mechanism of action of QRF in OA were predicted and analyzed, and it was concluded that QRF may act on targets such as IL-6, TNF and IL-1β through active ingredients such as G Re, G Rb1, GRg1 and GRd, regulating IL-17 signal pathways, TNF signaling pathway, Th 17 differentiation, Toll-like receptor signaling pathway, and osteoclast differentiation signaling pathway, etc. to achieve intervention in OA, providing a reference for clinical research on QRF intervention in OA. Huoxue Zhuyu Capsule is a traditional Chinese medicine formula composed of Angelica sinensis, Panax notoginseng, Boswellia serrata, borneol, Rhizoma Corydalis and Astragalus membranaceus in a ratio of 20:10:4:4:6:1. The main active ingredients are G Rg1 and G Rb1, etc. [2]. Jua et al. [2] found that Huoxue Zhuyu Capsule can improve the development of OA in a rat OA model induced by MIA.
4 Summary and outlook
Ginseng, as a traditional Chinese medicine, has broad clinical application prospects. Research on the pharmacological effects of ginsenosides has focused mainly on anticancer, antioxidant and immunostimulatory activities. However, many studies have provided evidence that ginsenosides can prevent and treat various inflammatory diseases, including OA, through anti-inflammatory effects.
OA, as a disease that mostly affects the elderly, has attracted increasing attention from researchers. Current studies have found that ginsenosides can exert therapeutic effects on OA through multiple pathways. Ginsenosides can regulate the expression ratio of the key factors Bax/Bcl-2 in chondrocyte apoptosis, inhibit chondrocyte apoptosis, or promote chondrocyte proliferation to protect chondrocytes. However, the related regulatory signal pathways are not yet clear and require further research. Secondly, ginsenosides can inhibit the expression of inflammatory signal factors in OA cells through signal pathways such as NF-KB, Notch and p38 MAPK, thereby exerting an anti-inflammatory effect. In addition, ginsenosides can inhibit chondrocyte senescence, thereby preventing the progression of OA cartilage damage. In addition to its anti-inflammatory effect, ginsenosides also have a certain degree of antioxidant stress [49]. Antioxidant stress is also a viable method for treating OA [50], but there have been few studies on whether ginsenosides have an effect on OA in terms of antioxidant stress, and its specific mechanism still needs further research.
In summary, with the deepening of research on ginsenosides, ginsenosides and their metabolites and derivatives can be used as effective drugs for the prevention and treatment of OA. Further research is needed on their therapeutic mechanisms to help develop OA treatment drugs and the clinical application of ginsenosides.
Reference:
[1] YI Y S. Ameliorative effects of ginseng and ginsenosides on rheumatic diseases[J]. Journal of Ginseng Research, 2019, 43(3): 335-341.
[2] JUA L J, HUA P P, CHEN P, et al. Huoxuezhitong capsule ameliorates MIA-induced osteoarthritis of rats through sup- pressing PI3K/ Akt/ NF-B pathway[J]. Biomedicine & Phar- macotherapy, 2020, 129:110471-110482.
[3] Duan Chao, Zhou Xijiang, Chen Yan, et al. Effects of ginsenoside Rgl on apoptosis of chondrocytes in rabbit knee osteoarthritis [J]. Chinese Medical Journal, 2018, 33(12): 2387-2392. [4] Wang Dongxue, Wu Xinmin, Lin Dongmei, et al. Research progress on the anti-gastric cancer effect of ginsenosides [J]. Specialty Research, 2022, 44(3): 118-123+128.
[5] Li Qian, Chai Yihui, Gao Jie, et al. Research progress on the modern pharmacological effects of ginseng [J]. Guiyang Journal of Traditional Chinese Medicine, 2019, 41(5): 89-92.
[6] Bao Lisha, Wang Huafang, Yang Jinying, et al. The effect of ginsenosides on osteoarthritis in rats [J]. Zhejiang Clinical Medicine, 2021, 23(1): 4-7.
[7] JI H K, YOUNG S Y, MI Y K, et al. Role of ginsenosides, the main active components ofpanar ginseng, in inflammatory re- sponses and diseases[J]. Journal of Ginseng Research, 2017, 41(4): 435-443.
[8] Gao Jian, Lv Shaowa. Research progress on the chemical composition and pharmacological effects of ginseng [J]. Chinese Medicine Herald, 2021, 27( 1): 127-130+137.
[9] AHMED T, RAZA S H, MARYAM A, et al. Ginsenoside Rb1 as a neuroprotective agent: a review [J]. Brain Research Bull- etin, 2016, 125: 30-43.
[ 10] MOHANAN P, SUBRAMANIYAM S, MATHIYALAGAN R, et al. Molecular signaling of ginsenosides Rb 1, Rg1, and Rg3 and their mode of actions [J]. Journal of Ginseng Re- search, 2018, 42(2): 123-132.
[ 11] O ’BRIEN M S, MCDOUGALL J J. Age and frailty as risk factors for the development of osteoarthritis[J]. Mechanisms ofAgeing and Development, 2019, 180: 21-28.
[ 12] LINN S, MURTAUGH B, CASEY E. Role of sex hormones in the development of osteoarthritis[J]. PM&R, 2012, 4(5): S169-S173.
[ 13] FRANCISCO V, PEREZ T, PINO J, et al. Biomechanics, ob- esity, and osteoarthritis. the role of adipokines: when the levee breaks [J]. Journal of Orthopaedic Research, 2018, 36 (2): 594-604.
[ 14] ALIZAI H, ROEMER F W, HAYASHI D, et al. An update on risk factors for cartilage loss in knee osteoarthritis assessed using MRI-based semiquantitative grading methods [J]. Euro- pean Radiology, 2015, 25(3): 883-893.
[ 15] FRANCISCO V, PEREZ T, PINO J, et al. Biomechanics, ob- esity, and osteoarthritis. the role of adipokines: when the levee breaks[J]. Journal ofOrthopaedic Research, 2018, 36(2): 594-604.
[ 16] ZHENG L L, Zhang Z J, SHENG P, et al. The role of metab- olism in chondrocyte dysfunction and the progression of osteoarthr- itis[J]. Ageing Research Reviews, 2021, 66: 101249-101266.
[ 17] ZHAI G J, Alteration of metabolic pathways in osteoarthritis[J]. Metabolites, 2019, 9(1): 11-22.
[ 18] WANG H, WANG Q, YANG M, et al. Histomorphology and innate immunity during the progression of osteoarthritis: does synovitis affect cartilage degradation[J]. Journal of Cellular Physiology, 2018, 233(2): 1342-1358.
[ 19] SKOU S T, ROOS E M, Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice[J]. Clinical and Experimental Rheumat- ology, 2019, 37( 120): 0112-0117.
[20] Yang X, Lin XJ. Research progress in the treatment of osteoarthritis [J]. Chinese Journal of Orthopaedics and Traumatology, 2019, 34(9): 900-903.
[21] HERMANN W, LAMBOVA S, MULLER-LADNER U, Current treatment options for osteoarthritis[J]. Current Rheumat- ology Reviews, 2018, 14:108-116.
[22] ZHU N, HOU J, MA G, et al. Network pharmacology identif- ies the mechanisms of action of shaoyao gancao decoction in the treatment of osteoarthritis[J]. Medical Science Monitor, 2019, 14(25): 6051-6073.
[23] CULLIFORD D J , MASKELL J, KIRAN A, et al. The life- time risk of total hip and knee arthroplasty: results from the UK general practice research database [J]. Osteoarthritis and Cartilage, 2012, 20: 519-524.
[24] HOU PW, FUP K, HSU H C, et al. Traditional Chinese medi- cine inpatients with osteoarthritis ofthe knee[J]. Journal ofTra- ditional and Complementary Medicine, 2015, 5(4): 182-196.
[25] ZHU Q G, LI J H, FANG M, et al. Effect of Chinese massage (Tui Na) on isokinetic muscle strength in patients with knee osteoarthritis[J]. Journal ofTraditional Chinese Medicine, 2016, 36(3): 314-320.
[26] WING S S, WAI T S, WEN C, et al. Topical application of Chinese herbal medicine DAEP relieves the osteoarthritic knee pain in rats[J]. Chinese Medicine, 2019, 14: 55.
[27] Pan B, Zhou Y, Fang F, et al. Current research status and treatment progress of osteoarthritis at home and abroad [J]. Chinese Journal of Basic Medicine in Traditional Chinese Medicine, 2019, 27(5): 861-865.
[28] CHENG W D, WU D Y, ZUO Q, et al. Ginsenoside Rb1 pre- vents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes[J]. International Orthopaed- ics, 2013, 37( 10): 2065-2070.
[29] ARAVINTHAN A, HOSSAIN M A, KIM B, et al. Ginseno- side Rb 1 inhibits monoiodoacetate-induced osteoarthritis in postmenopausal rats through prevention of cartilage degrada- tion[J]. Journal of Ginseng Research, 2021, 45(2): 287-294.
[30] Gao Z. Effects of the active ingredient ginsenoside Rb1 from the medicinal plant ginseng on osteoarthritis inflammation and proteoglycan degradation in rats with anterior cruciate ligament transection [J]. Molecular Plant Breeding, 2022, 20(14): 4800-4806.
[31] Wang W. Experimental study on the regulation of matrix metalloproteinase-13 by ginsenoside Rb1 through the Notch signaling pathway to prevent osteoarthritis [D]. Chongqing: Chongqing Medical University, 2015.
[32] HOSSAIN M A, ALAM M J, KIM B, et al. Ginsenoside-Rb1 prevents bone cartilage destruction through down-regulation ofp-Akt, p-P38, and p-P65 signaling in rabbit[J]. Phytomedi- cine, 2022, 100: 154039.
[33] Huang Yumin. Therapeutic effect and related mechanism of ginsenoside Rg1 on rat osteoarthritis [D]. Nanjing: Nanjing Medical University, 2015.
[34] HUANGYM, WU DY, FAN WM. Protection ofginsenoside Rg1 on chondrocyte from IL-1b-induced mitochondria-activ- ated apoptosis through PI3K/Akt signaling[J]. Molecular and Cellular Biochemistry, 2014, 392( 1): 249-257.
[35] CHENG W D, JING J H, WANG Z, et al. Chondroprotective effects of ginsenoside Rg 1 in human osteoarthritis chond- rocytes and a rat model of anterior cruciate ligament transec- tion[J]. Nutrients, 2017, 9(3): 263-275.
[36] WON H J, KIM H I, PARK T, et al. Non-clinical pharmaco- kinetic behavior of ginsenosides[J]. Journal of Ginseng Re- search, 2019, 43(3): 354-360.
[37] SO M W, LEE E J, LEE H S, et al. Protective effects ofginse- noside Rg3 on human osteoarthritic chondrocytes[J]. Modern Rheumatology, 2012, 23( 1): 104-111.
[38] MA C H, CHOU W C, WU C H, et al. Ginsenoside Rg3 At- tenuates TNF-a-Induced damage in chondrocytes through regulating SIRT1-mediated anti-apoptotic and anti-inflamma- tory mechanisms[J]. Antioxidants, 2021, 10( 12): 1972-1986.
[39] XU H L, CHEN G H, WU Y T, et al. Ginsenoside Ro, an ole- anolic saponin of pamnr ginseng, exerts antiinflammatory ef- fect by direct inhibiting toll like receptor 4 signaling pathway[J]. Journal of Ginseng Research, 2022, 46: 156-166.
[40] ZHANG X H, XU X X, XU T. Ginsenoside Ro suppresses in- terleukin-1β-induced apoptosis and inflammation in rat chondrocytes by inhibiting NF-KB [J]. Chinese Journal of Natural Medicines, 2015, 13(4): 283-289.
[41] LEE S Y, Anti-Metastatic and anti-inflammatory effects of matrix metalloproteinase inhibition by ginsenosides [J]. Bio- medicines, 2021, 9(2): 198-218.
[42] LEE J H, LIM H, SHEHZAD O , et al. Ginsenosides from ko- rean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation [J]. European Journal of Pharmacology, 2014, 724( 1): 145-151.
[43] YANG N, ZHANG X, LI L F, et al. Ginsenoside rc promotes bone formation in ovariectomy-induced osteoporosis in vivo and osteogenic differentiation in vitro[J]. International Jour- nal ofMolecular Sciences, 2022, 23( 11): 6187-6209.
[44] Li L, Li X, Si Y, et al. Research on the mechanism of ginsenoside Rb2 in protecting articular chondrocytes against oxidative stress [J]. Chinese Journal of Orthopaedics, 2018, 26(9): 845-849.
[45] ZHANG P. Ginsenoside-Rg5 treatment inhibits apoptosis of chondrocytes and degradation of cartilage matrix in a rat model of osteoarthritis[J]. Oncology Reports, 2017, 37(3): 1497-1502.
[46] KIM Y O, LEE S W, KIM D H, et al. Pharmaceutical compo- sition for preventing or treating bone diseases such as rheuma- toid or degenerative bone disease, comprises complex ginsenoside consisting ofginsenoside Rd and ginsenoside Re as active ingredient: KR1856477-B1[P]. 2018-05-14.
[47] SIDDIQIMH, SIDDIQI MZ, AHN S, et al. Stimulative effect of ginsenosides Rg5: Rk1 on murine osteoblastic MC3T3-E1 cells[J]. Phytotherapy Research, 2014, 28: 1447-1455.
[48] Su J, Zhou R, Wang M, et al. Prediction of active ingredients and mechanisms of action of Qinci rheumatism formula intervention in osteoarthritis based on network pharmacology [J]. Chinese Journal of Modern Traditional Chinese Medicine, 2022, 24(3): 476-487.
[49] Heng Ke. Experimental study on the prevention of hormone-induced femoral head necrosis by Rg1 inhibition of oxidative stress [D]. Nanjing: Nanjing Medical University, 2018.
[50] Xia Ganqing, Zhou Panghu. Research progress on the correlation between oxidative stress response and osteoarthritis and the application of antioxidant stress drugs [J]. Journal of Intractable Diseases, 2021, 20 (1): 94-98.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






