Study on Ginseng Extract Ginsenoside for Liver Protection
Ginseng is a medicinal plant in the genus Panax of the Araliaceae family. As a traditional precious medicinal herb, it has extremely high medicinal value and can tonify the vital energy, invigorate the spleen and benefit the lungs, engender body fluids, calm the mind and improve intelligence. Panaxoside is the main active ingredient in ginseng. Previous studies have mainly focused on its effects on the nervous system, endocrine system, anti-tumor synergy, and lowering blood sugar [1-2]. In recent years, ginsenosides have been increasingly valued for their application in liver diseases, and related research has involved liver cancer, liver fibrosis, liver damage, hepatitis and other diseases. Studies have shown that the mechanism of action of ginsenosides in different liver diseases is quite different, and the research on the therapeutic application of different ginsenoside monomers in various liver diseases is also different. Therefore, an in-depth exploration of the therapeutic effects and mechanisms of ginsenosides in different liver diseases is of great significance for the clinical application of ginsenosides in the treatment of liver diseases. The author combines domestic and foreign literature to review the application and related mechanisms of ginsenosides in liver diseases, with a view to further improving the clinical application value of ginsenosides.

1 Classification and pharmacological effects of ginsenosides
To date, more than 150 ginsenoside monomers have been isolated [3]. According to the different aglycones, ginsenosides can be divided into two types: oleanane and damarane. The chemical structures are shown in Figure 1. Except for ginsenoside Ro, which belongs to the oleanane type, the rest of the ginsenosides are all of the damarane type. Dammarane-type ginsenosides are further divided into diol-type and triol-type ginsenosides, the structural difference between the two being whether or not there is a hydroxyl group at C-6 [4]. Diol-type saponins include ginsenosides Ra1, Ra2, Ra3, Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, Rs1, and Rs2, as well as propanoyl-ginsenosides Rb1, Rb2, Rc, and Rd, notoginseng saponin R4, American ginseng saponin R1, 20(S)-ginsenoside Rg3, 20(R)-ginsenoside Rh2, 20(S)-ginsenoside Rh2; triterpenoid saponins including ginsenosides Re, Rf, Rg1, Rg2, Rh1, Rh3, Rf1, 20-glucosylginsenoside Rf, 20(R)-ginsenoside Rg2, 20(R)-ginsenoside Rh1, notoginsenoside R1, pseudoginsenoside R11, Rp1, Rt1, pseudoginsenoside R11, Rp1, Rt1,ginsenoside IV and IVa, and 20(R) protopanaxatriol[2]. The ginsenoside monomers that have been studied more are Rh2, Rg3, Rg1, Rb1, and Rh1. Due to the differences in structure between the ginsenoside monomers, their pharmacological effects also differ somewhat.
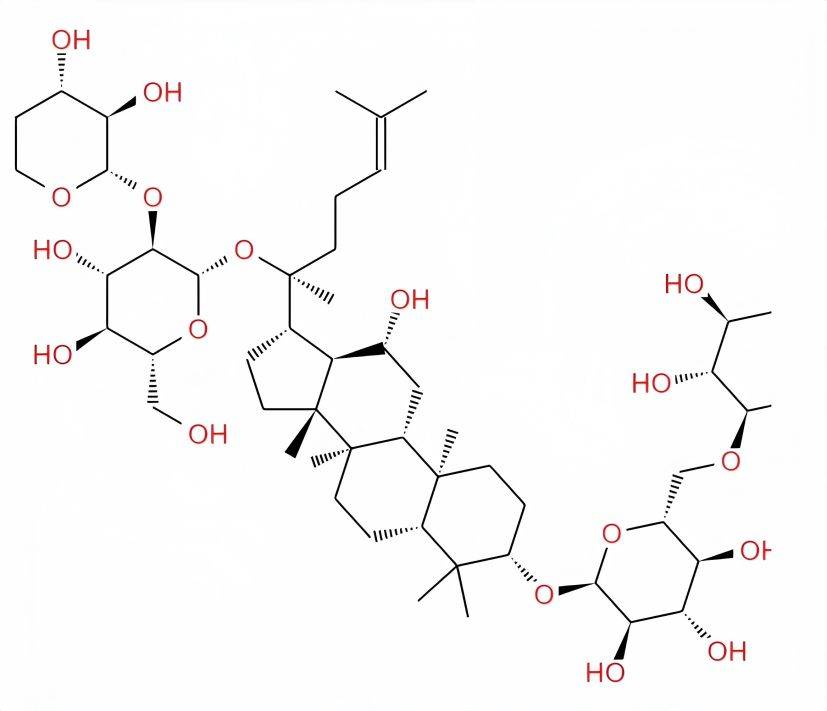
Among the above-mentioned monomers, ginsenosides Rh2 and Rg3 are currently commonly used anti-tumor drugs in clinical practice. Rh2 has the effect of enhancing the body's immunity and quickly restoring physical fitness, and can inhibit the metastasis of tumor cells to other organs. Rg3 can act on the G2 phase of the cell cycle, inhibit the synthesis of mitotic proteins and ATP in tumor cells, and promote apoptosis of tumor cells, inhibit the growth and infiltration of tumor cells, inhibit tumor cell metastasis [5-6]. In addition, Rg1 can quickly restore fatigue, improve learning and memory, delay aging, excite the central nervous system and inhibit platelet aggregation. Rb1 has the function of enhancing the cholinergic system, increasing the synthesis and release of acetylcholine and improving memory. Rh1 has the function of promoting liver cell proliferation and DNA synthesis, and can be used to treat and prevent hepatitis and cirrhosis [5-6].
2. The effect of ginsenosides on liver disease
Ginsenosides have been shown to have a beneficial effect in a variety of liver diseases. Their mechanism of action is complex and diverse, and they protect liver cells and regulate liver function mainly through antioxidant, anti-inflammatory and cytochrome P450 inhibition effects.
2.1. Liver cancer
Primary liver cancer is one of the most common malignant tumors in clinical practice. Ginseng saponins have a mild anti-tumor effect and relatively few adverse reactions, giving them unique advantages in the treatment of primary liver cancer. The results of multiple clinical studies have shown that ginsenosides can significantly enhance the body's immune function, relieve symptoms related to liver cancer, reduce the adverse reactions of interventional therapy, and can be used in conjunction with transcatheter arterial chemoembolization (TACE) to treat primary liver cancer, prolong survival, and improve quality of life [7-9]. Hepatitis and cirrhosis are very likely to progress to liver cancer. Among 300 patients with hepatitis or cirrhosis, those who took 1 g of red ginseng (the main active ingredient is ginsenoside) daily for 5 years had a higher survival rate than those who did not take red ginseng [8]. In addition, in a model of liver cancer induced by diethylnitrosamine in SD rats, it was found that after 10 weeks of consuming food containing 1% red ginseng extract, the experimental group could significantly improve liver function by regulating the redox environment of cells and reducing oxidative damage to cells, indicating that it also has a good hepatoprotective and antiviral effect in liver cancer patients [10]. In terms of cancer prevention, it was found that taking wild ginseng water extract (50 mg·kg-1·d-1) for 4 weeks can selectively inhibit the activity of cytochrome P450 in SD rats, inhibit the increase in CYP1A1 activity induced by benzo[a]pyrene by downregulating its gene expression, and thus inhibit the carcinogenic activity of benzo[a]pyrene [11].
A large number of studies have also been conducted on the anti-liver cancer effects of different ginsenoside monomers. Ginsenoside Rg3 is currently the most studied ginsenoside monomer in the field of anti-liver cancer, is often used in combination with chemotherapeutic drugs to achieve better efficacy. Zhou et al. [12] found that after oral administration of ginsenoside Rg3 (1 mg·kg-1) for 4 weeks in a rat model of Buffalo liver cell carcinoma, the overexpression of hepatocellular carcinoma after TACE in patients with orthotopic liver transplantation can be reduced. Yu et al. [13] used the liver VX2 tumor model in adult rabbits and found that Rg3 combined with TACE to treat liver cancer by regulating the hepatic artery. It has also been shown that ginsenoside Rg3 (5 mg·kg-1·d-1) given continuously by gavage for 10 days has a significant inhibitory effect on the formation of liver cancer blood vessels in Kunming mice, and combined with 5-fluorouracil, the efficacy is significantly better than 5-fluorouracil alone [14].
The mechanism of action may be that ginsenoside Rg3 inhibits the expression of vascular endothelial growth factor, basic fibroblast growth factor and matrix metalloproteinase-2, which are tumor vascular growth regulatory factor proteins [15]. In addition, ginsenoside monomers also have the effect of improving the body's immune function. By comparing the clinical efficacy, T lymphocyte and subgroup changes of the test group (61 cases) and the control group (30 cases) taking Ganshenyi Capsule (the active ingredient is ginsenoside Rg3), it is proved that the application of Ganshenyi Capsule during chemotherapy in breast cancer patients can significantly improve the symptoms of qi deficiency in tumor patients and improve their immune function [16].
As a degradation product of ginsenoside Rg3, ginsenoside Rh2 has a more obvious anti-tumor cell metastasis effect. It can inhibit the metastasis of human HepG2 liver tumor cells by restoring histone deacetylase and inhibiting the activation protein 1 transcription factor, and also has a unique effect in the treatment of liver cancer [17]. Li Linjun et al. [18] selected a total of 60 patients with advanced primary liver cancer and randomly divided them into a treatment group and a control group. Both groups were given transarterial chemoembolization, and the treatment group was given ginsenoside Rh2 in addition. It was found that this could prolong the time to disease progression and overall survival time in patients, with no significant adverse reactions. Fan Guanghua et al. [19] used flow cytometry to confirm that ginsenoside Rh2 has an apoptosis-inducing effect on the liver cancer cell line Bel-7047. The apoptosis rate increased with the concentration of ginsenoside Rh2 and the duration of the effect, and it was found that ginsenoside Rh2 induced apoptosis by blocking the cell cycle at the G1 phase induces apoptosis. Cheong et al. [20] found that the inhibition of liver tumor cells by different stereoisomers of Rg3 and Rh2 is different. The 20(S) configuration of Rg3 and Rh2 significantly induces autophagy and apoptosis in human liver tumor cells through mitochondria and calcium-related channels, and the 20(S) isomer of Rg3 and Rh2 has stronger anti-tumor activity than the 20(R) isomer, which suggests that isolating its isomers will help improve the efficacy of liver cancer treatment. In addition to Rg3 and Rh2, recent studies have found that Rg1 can inhibit the epithelial mesenchymal transition induced by transforming growth factor-β1 in liver cancer cells to reduce tumor cell invasion and metastasis [21].
2.2 Liver damage
Acute and chronic hepatotoxicity caused by drugs, alcohol, infections and poisoning may cause liver damage and liver fibrosis. Studies have found that ginsenosides not only have a protective effect on in vitro liver cell damage models, but also against hydrogen peroxide [22], alcohol [23], CCl4 [22-25], aflatoxin B1[26-27], fumonisin[27], tert-butyl hydroperoxide[28], cadmium chloride[29-30], benzo[a]pyrene[11], and thioacetamide[31]. Studies have reported that ginsenosides can increase the activity of superoxide dismutase, catalase, glutathione peroxidase, etc., and the mechanism of ginsenosides' liver protection is closely related to their antioxidant properties [32-33]. In a rat model of liver damage induced by CCl4, ginsenoside CK at a low dose (0.3 mg·kg-1) can reduce the serum ALT and AST levels, increase the content of superoxide dismutase in the serum, and reduce the MDA content; CK has no significant effect at medium or high doses. This indicates that low-dose ginsenoside CK has a protective effect against chronic liver damage caused by CCl4, which may be related to its antioxidant effect [34].
CYP is a key enzyme involved in the metabolism of endogenous substances and exogenous substances, including drugs and environmental compounds, that is present in liver cells. As mentioned above, ginsenosides inhibit the transcription and expression of CYP, thereby playing an important role in reducing liver damage caused by toxins. In addition, some studies have shown that after ginseng saponins are taken orally, this effect is not caused by the ginseng saponins themselves, but by some metabolized ginseng saponins (such as ginsenoside CK), which ultimately inhibit the metabolic activity of CYP [35].
2.3 Liver fibrosis
Liver fibrosis refers to the excessive deposition of fibrous connective tissue in the liver, which is the result of an imbalance between fibrous hyperplasia and fibrous decomposition. Fibrous hyperplasia is the body's repair response to damage. Repeated or persistent chronic inflammation and necrosis of the liver parenchyma due to various causes can lead to persistent fibrous hyperplasia of the liver and the formation of liver fibrosis. Ginseng saponins can reduce liver fibrosis and liver damage. Studies have found that ginsenoside Rb1 (0.05 g·kg-1 orally) can reduce elevated ALT and AST plasma concentrations in liver damage in rats, and can inhibit the accumulation of triglycerides in the liver, thereby reducing CCl4-induced liver cell fibrosis in rats [25], which indicates that ginsenosides can accelerate liver cell repair and reduce liver cell damage. Rg1 can prevent liver fibrosis induced by thioacetamide and CCl4, significantly inhibit liver fibrosis markers in the serum, and treat the increase in hydroxyproline in the liver tissue of rats caused by thioacetamide. In cell culture experiments, Rg1 significantly inhibited the proliferation and activation of hepatic stellate cells, and the effect of inhibiting hepatic fibrosis became more pronounced with increasing dose (15, 50, 100 mg·kg-1) [31]. The mechanism may be that it exerts an anti-fibrosis effect by activating the Nrf2 pathway and increasing the expression of antioxidant enzymes [36].
2.4 Liver ischemia-reperfusion injury
Liver ischemia-reperfusion injury often occurs during shock and liver surgery that requires interruption of the blood flow in the hepatic pedicle. It is one of the important factors affecting liver function after liver transplantation and segmental resection. Liver ischemia-reperfusion injury may be caused partly by the damage produced during liver ischemia and partly by a series of damages caused when the ischemic liver is reperfused with blood [37]. Guo et al. [38] found that that oral administration of Rh1 (20 mg·kg-1) after liver ischemia-reperfusion in mice significantly reduced liver function and morphological damage caused by ischemia-reperfusion, and significantly reduced serum ALT. At the same time, Rb1 significantly reduced MDA concentration, and increased nitric oxide and inducible nitric oxide synthase concentrations. This shows that Rb1 can prevent and treat liver ischemia-reperfusion injury by anti-oxidative damage. Other studies have also shown that intravenous administration of Rg1 (20 mg·kg-1) can protect mice from liver ischemia-reperfusion injury by anti-inflammatory and anti-apoptotic effects [39].
2.5 Hepatitis
Ginsenosides have a good anti-inflammatory effect and can reduce liver cell damage. Studies have found that ginsenosides can inhibit the activity of inflammatory cytokines (interleukin-1β, interferon-γ) and chemokines (monocyte chemotactic protein-1, macrophage inflammatory protein-2β) in rats treated with CCl4 [40]. It has also been found that ginsenoside Rd can inhibit TNF-α-induced activation of transcription factors, which further inhibits the gene expression of inducible nitric oxide synthase and cyclooxygenase-2 in tumor cells, thereby producing significant anti-inflammatory activity [41]. In addition, for viral hepatitis, ginsenosides also have a significant protective effect on liver cells and can improve a range of symptoms of viral hepatitis [42].
2.6 Fatty liver
Liver adipose tissue plays an important role in lipid and glucose metabolism. Studies have found that rats that consume a certain amount of ginseng extract show significant changes in lipid and glucose metabolism [43-44]: the liver cholesterol and triglyceride content of rats decreases, while the phospholipid content increases, indicating that ginsenosides can effectively improve fatty liver symptoms [45]. A high-fat diet causes fatty liver in rats. After intraperitoneal injection of Rb1 (10 mg·kg-1) for 4 weeks, it was found that Rb1 significantly improved the accumulation of liver fat in obese rats induced by a high-fat diet, reduced liver weight, and reduced the content of triglycerides in the liver. Histological evaluation was performed using HE staining and oil red staining of liver sections. the results showed that ginsenoside Rb1 mainly alleviates liver fat accumulation and liver hypertrophy by activating adenosine monophosphate kinase, thereby treating fatty liver [46].
2.7 Other
Acute rejection is common after liver transplantation. Inflammatory cytokines such as TNF-α play an important role in the pathogenesis of graft-versus-host disease. Ginsenosides can effectively inhibit pro-inflammatory cytokines such as TNF-α, effectively treat acute graft-versus-host rejection, and improve the survival rate of transplanted organs. Twelve liver transplant patients were suspected of developing graft-versus-host disease. After one patient took Korean ginseng, the inflammatory indicators decreased compared to before taking it, and immunosuppression improved, temporarily restoring immune balance in the body [47].
In addition, by monitoring liver weight and hepatocyte proliferation rate, ginsenosides can significantly increase the liver regeneration capacity of dogs undergoing partial hepatectomy [48]. Specifically, 15 beagle dogs were divided into three groups: a control group (40% hepatectomy, no medication), an experimental group 1 (40% hepatectomy, oral administration of 250 mg·kg-1 of Korean ginseng aqueous solution for 1 week), and an experimental group 2 (40% hepatectomy, oral administration of 500 mg·kg-1 of Korean ginseng aqueous solution for 1 week). In the experiment, liver tissue regeneration, histological examination, routine blood test, and liver function test were observed. The results showed that the liver tissue regeneration rate of the experimental group was higher than that of the control group, and that it was the main effective component ginsenoside that was playing a role.
Acute liver failure is characterized by its rapid onset and high mortality rate among liver diseases. Studies have shown that ginsenoside Rg1 can significantly reduce liver damage in a mouse model of acute liver failure, thereby alleviating the symptoms of acute liver failure. This provides a theoretical basis for the use of ginsenosides in the treatment of clinical acute liver failure [49].

3 Conclusion
In summary, ginsenosides, the extract of ginseng, have a wide range of pharmacological effects in the treatment of liver cancer, liver fibrosis, liver damage, hepatitis, liver ischemia reperfusion, fatty liver and other diseases. The mechanism of action has been partially studied and discussed, and some progress has been made. However, the current analysis of its liver protection mechanism is still focused on several aspects such as antioxidant, anti-inflammatory, inhibition of CYP450 and promotion of liver cell regeneration. Although the current therapeutic effects show certain advantages, there is a lack of more extensive clinical studies to further confirm their efficacy. Therefore, the application of ginsenosides in liver diseases requires further research. In addition, it is also important to explore the possible synergistic effects of ginsenosides when used in combination with other drugs for liver diseases. The author believes that with further research, ginsenosides will have a wider application in liver diseases.
Reference:
[1]WU D D, LIU Q. Synergistic effects of ginsenoside Rg3 with cyclophosphamide on tumor-bearing mice [J]. Her Med, 2010, 29(7): 850-853.
[2] Li Yang, Zhang Tiejun, Liu Suhuang, et al. Research progress on the chemical composition and pharmacological effects of ginseng [J]. Chinese Herbal Medicine, 2009, 40(1): 164-166.
[3]HRISTENSEN L P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects [J]. Adv Food Nut Res, 2009, 55: 1-99.
[4] HUU TUNG N, UTO T, MORINAGA O, et al. Pharmacological effects of ginseng on liver functions and diseases: a minireview [J]. Evid Based Complement Alternat Med, 2012, 2012: 173297. Doi:10.1155/2012/173297
[5] DOU D, WEN Y, PEI Y P, et al. Studies on the minor saponins from leaves of Panax ginseng C.A.Meyer [J]. China J Chin Mater Med, 1997, 22(1): 36-37.
[6] Wang Hongyan, Xu Suixu, Chen Yingjie, et al. New progress in the pharmacological activity research of ginseng monomer components [J]. Chinese Journal of Medicinal Chemistry, 1992, 2(3): 73-78.
[7] HELMS S. Cancer prevention and therapeutics: Panax ginseng [J]. Altern Med Rev, 2004, 9(3): 259-274.
[8] Ginseng-HCC Chemopreventive Study Osaka Group. Study on chemoprevention of hepatocellular carcinoma by ginseng: an introduction to the protocol [J]. J Korean Med Sci, 2001, 16(Suppl): S70-S74.
[9] Zhao Gang. Effects of ginsenosides on the immune microenvironment of primary liver cancer [D]. Nanjing: Nanjing University of Traditional Chinese Medicine, 2009.
[10] KIM H, HONG MK, CHOI H, et al. Chemopreventive effects of korean red ginseng extract on rat hepatocarcinogenesis [J]. J Cancer, 2015, 6(1): 1-8.
[11]GUM S I, JO S J, AHN S H, et al. The potent protective effect of wild ginseng (Panax ginsengC.A. Meyer) against benzo[alpha]pyrene-induced toxicity through metabolic regulation of CYP1A1 and GSTs [J]. J Ethnopharmacol, 2007, 112(3): 568-576.
[12] ZHOU B, WANG J H, YAN Z P. Ginsenoside Rg3 attenuates hepatoma VEGF overexpression after hepatic artery embolization in an orthotopic transplantation hepatocellular carcinoma rat model [J]. Onco Targets Ther, 2014(7): 1945-1954.
[13] YU Y, ZHANG C, LIU L, et al. Hepatic arterial administration of ginsenoside Rg3 and transcatheter arterial embolization for the treatment of VX2 liver carcinomas [J]. Exp Ther Med, 2013, 5(3): 761-766.
[14] MA Y, LI Y L, KONG L. Effect of ginsenoside Rg3 on expression of VEGF in mice with hepatocellular carcinoma [J]. Chin J Integr Tradit West Med Liver Dis, 2014, 24(1): 41-42, 46.
[15] CHEN M W, NI L, ZHAO X G, et al. The inhibition of 20(R)-ginsenoside Rg3 on the expressions of angiogenesis factors proteins in human lung adenocarcinoma cell line A549 and HUVEC304 cell [J]. China J Chin Mater Med, 2005, 30(5): 357-360.
[16] LIU J W, SUN L X, ZHAO Y, et al. Clinical phase Ⅱ study on immunoimprovement of patients with breast cancer treated by Shenyi capsule [J]. Chin J Clin Oncol, 2000, 27(7): 534-536.
[17] SHI Q, LI J, FENG Z, et al. Effect of ginsenoside Rh2 on the migratory ability of HepG2 liver carcinoma cells: recruiting histone deacetylase and inhibiting activator protein 1 transcription factors [J]. Mol Med Rep, 2014, 10(4): 1779-1185.
[18] Li Linjun, Yu Jianyun, Li Min, et al. Clinical study on the treatment of liver-stagnation-qi-stagnation type advanced hepatocellular carcinoma with ginsenoside Rh2 combined with transarterial chemoembolization [J]. Chinese Medicine Herald, 2015, 21(1): 81-83.
[19] FAN G H, JIANG H, OU W S. The Effect of ginsenoside-Rh2 in induction of apoptosis on Bel-7404 cell line [J]. Pract J Cancer, 2003, 18(1): 16-18.
[20] CHEONG J H, KIM H, HONG M J, et al. Stereoisomer- specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers [J]. Biol Pharm Bull. 2015, 38(1): 102-108.
[21] YU M, YU X, GUO D, et al. Ginsenoside Rg1 attenuates invasion and migration by inhibiting transforming growth factor-β1-induced epithelial to mesenchymal transition in HepG2 cells [J]. Mol Med Rep, 2015, 11(4): 3167-3173.
[22] BAK M J, JUN M, JEONG W S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated hepG2 cells and CCl(4)-treated mice [J]. Int J Mol Sci, 2012, 13(2): 2314-2330.
[23] ZUIN M, BATTEZZATI P M, CAMISASCA M, et al. Effects of a preparation containing a standardized ginseng extract combined with trace elements and multivitamins against hepatotoxin-induced chronic liver disease in the elderly [J]. J Int Med Res, 1987, 15(5): 276-281.
[24] EL DENSHARY E S, AL-GAHAZALI M A, MANNAA F A, et al. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats [J]. Exp Toxicol Pathol, 2012, 64(7/8): 753-760.
[25] HOU Y L, TSAI Y H, LIN Y H, et al. Ginseng extract and ginsenoside Rb1 attenuate carbon tetrachloride-induced liver fibrosis in rats [J]. BMC Complement Altern Med, 2014, 14:415.
[26] KIM Y S, KIM Y H, NOH J R, et al. Protective effect of korean red ginseng against aflatoxin B1-induced hepatotoxicity in rat [J]. J Ginseng Res, 2011, 35(2): 243-249.
[27] ABDEL-WAHHAB M A, HASSAN N S, EL-KADY A A, et al. Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats [J]. Food Chem Toxicol, 2010, 48(2): 733-742.
[28] LEE H, KIM J, LEE S Y, et al. Processed Panax ginseng, Sun ginseng, decreases oxidative damage induced by tert-butyl hydroperoxide via regulation of antioxidant enzyme and anti-apoptotic molecules in HepG2 cells [J]. J Ginseng Res, 2012, 36(3): 248-255.
[29] SHUKLA R, KUMAR M. Role of Panax ginseng as an antioxidant after cadmium-induced hepatic injuries [J]. Food Chem Toxicol, 2009, 47(4): 769-773.
[30] KARADENIZ A, CEMEK M, SIMSEK N. The Effects of panax ginseng and spirulina platensis on hepatotoxicity induced by cadmium in rats [J]. Ecotoxicol Environ Saf, 2009, 72(1): 231-235.
[31] GENG J, PENG W, HUANG Y, et al. Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis induced by thioacetamide in rats [J]. Eur J Pharmacol, 2010, 634(1/3): 162-169.
[32] KANG K S, YAMABE N, KIM H Y, et al. Effect of sun ginseng methanol extract on lipopolysaccharide-induced liver injury in rats [J]. Phytomedicine, 2007, 14(12): 840-845.
[33] YOKOZAWA T, KANG K S, YAMABE N, et al. Therapeutic potential of heat-processed Panax ginseng with respect to oxidative tissue damage [J]. Drug Discov Ther, 2007, 1(1): 30-44.
[34] ZHANG L M, FU F H, WANG T, et al. Effect of Compound K on chronic hepatic injury induced by carbon tetrachloride(CCl4) in rats [J]. Lishizhen Med Mater Med Res, 2006, 17(1): 38-39.
[35] LIU Y, ZHANG J W, LI W, et al. Ginsenoside metabolites, rather than naturally occurring ginsenosides, lead to inhibition of human cytochrome P450 enzymes [J]. Toxicol Sci, 2006, 91(2): 356-364.
[36] LI J P, GAO Y, CHU S F, et al. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCl4-induced hepatic fibrosis [J]. Acta Pharmacol Sin, 2014, 35(8): 1031-1044.
[37] Wang B. Research progress on the pathogenesis of liver ischemia-reperfusion injury [J]. Organ Transplantation, 2010, 1(5): 317-320.
[38] GUO Y, YANG T, LU J, et al. Rb1 postconditioning attenuates liver warm ischemia-reperfusion injury through ROS-NO-HIF pathway [J]. Life Sci, 2011, 88(13/14): 598-605.
[39] TAO T, CHEN F, BO L, et al. Ginsenoside Rg1 protects mouse liver against ischemia-reperfusion injury through anti-inflammatory and anti-apoptosis properties [J]. J Surg Res, 2014, 191(1): 231-238.
[40] SHIM J Y, KIM M H, KIM H D, et al. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response [J]. ToxicolAppl Pharm, 2010, 242(3): 318-325.
[41] SONG S B, TUNG N H, QUANG T H, et al. Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by dammarane-type saponins from Panax ginseng leaves [J]. J Ginseng Res, 2012, 36(2): 146-152.
[42] ABDEL-WAHHAB M A, GAMIL K, AHMED A, et al. Therapeutic effects of korean red ginseng extract in egyptian patients with chronic liver diseases [J]. J Ginseng Res, 2011, 35(1): 69-79.
[43] YOKOZAWA T, SENO H, OURA H. Effect of ginseng extract on lipid and sugar metabolism. I. Metabolic correlation between liver and adipose tissue [J]. Chem Pharm Bull (Tokyo), 1975, 23(12): 3095-3100.
[44] YOKOZAWA T, KANAI K, TAKEFUJI M, et al. Effect of ginseng saponin on liver glycogen content [J]. Chem Pharm Bull (Tokyo), 1976, 24(12): 3202-3204.
[45]YAMAMOTO M, UEMURA T, NAKAMA S, et al. Serum HDL-cholesterol-increasing and fatty liver-improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man [J]. Am J Chin Med, 1983, 11(1/4): 96-101.
[46] SHEN L, XIONG Y, WANG D Q, et al. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats [J]. J Lipid Res, 2013, 54(5): 1430-1438.
[47] XU X, LING Q, WEI Q, et al. Korean red ginseng: a new approach for the treatment of graft-versus-host disease after liver transplantation [J]. Transplant Proc, 2011, 43(7): 2651-2655.
[48] KWON Y S, JANG K H, JIANG I H. The effects of Korean red ginseng (Ginseng radix rubra) on liver regeneration after partial hepatectomy in dogs [J]. J Vet Sci, 2003, 4(1): 83-92.
[49] ZHAO J, SHI Z, LIU S, et al. Ginsenosides Rg1 from Panax ginseng: a potential therapy for acute liver failure patients? [J]. Evid Based Complement Alternat Med, 2014, 2014: 538059.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






