Study on Lycopene for Men's Health
Lycopene is a needle-like dark red crystal [1] that is soluble in chloroform, benzene and oil, but not in water. Its molecular formula is C40 H56, relative molecular mass 536.85, and the molecular structure has 11 conjugated double bonds and 2 non-conjugated double bonds, making it a straight-chain hydrocarbon [2]. Lycopene is widely found in the fruits and vegetables in our daily lives, especially in ripe tomatoes. It is the pigment with the highest degree of unsaturation in the carotenoid family.
In natural fruits and vegetables, lycopene mainly exists in the all-trans configuration. It has strong antioxidant activity, but the human body cannot synthesize it on its own, so it needs to be obtained from the outside [3⁃4]. After entering the human body, lycopene is widely distributed in tissues and organs such as the blood, adrenal glands, liver, testes, prostate, ovaries, mammary glands, uterus and digestive tract [5]. In addition to its antioxidant function, lycopene also has the effects of lowering lipids, improving immunity, protecting cardiovascular and cerebrovascular health and preventing cancer [6]. In recent years, there has been a research boom in China and abroad on the prevention and treatment of animal and human diseases with lycopene, and a series of important research results have been obtained. This article provides a brief overview of the research progress of lycopene in the field of male reproduction.

1 Lycopene and prostate-related diseases
The prostate is a male accessory gland organ. Diseases caused by prostate abnormalities such as benign prostatic hyperplasia, chronic prostatitis and prostate cancer pose a great threat to male reproductive health. Research on lycopene and prostate-related diseases focuses on the following aspects:
1.1 Benign prostatic hyperplasia (BPH)
Benign prostatic hyperplasia (BPH) is a disease caused by abnormal proliferation of the stromal and epithelial cells of the prostate, resulting in enlargement of the prostate and compression of the urethra [7]. In China, the incidence of BPH tends to increase with age. Because the initial symptoms of BPH are not obvious, many patients do not take them seriously. Over time, the symptoms of BPH gradually worsen, and the risk of surgery also significantly increases [8⁃9]. Some studies have shown [10] that lycopene treatment of mice with BPH significantly reduced testosterone levels, dihydrotestosterone levels, and the ratio of testosterone to estradiol. Other studies have also shown [11] that lycopene inhibits the expression of cyclin A and B and other proteins, causing the cells of the prostate epithelium to stop dividing and thus inhibiting the growth of the prostate gland.
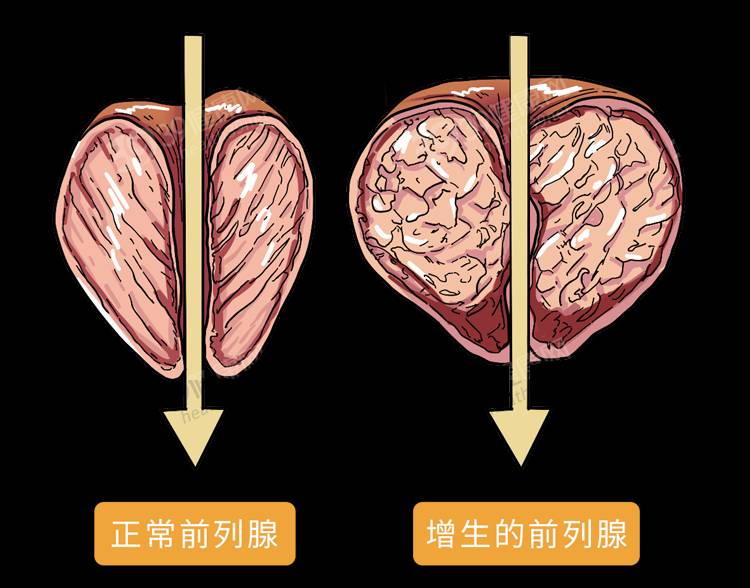
WERTZ K et al. [12] found in their research that lycopene can inhibit the proliferation of normal cells and cancer cells in the prostate epithelium by inhibiting the IGF-I signaling pathway and androgen signaling pathway in the prostate, reduce DNA damage, and improve the oxidative stress resistance of cells, thereby effectively preventing BPH and preventing the transformation of prostatic hyperplasia into prostate cancer. MASEREJIAN et al. [13] conducted a cross-sectional multivariate analysis of 1,499 cases of men aged 30-79 in the Boston Community Health Survey (2002-2005) in the United States and found that among men with BPH who consumed more lycopene in their diet or high-lycopene products, the incidence of lower urinary tract symptoms (LUTS) such as frequent urination, urinary symptoms such as frequency, urgency and pain (LUTS) were reduced by about 40% to 50%. Li Yize et al. [14] treated 127 patients with confirmed BPH with lycopene for 16 consecutive weeks. The clinical research results found that after 8 weeks of treatment, the patients' scores on the International Prostate Symptom Score (IPSS) and Quality of Life (QOL) improved significantly, and the concentration of prostate-specific antigen (PSA) decreased significantly. After 16 weeks of treatment, the difference between the patients and the pre-treatment results was even more significant in all of the above indicators, that is, lycopene has a significant effect on relieving the symptoms of BPH.
1.2 Chronic prostatitis (CP)
Chronic prostatitis is common in adult men under 50 years old who are under high pressure, have an irregular work-rest schedule, or do not pay attention to their diet. Clinical manifestations include localized pain in the groin on both sides of the perineum, LUTS, and sexual dysfunction. If not treated in time, it will seriously affect the quality of life and increase the financial pressure on patients. In addition, due to the large number of people suffering from this disease, it has been listed by the US National Institutes of Health as one of the chronic diseases that most seriously affect people's quality of life [15]. Zhao et al. [16] found that lycopene can inhibit bacterial growth and tissue inflammation, and has a certain therapeutic effect on chronic prostatitis. It can down-regulate the production of cytokines (such as TNF-α and IL-2) and chemokines (such as MCP-1 and MIP-1α), and up-regulate the expression of antioxidant genes. In addition, Zhao Qinxuan et al. [17] found that compared with the addition of a single antioxidant lycopene, the combination of quercetin and curcumin had a better therapeutic effect on chronic prostatitis in rats, with significantly reduced expression levels of inflammatory factors and significantly increased activity of antioxidant enzymes.
1.3 Prostate cancer
Prostate cancer is common in middle-aged and elderly men, and the incidence generally increases with age. At the same time, the occurrence of prostate cancer is closely related to family inheritance [18]. Most prostate cancers are caused by the amount of free radicals in the body exceeding the upper limit of its own ability to clean up and generating oxidative stress [19]. Recent studies have shown that lycopene may play a role in fighting prostate cancer by means of antioxidant protection, quenching singlet oxygen[20], inhibiting the proliferation, adhesion and migration of tumor cells, and affecting the expression of tumor-related genes and proteins[21]. In addition, lycopene, as a strong antioxidant, can protect lymphocytes and macrophages from the attack of a large number of free radicals and thus has immunoprotective functions [22].
Giovannucci [23] conducted a follow-up study on 48,000 medical personnel in the United States from 1986 to 1992 and confirmed that lycopene is negatively correlated with the occurrence of prostate cancer. Several studies have confirmed that adding a moderate amount of lycopene to the daily diet plays a key role in the prevention of prostate cancer. The incidence of prostate cancer in people who regularly eat tomatoes or foods high in lycopene is significantly lower than in people who occasionally or do not consume lycopene at all [24⁃27]. In addition to being used alone, lycopene also shows good results in combination with other drugs. For example, lycopene and maca extract combined at low doses can inhibit prostate hyperplasia and thus control the incidence of prostate cancer [28]. It can be seen that healthy people can prevent prostate-related diseases, reduce the incidence of prostate-related diseases, and ultimately delay or stop prostate cancer by changing their eating habits and eating more lycopene-rich fruits and vegetables as well as health supplements. This is of great significance for promoting men's reproductive health.
2 Lycopene and reproductive toxic damage
There are many factors in daily life that can cause reproductive toxic damage, such as smoking, heat stress in high-temperature environments, inorganic and organic toxic pollutants, and radiation[29⁃30] . Reproductive toxic damage is closely related to the oxidative stress caused by the above factors, and lycopene can slow down possible reproductive toxic damage by regulating the antioxidant reduction level of cells. Important progress has been made in the following areas:
2. 1 Nano⁃TiO2
Nano⁃TiO2, commonly known as titanium dioxide, is widely used in fields such as food coloring, sunscreens and nanomedicine[31]. In recent years, the toxic effects of Nano⁃TiO2 on the human body have gradually attracted attention and attention. After Nano⁃TiO2 enters the digestive tract, it penetrates the blood⁃testis barrier and gradually accumulates in the testes over time, eventually causing reproductive problems such as atrophy of the seminiferous tubules, reduction in the thickness of the seminiferous layer, decreased semen quality in the epididymis, and damage to sperm cells [32]. Previous studies [33–37] have shown that after the male reproductive system is stimulated by Nano-TiO2, the level of reactive oxygen species (ROS) in testicular support cells increases, and the levels of enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) decrease significantly, resulting in oxidative stress and tissue damage. An Hongmei et al. [38] showed that after subacute nano-titanium dioxide poisoning, oxidative damage occurred in the reproductive system of male rats. However, after lycopene was added to intervene, the sperm activity rate and the quality of the seminal fluid in the epididymis of mice were significantly improved, the histopathological damage to the testes was reduced, and the activities of antioxidant enzymes such as SOD, CAT and GPX were increased. The above study shows that lycopene can indeed relieve the oxidative stress caused by nano-TiO2 through its antioxidant capacity, thereby reducing the damage to testicular tissue.

2. 2 Trans fatty acids (TFA)
Trans fatty acids can reduce male hormone secretion and cause a decrease or damage to rat sperm. Recent studies on TFA at home and abroad have shown that the intake of TFA is inversely proportional to sperm concentration and total sperm count. High TFA intake (more than 1% of energy consumption) is currently one of the risk factors for infertility [39]. In the study by Yao Mingyan et al. [40], after administering different concentrations of lycopene to rats with high trans fatty acid intake by gavage, testicular tissue sections were taken for pathological analysis. It was found that compared with the control group, the testes of the TFA-infected group showed degeneration and necrosis of some spermatogonial epithelial cells, chaotic cell arrangement, atrophy of seminiferous tubules, sperm count decreased and basement membrane thinned. However, when lycopene was added to the TFA-treated group, the above pathological changes were alleviated, and a certain dose-effect relationship was observed. The author speculated that the mechanism of action may be related to the anti-lipid, anti-oxidation and free radical scavenging effects of lycopene itself. In other words, lycopene has a certain repair effect on the reproductive damage caused by TFA.
2. 3 Environmental pollution and radiation
2. 3. 1 Cadmium
Cadmium is a heavy metal whose compounds are widely used in preservatives, pigments and cadmium batteries. Improper recycling and disposal of these compounds can enter the human body through the digestive tract and respiratory tract [41]. The cadmium content of most foods is very low, but the damage caused by its long-term accumulation to the body should not be underestimated [42]. The male reproductive system is an important target of cadmium. The testes are very sensitive to cadmium, and low concentrations of cadmium can cause damage to testicular tissue, mainly manifested as a decrease in sperm count in adults, a decrease in SOD and GSH-Px activity in testicular tissue, and an increase in MDA content. Cadmium can also cause tumors and disrupt the reproductive endocrine system.
Wu Bo et al. [46] showed that in cadmium-poisoned rats, the activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and the level of testosterone (T) in the testes, seminal vesicles and epididymis were significantly reduced, while the level of luteinizing hormone (LH) in the serum was significantly increased. After lycopene intervention, the antioxidant enzyme SOD, GSH-Px and MDA activities and reproductive hormone levels in rats rebounded, and the alleviating effect was positively correlated with the concentration of lycopene. Zhang Ming et al. [47] found that cadmium can inhibit the activity of the oxidase in testicular support cells, damage the DNA of support cells and testicular tissue, and ultimately lead to infertility. In addition, studies have shown that lycopene also has a certain mitigating and repairing effect on the oxidative damage caused to the body by other heavy metals in the environment, such as iron [48], lead [49] and mercuric chloride [50].

2. 3. 2 Perfluorooctane sulfonate (PFOS)
Perfluorooctane sulfonate is an important environmental pollutant that has attracted global attention in recent years. A large number of animal studies have shown that PFOS can cause damage to multiple organs such as the reproductive system, liver and nervous system. Toxicological studies have shown that rats exposed to PFOS have lower sperm quality, which is manifested by a significant decrease in sperm count and a decrease in motility, as well as asthenospermia and an increase in the rate of deformities [51]. SIMON GIAVAROTTI KA et al. [52] found that PFOS can directly damage reproductive cells through lipid peroxides (LPO), leading to a decrease in the testicles' ability to remove free radicals and a large accumulation of lipid peroxides, thus causing damage to the reproductive glands. LIU Guoqing et al. [53] found that after administering low, medium and high doses of lycopene to PFOS-intoxicated mice by gavage, found that compared with the PFOS-treated group, the low, medium and high lycopene-treated groups had significantly higher sperm counts, sperm motility and serum testosterone concentrations, significantly restored SOD and CAT enzyme activity, and significantly reduced MDA levels. This indicates that a certain dose of lycopene can antagonize the damage caused by PFOS to the reproductive system by its antioxidant properties and ability to regulate testosterone secretion.
Coal-burning fluorine poisoning is a type of fluorine poisoning that occurs frequently in China. Fluorine poisoning can lead to changes in the structure and function of spermatogonia in the testes and increase the number of apoptotic spermatogonia [54]. Tian Yuan et al. [55] showed through experiments that compared to the control group, the fluorine content in the testes, the degree of histopathological damage to the testes, and the index of apoptosis of spermatogonia in the testes of rats treated with low, medium, and high concentrations of fluoride were significantly increased, while the level of clusterin protein decreased. However, after lycopene was added to the high fluoride group, all of the above indicators were significantly reversed, suggesting that lycopene can alleviate reproductive damage caused by coal-burning fluorine poisoning.

2. 3. 3 Radiation
The vigorous development of modern nuclear medicine and ionizing radiation technology has also brought hidden dangers to human health. Short-term ionizing radiation can cause serious damage to tissues and organs, as well as serious harm to the immune system, blood system, and reproductive system [56], with the testicles being the most severely damaged in men [57]. Common sources of ionizing radiation in life can produce ROS in the reproductive system. When the production of ROS exceeds the body's ability to remove it, it may damage the integrity of the biological membrane, leading to sperm DNA damage or chromosomal abnormalities [58].
Li Yize's[59] research shows that after different concentration gradients of lycopene were administered to rats for 24 days, which had been made listless, lethargic and slow to respond by ionizing radiation, the sperm concentration and sperm activity of the rats recovered significantly. The structure of the testicles and prostate tended to normalise, and the levels of interleukin 6 (IL-6), tumour necrosis factor (TNF-α), interleukin 8 (IL⁃8) and MDA levels were up-regulated and tended to normal, indicating that lycopene alleviated the inflammatory symptoms of the reproductive system to a certain extent, enhanced immunity, alleviated oxidative stress damage to the reproductive system, and promoted the occurrence and maturation of sperm. In other words, lycopene can indeed reduce the reproductive damage caused by radiation in rats by regulating oxidative stress.
2. 3. 4 Acrylonitrile (ACN)
Acrylonitrile is an important raw material for the production of acrylic fibers, acrylonitrile-butadiene-styrene terpolymer resins, and nitrile rubber. It is toxic to various organs and systems, such as the nervous system and digestive system, and can even cause cancer [60]. Studies have found that acrylonitrile is also harmful to the reproductive system, and the main mechanism causing disease is oxidative stress damage. Acrylonitrile can stimulate the testes to produce excessive ROS[61] , and these ROS act on proteins, lipids and nucleic acids, causing toxic damage[62] . Li Xiuju et al.[63] studied the mechanism of lycopene intervention in reproductive damage caused by ACN. Rats fed with different concentrations of lycopene had significantly higher body weight and testis weight than the ACN-injected group, and there was a dose effect. After lycopene feeding, the MDA content decreased, and the GSH and SOD activities increased significantly compared with the control group. After treatment with medium and high doses of lycopene, the structure of the seminiferous tubules was clearer, the arrangement of spermatogenic cells was more regular, the spermatogenic cells at all levels were well differentiated, and the quality of spermatozoa was significantly improved compared with the control group.
3 Lycopene and livestock sperm cryopreservation
Artificial insemination and supporting technology have important promotional value in production [64]. Lycopene powder as a highly effective antioxidant, plays an important role in semen cryopreservation. Sperm cryopreservation also plays an important role in animal husbandry reproduction and breeding, and in the preservation of precious genetic resources [65]. A previous study [66] reported that the addition of lycopene to cryopreserved turkey sperm can improve sperm activity, survival index, and DNA integrity. Wang Xiaojuan et al. [67] used different concentrations of lycopene to dilute chicken mixed fresh sperm at a ratio of 1:1, and found that the sperm activity was improved after adding semen dilution with lycopene at low temperature. Wang Yanhua et al. [68] added a certain concentration of lycopene to the frozen dilution of goat sperm, tested various indicators of sperm quality after thawing, and measured the activities of SOD, CAT and GSH-Px. The results showed that lycopene has a good protective effect on the cryopreservation of goat semen. Fan Yuanjing et al. [69] showed that lycopene can upregulate the activity of endogenous antioxidant enzymes, which can effectively remove excessive ROS produced during semen freezing and thawing, thereby reducing sperm damage. Bucak et al. [70] found that lycopene can enhance the mitochondrial activity of bull sperm dilution freezing solution. Wang Jie [71] found that after lycopene was added to pig semen during freezing, the activity of antioxidant enzymes in the thawed sperm was improved. The effect was even better when lycopene was combined with other substances such as sesamin. The above research results clearly show that lycopene can protect semen from oxidative damage that may occur during cryopreservation.
4 Outlook
Lycopene, which is recognized worldwide as one of the most powerful antioxidants, plays an important role in the prevention and treatment of male reproductive diseases. Existing research has confirmed the clinical value of lycopene, providing theoretical support for the development of lycopene-related health products and subsequent product marketing. This paper will help to further promote the application of lycopene in the field of male reproduction, and will be of certain reference value for research on safeguarding male reproductive health and promoting the breeding of superior livestock and poultry.
References
[1] Wang Jingzi, Lin Xinying. The relationship between lycopene and related diseases [J]. Food and Drugs, 2005(9): 14⁃16.
[2] Sui Haixia, Xu Haibin, Yan Weixing. Antioxidant and antitumor effects of lycopene [J]. Chinese Journal of Food Hygiene, 2003(6) :544⁃547.
[3] He Chunmei, Lin Ying. Research status of lycopene [J]. Anhui Agricultural Bulletin (First Half Month), 2009 , 15(17) :35 ⁃36 + 145.
[4] Dong Hongchun, Fu Cong, Yang Xianqing, et al. Research progress on the characteristics, preparation and evaluation of carotenoid liposomes [J]. Food and Fermentation Industry, 2022, 48(14): 303-310.
[5] Ma Yuping, Wang Xingtai. Research progress on the physiological functions of lycopene [J]. Endemic Disease Bulletin, 2007(4) :90⁃91 .
[6] Zhu Yuan, Zhang Yongying, Zhu Haibo, et al. Research progress on the biological functions of lycopene [J]. Food Research and Development, 2020 ,41(18) :202⁃207.
[7] KAHOKEHR A , GILLING P J. Landmarks in BPH — from aetiology to medical and surgical management [ J] . Nature Reviews Urology , 2014 , 11(2) : 118 ⁃122.
[8] PAGANO E , LAUDATO M , GRIFFO M , et al. Phytotherapy of benign prostatic hyperplasia[ J] . Phytother Research : A mini review , 2014 , 28 (7) :949 ⁃955.
[9] Liu Heqian, Chen Yisheng. New progress in the treatment of lower urinary tract symptoms with drugs [J]. Chinese Journal of Andrology, 2015, 29(4): 66-69.
[10] ZOU Y , SUN Q , LI J , et al. Effects of E/Z isomers of lycopene on ex⁃ perimentalprostatic hyperplasia in mice[ J] . Fitoterapia, 2014 , 99 : 211 ⁃217.
[11] OBERMULLER⁃JEVIC U C , OLANO⁃MARTIN E , CORBACHO A M , et al. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro[ J] . The Journal of Nutrition ,2003 , 133(11) :3356 ⁃3360.
[12] WERTZ K , SILER U , GORALCZYK R. Lycopene: Modes of action to promote prostate health[ J] . Archives of Biochemistry and Biophysics , 2004 ,430(1) : 127 ⁃134.
[13] MASEREJIAN N N , GIOVANNUCCI E L , MCVARY K T , et al. Dieta⁃ ry , but not supplemental , intakes of carotenoids and vitamin C are asso⁃ ciated with decreased odds of lower urinary tract symptoms in men[ J] . The Journal of Nutrition ,2011 , 141(2) :267 ⁃273 .
[14] Li Yize, Liang Weining, Zhang Guowei, et al. Analysis of the effectiveness and safety of lycopene in the treatment of benign prostatic hyperplasia with lower urinary tract symptoms [J]. Chinese Journal of Andrology, 2019, 25(11): 1001-1004.
[15] Sun Zixue, Song Chunsheng, Xing Junping, et al. Guidelines for the diagnosis and treatment of benign prostatic hyperplasia with integrated traditional Chinese and Western medicine (trial version) [J]. Chinese Journal of Andrology, 2017, 23(03): 280⁃285.
[16] ZHAO QX , YANG FY , MENGLQ , et al. Lycopene attenuates chronic
peostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interraction of NF⁃Kb , MAPKs , and Nrf2 sig⁃ naling pathways in rats[ J] . Andrology,2020 ,8(3)747 ⁃755.
[17] Zhao Q, Yang F, Chen D, et al. Preliminary study on the therapeutic effect and mechanism of lycopene combined with quercetin and curcumin in rats with chronic prostatitis/chronic pelvic pain syndrome [J]. Chinese Journal of Andrology, 2021, 27(02): 99⁃105.
[ 18 ] FREDDIE B , MELISSA M C. Global cancer statistics [ J ] . CA : A Cancer Journal for Clinicians ,2011 ,61(2) :69 ⁃90.
[ 19 ] Liu Wei, Wang Linjun, Li Diyin, et al. Research status of lycopene in the prevention and treatment of benign prostatic hyperplasia and prostate cancer [J]. Chinese Journal of Clinical Pharmacology, 2018, 34 (20): 2477-2480.
[20] CHEN L , STACEWICZ⁃SAPUNTZAKIS M , DUNCANC , et al. Oxida⁃ tive DNA damage in prostate cancer patients consuming tomato sauce⁃ based entrees as a whole⁃food intervention[ J] . Journal of the National Cancer Institute ,2001 ,93(24) : 1872⁃1879.
[21] SOARES N D A C , TEODORO A J , OLIVEIRA F L , et al. Influence of lycopene on cell viability , cell cycle , and apoptosis of human prostate
cancer and benign hyperplastic cells[ J] . Nutrition and Cancer, 2013 , 65(7) : 1076 ⁃1085.
[22] Conglin, Zhu Jinghua, Zhang Zhaocheng. A brief discussion on the efficacy and use of lycopene [J]. Track and Field, 2020(08):84.
[23] GIOVANNUCCI E , ASCHERIO A , RIMM E B , et al. Intake of carote⁃ noids and retinol in relationship to risk of prostate cancer[ J] . Journal of the National Cancer Institute , 1995 ,87(23) : 1767 ⁃1776.
[24] GRAINGER E M , MORAN N E , FRANCIS D M , et al. A novel toma⁃ tosoy juice induces a dose⁃response increase in urinary and plasma phy⁃ tochemical biomarkers in men with prostate cancer[ J] . The Journal of Nutrition ,2019 , 149(1) :26 ⁃35.
[25] ZUNIGA K B , HAN J M , RYAN C J , et al. Diet and lifestyle consider⁃ ations for patients with prostate cancer[ J] . Urologic Oncology ,2020 ,38(3) : 105 ⁃117.
[26] BEYNON R A , RICHMOND R C , SANTOS FERREIRA D L , et al. In⁃ vestigating the effects of lycopene and green tea on the metabolome ofmen at risk of prostate cancer: The ProDiet randomised controlled trial [ J] . International Journal of Cancer,2019 , 144(8) : 1918 ⁃1928.
[27] LANE J A , Er V , AVERY K N L , et al. ProDiet: A phase II randomized placebo⁃controlled trial of green tea catechins and lycopene in men at increased risk of prostate cancer [ J ] . Cancer Prevention Research ( Philadelphia,pa) ,2018 , 11(11) :687 ⁃696.
[28] Zou Y. Lycopene and maca extract combined to improve mouse prostate hyperplasia [D]. Wuxi, Jiangsu: School of Food Science and Technology, Jiangnan University, 2015.
[29] Wei Lun, Zhang Xiaofang, Hao Jianhong, et al. Determination of the coefficients of major organs and growth indicators of Kunming mice [J]. Journal of Hebei University of Northern Science (Natural Science Edition), 2019, 35 (02): 1-4 +19.
[30] HONG F , ZHAO X Y , SI W H , et al. Decreased spermatogenesis led to alterations of testis⁃specific gene expression in male mice following Nano⁃TiO 2 expression[ J] . Journal of Hazardous Materials ,2015 ,300 : 718 ⁃ 728.
[31] Jia Fang. Research on the effect and mechanism of nano-titanium dioxide on testosterone production in adolescent mice [D]. Jinzhong, Shanxi: Shanxi Agricultural University, 2013.
[32] Wang Yan, Wang Yu, Ge Shaoqin, et al. Effect of nano-titanium dioxide on male mice reproduction [J]. Journal of Environment and Health, 2011, 28(03): 262⁃264.
[33] MEENA R , KAJAL K , R P. Cytotoxic and genotoxic effects of titanium dioxide nanoparticles in testicular cells of male wistar rat [ J] . Applied Biochemistry andBiotechnology,2015 , 175(2) :825 ⁃840.
[34] MORGAN A M , IBRAHIM M A , NOSHY P A. Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of tiron in adult male rats[ J] . Biochemical and Biophysical and Research Communications ,2017 ,486(2) :595 ⁃600.
[35] GAO G , ZE Y , ZHAO X , et al. Titanium dioxide nanoparticle⁃induced testicular damagespermatogenesis suppressionand gene expression alter⁃ ations in male mice [ J ] . Journal of Hazardous Materials , 2013 , 258 ⁃ 259 : 133 ⁃143 .
[36] Guo Lili, Liu Xiaohui, Qin Dingxia, et al. Effects of nano-titanium dioxide on the reproductive system of male mice [J]. Chinese Journal of Andrology, 2009, 15(06): 517⁃522.
[37] Zuo Zhaoyun. The physiological functions of lycopene and its application in animal production [J]. Chinese Journal of Animal Science, 2021, 57(02): 40⁃45.
[38] An Hongmei. Lycopene inhibits the male reproductive toxicity induced by nano-titanium dioxide and discusses the mechanism [D]. Shihezi, Xinjiang: Shihezi University, 2020.
[39] HANDE C , YA S A. The association between trans fatty acids , infertil⁃ ity and fetal life : a review[ J] . Human Fertility,2018 : 1 ⁃10.
[40] Yao Mingyan, Shen Yue, Liu Juan, et al. The effect of lycopene on the reproductive toxicity of trans fatty acids in male SD rats [J]. Food Safety Herald, 2018(36): 159⁃160.
[41] ATSDR. Toxicological profile for cadmium [ M] . Atlanta, GA : Agency⁃ for Toxic Substances and Disease Registry, 1999 : 182⁃186.
[42] JIN T , NORDBERG G , YE T , et al. Osteoporosis and renal dysfunction in ageneral population exposed to cadmium in China[ J] . Environmental Research ,2004 ,96 :353 ⁃359.
[43] Li Huangyuan, Yan Ping, Xia Pincang, et al. Effects of cadmium on spermatogenesis and sex hormone levels in rat testes [J]. Chinese Journal of Public Health, 2007, (11): 1371-1373.
[44] BAKER M A , AITKEN R J. Reactive oxygen species in spermatozoa : methods for monitoringand significance for the origins of genetic disease and infertility [ J ] . Reproductive Biology and Endocrinology , 2005 , 3 : 67.
[45] Liu Qing, Zhen Jianwei, Wang Yajun, et al. Effects of short-term oral cadmium exposure on reproductive hormone levels in plasma and testicular tissue of male rats [J]. Journal of Toxicology, 2012, 26 (04): 294⁃296.
[46] Wu Bo, Wang Yilong, Zhao Huiling, et al. Effects of lycopene on the activities of antioxidant enzymes and levels of reproductive hormones in rats with cadmium-induced testicular damage [J]. Guangdong Trace Element Science, 2016, 23(11): 11-14.
[47] Zhang Ming, Wu Jing, Deng Sijun, et al. Cadmium induces decreased oxidative enzyme activity and DNA damage in Sertoli cells of piglets [J]. Acta Ecologica Sinica, 2010, 19(03): 576-579.
[48] Wang Rui, Zhang Hong, Wu Bo, et al. Effect of lycopene on the antioxidant function of rats with iron overload [J]. Chinese Journal of Public Health, 2012, 28(11): 1457-1459.
[49] Qiu Xianghong, Li Hui, Pan Hongzhi. Protective effect of lycopene on lipid peroxidation in lead-poisoned mice [J]. Trace Elements and Health Research, 2005(05) : 1 ⁃2.
[50] Yang Haibo, Xu Zhaofa, Liu Wei, et al. Protective effect of lycopene on mercury chloride-induced renal injury in rats [J]. Chinese Journal of Public Health, 2011 ,27(10) : 1279⁃1280.
[51] FAN Y, JIN Y, MA Y, et al. Effects of perfluorooctane sulfonate on spermatogenesis in male rats [J]. Health Research, 2005, (01): 37-39.
[52] SIMON GIAVAROTTI K A , RODRIGUES L , RODRIGUES T , et al. Liver microsomal parameters related to oxidative stressand antioxidant systems in hyperthyroid rats subjected toacutelindane treatment [ J ] . Free Radical Research , 1998 ,29(1) :35 ⁃42.
[53] Liu Qingguo, He Yinni, Zou Xuemin, et al. Effects of lycopene on the reproductive toxicity of PFOS in male rats [J]. Journal of Nanhua University (Medical Sciences), 2010, 38 (02): 163-165 +169.
[54] Zhang Boyou, Li Dasheng, Zhang Nianheng, et al. Analysis of the prevalence of fluorosis in children aged 8 to 12 in three counties in Guizhou Province with endemic fluorosis caused by coal combustion [J]. Chinese Journal of Endemiology, 2014, 33(2): 167-169.
[55] Tian Yuan, Sun Fa, Xiao Yuehai, et al. Effect and mechanism of lycopene and vitamin E on the expression of clusterin protein in testicular tissue of rats with coal-burning fluorosis [J]. Chinese Journal of Endemic Disease Prevention and Control, 2017, 32(08): 856-857.
[56] Liu He. Research status of the effects of low-dose ionizing radiation on the human body (review) [J]. China Urban and Rural Enterprise Health, 2012, 27(02): 107-108.
[57] Wang Yujue, Yue Min, Lin Jihong, et al. Effect of different doses of X-ray radiation on the biological effects of the testis [J]. Journal of Practical Medicine, 2015, 31(01): 17-20.
[58] Xue Xiaolei. Protective effect and mechanism of antioxidants against radiation-induced damage to the hematopoietic system in mice [D]. Beijing: Peking Union Medical College, 2017.
[59] Li Yize. Protective effect of lycopene on radiation-induced reproductive damage in male rats [D]. Guangzhou, Guangdong: The First Clinical Medical College of Southern Medical University, 2020.
[60] ABBASI F A. Acrylonitrile⁃induced gastric toxicity in rats: the role of xanthine oxidase [J]. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 2012, 18(6): 208⁃214.
[61] PU X, KAMENDULIS L M, KLAUNIG J E. Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats [J]. Narnia, 2009, 111(1): 64-71.
[62] JACOB K, PERIAGO M J, OHM V, et al. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation [J]. British Journal of Nutrition, 2008, 99(1): 137-146.
[63] Li Xiu-ju, Wang Peng, Zhou Yuan-ling, et al. Research on the protective mechanism of lycopene on the testis of rats poisoned by acrylonitrile [J]. Industrial Hygiene and Occupational Diseases, 2017, 43(03): 164-168.
[64] Hu Yan-juan, Bai Yin-shan, Li Li, et al. Analysis of the effect of semen dilution and cryopreservation in chickens [J]. China Poultry, 2010, 32(23): 54-56.
[65] LIU Bifan, JI Yingli, WANG Yaodong, et al. Biological functions of lycopene and its application in animal production [J]. Journal of Animal Nutrition, 2019, 31(02): 584-590.
[66] ROSATO M P, CENTODUCATI G, SANTACROCE M P, et al. Effects of lycopene on invitro quality and lipid peroxidation in refrigerated and cryopreserved turkey spermatozoa[J]. British Poultry Science, 2012, 53 (4): 545-552.
[67] Wang Xiaojuan, He Yu, Tang Bing, et al. Research on the protective effect of lycopene added to the release solution on chicken sperm [J]. Heilongjiang Animal Husbandry and Veterinary Medicine, 2015(22)56 + 59.
[68] Wang Yanhua, Zhu Haijing, Han Cong, et al. Effect of lycopene on the cryopreservation of goat semen [J]. Journal of Northwest Agricultural University, 2017, 26(03): 336⁃341.
[69] FAN Y J, HUANG L. Research on the mechanism of lycopene absorption and in vivo anti⁃oxidation [J]. Food Science, 2007(11): 545⁃548.
[70] BUCAK M N , ATAMAN M B , BASPINAR N , et al. Lycopene and resveratrol improve post-thaw bull sperm parameters: sperm motility, mitochondrial activity and DNA integrity [J]. Andrologia, 2015, 47 (5): 545–552.
[71] Wang J. The effect of lycopene, sesaminol and baicalein on the cryopreservation of pig sperm [D]. Yangling, Shaanxi: Northwest A&F University, 2017.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






