Study on Hyaluronic Acid Modification and Compounding
Hyaluronic acid (HA) is a type of glycosaminoglycan that belongs to the group of acidic mucopolysaccharides. It is widely distributed in various parts of the human body, and the skin also contains a large amount of hyaluronic acid. In 1934, Professor Meyer of Columbia University in the United States was the first to isolate hyaluronic acid from the vitreous humor of cattle [1]. In the body, hyaluronic acid is a multifunctional matrix that exhibits a variety of important physiological functions, such as regulating proteins, assisting in the diffusion and transport of water and electrolytes, lubricating joints, regulating the permeability of blood vessel walls, promoting wound healing, etc. Most importantly, hyaluronic acid has a special water-retaining effect. It is currently the best moisturizing substance found in nature, and is known as the ideal natural moisturizing factor (NMF). (A 2% aqueous solution of pure hyaluronic acid can firmly retain 98% of the moisture. ) Due to its unique physical and chemical properties and physiological functions, hyaluronic acid has been widely used in medicine and biological materials.
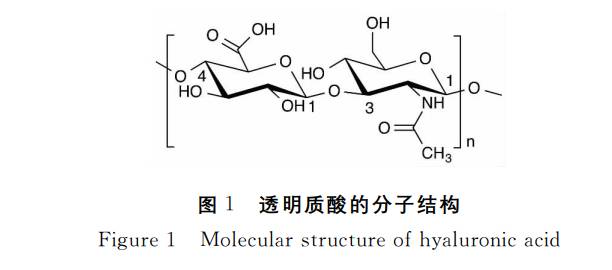
The chemical structure of hyaluronic acid was elucidated by Karl Mayer's laboratory in the 1950s [1]. Hyaluronic acid is a polymer. It is a high-molecular straight-chain mucopolysaccharide composed of a unit of D-glucuronic acid and N-acetylglucosamine. The D-glucuronic acid and N-acetylglucosamine are linked by β-1,3-glycosidic bonds, and the disaccharide units are linked by β-1,4-glycosidic bonds. The two monosaccharides in the molecule are composed in a 1:1 molar ratio. There can be up to 25,000 disaccharide units. In the human body, the molecular weight of hyaluronic acid ranges from 5,000 to 20,000,000 daltons [2, 3]. The structural formula of hyaluronic acid is shown in Figure 1.
Hyaluronic acid is soluble in water but insoluble in organic solvents. It has many properties in common with other natural mucopolysaccharides. Hyaluronic acid extracted from living organisms is white in color, odorless and highly hygroscopic. Hyaluronic acid in a sodium chloride solution dissociates due to the carboxyl group in glucuronic acid, producing H+ and making it appear as an acidic polyionic anion state, giving hyaluronic acid the properties of an acidic mucopolysaccharide [4, 5]. Although the hydroxyl groups on the hyaluronic acid molecule are arranged in a continuous orientation, forming hydrophobic areas on the molecular chain, the presence of hydrogen bonds between the monosaccharides in the hyaluronic acid molecule chain results in a rigid columnar helical structure in space [6]. The presence of a large number of hydroxyl groups on the inside of the column makes hyaluronic acid highly hydrophilic. Therefore, the hydrophilic and hydrophobic properties of hyaluronic acid allow hyaluronic acid with a concentration of less than 1‰ to form a continuous three-dimensional honeycomb network structure[5].
Water molecules are locked in place within the hyaluronic acid network by polar and hydrogen bonds with hyaluronic acid molecules, and are not easily lost. Studies have shown that hyaluronic acid can adsorb about 1000 times its own weight in water, which is unmatched by other polysaccharide compounds. Therefore, hyaluronic acid, as a water-retaining agent, is currently the best natural substance found in nature for retaining water.
Hyaluronic acid combines with proteins to form proteoglycan molecules with a higher molecular weight, which are important components for maintaining the moisture in loose connective tissue. This gel-like structure of hyaluronic acid-protein-water bonds cells together, allowing them to carry out normal metabolic functions while retaining tissue moisture. It also protects cells from viruses and bacteria, preventing infection, and gives the skin a certain degree of resilience and elasticity [7, 8].
1 Preparation method of hyaluronic acid
The traditional method of preparing hyaluronic acid is the extraction method, which uses raw materials that are generally fresh animal tissues, such as human umbilical cords, animal vitreous bodies, roosters' combs, and whale cartilage. These raw materials are difficult to source and expensive, and the hyaluronic acid content in these materials is very low, which directly leads to a low yield. Moreover, the extraction process is complex and the operating units are cumbersome. A large amount of enzymes and organic solvents are used, and the high impurity content makes purification difficult, which to some extent increases the cost of hyaluronic acid. Therefore, the hyaluronic acid obtained by extraction cannot meet the ever-expanding research and application needs. In order to find new sources of HA and reduce costs, scientific researchers began to use the fermentation method to produce hyaluronic acid [7].
The fermentation method for preparing hyaluronic acid can be traced back to the 1970s, but it has not been developed on a large scale. It was not until 1985 that Shiseido in Japan first reported the use of Streptococcus to produce hyaluronic acid, and then the fermentation method for preparing hyaluronic acid made great progress. The reported hyaluronic acid-producing bacteria are mainly Streptococcus groups A and C in the Berger's Manual, such as Streptococcus pyogenes (group A), Streptococcus zooepidemicus (group 200), Streptococcus equi (group C), Streptococcus equi group C, Streptococcus agalactiae group C, and Clostridium perfringens. Group A is mainly pyogenic streptococcus, a human pathogen, and is not suitable as a production strain. It is currently rarely used. Group C streptococcus is not a human pathogen and is relatively suitable for industrial production. In recent years, the industrial production of hyaluronic acid using Streptococcus pyogenes has reached the industrial stage abroad. Table 1 compares the main differences between the extraction method and the fermentation method [7-9].
For the extraction method, the raw material is different, and the extraction and purification process is also different [9]. For example, the cockscomb has low fat content and high hyaluronic acid content. After being ground, it can be directly extracted with distilled water several times or heated to 40-50°C for extraction. A hyaluronic acid solution with a yield of 0.47% can be obtained. For human umbilical cords, the fat content is higher than that of chicken combs. They can be extracted several times with a dilute alkali solution (pH=8) at 60°C, or extracted with a mixture of water and chloroform (20:1/W:W), and washed with an equal volume of chloroform to further degrease. The yield of hyaluronic acid is 0.2%. The extraction of hyaluronic acid from vitreous humor generally uses a NaCl solution (0.1-1M) as the extraction solution, and the yield can reach 0.64-2.4%. Pigskin contains a lot of fat and is tough and not easily ground, so it is generally liquefied in a NaOH solution at 37°C for a period of time, and then neutralized with 50% acetic acid. Although the yield of hyaluronic acid can reach about 0.7%, the purification process is relatively complicated.
The quality of hyaluronic acid prepared using the fermentation method mainly depends on the following four aspects: strain selection, medium matching, fermentation process optimization and separation and purification process. The advantages of the biological fermentation method are that the product is not limited by raw material resources, the process is simple and the cost is low. Therefore, the fermentation method is currently preferred for the preparation of hyaluronic acid. The main bacteria used in the fermentation method for producing hyaluronic acid are Streptococcus zooepidemicus, Streptococcus equi and Streptococcus equi-like.
Fermentation methods for producing hyaluronic acid are divided into aerobic fermentation and anaerobic fermentation. Aerobic fermentation has a high yield and produces hyaluronic acid with a high molecular weight. During the fermentation process, the temperature is usually 37°C, and the pH value needs to be controlled within the range of 6.0-8.5. An environment with too much acid or alkali will affect the growth of the bacteria and reduce the yield of hyaluronic acid. Different oxygen dissolution rates can also be used at different fermentation stages to increase the yield of hyaluronic acid. In addition, the viscosity of the fermentation broth can directly reflect the yield of hyaluronic acid. The pseudoplasticity of hyaluronic acid causes the viscosity of the solution to decrease at high shear rates. High stirring rates can significantly increase the molecular weight of hyaluronic acid, but too high a speed can destroy the molecules and reduce the molecular weight of hyaluronic acid. Therefore, the stirring speed is usually controlled at 100–800 r/min. The yield of hyaluronic acid can also be increased by adding a small amount of uracil, glutamine and aspartic acid to the fermentation broth, or by adding lysozyme [10-13].
At present, the extraction of HA in China is still in the stage of using human umbilical cords and chicken combs as raw materials. Shanghai University has reported a method for extracting hyaluronic acid from pig skin, and the molecular weight of the hyaluronic acid produced is about 106. Some people use microbial fermentation to produce hyaluronic acid, and the yield has been reported to be 4.6 g/l, but the molecular weight is only 500,000. In addition, some people have also used γ-rays combined with magnetic field mutagenesis to obtain high-yielding strains of hyaluronic acid. For example, Chen Yonghao [14] used ultraviolet and 60Co-γ-ray irradiation to mutate and obtained a strain of non-hemolytic bacteria NC1150, which increased the yield and relative molecular weight of HA.
2 Improvement of hyaluronic acid properties as a biomaterial
Pure hyaluronic acid has the disadvantages of being easily soluble in water, rapidly absorbed, having a short residence time in tissues, and poor mechanical properties, which limits its use in situations where material hardness and mechanical strength are required. In order to make hyaluronic acid more widely used in the field of biomaterials, it is necessary to chemically modify it to optimize its properties and expand its scope of application. To improve the mechanical properties of hyaluronic acid and control its degradation rate, hyaluronic acid can be chemically modified or crosslinked. Hyaluronic acid has functional groups such as hydroxyl, carboxyl and acetamido, and can be modified by cross-linking, esterification, grafting, molecular modification and compounding. Chemically modified hyaluronic acid clearly possesses the main properties of carboxylic acids and/or alcohols. Carboxylic acids and alcohols are modified by esterification, and combined with hydrazine compounds, dithiothreitol or disulfides [3, 6]. After modification, hyaluronic acid is endowed with a series of good properties such as mechanical strength, viscoelasticity, rheological properties and resistance to hyaluronidase degradation, while maintaining its original biocompatibility.
2.1 Covalent cross-linking of hyaluronic acid with polyethylene glycol
Current research shows that the mechanical properties and degradation rate of cross-linked hyaluronic acid gels can be controlled by the degree of cross-linking and molecular weight of the cross-linking molecule. Hyaluronic acid can be covalently cross-linked with polyethylene glycol diamines at various degrees of cross-linking. Polyethylene glycol was chosen as the cross-linking molecule because it is biocompatible and hydrophilic. PEG is soluble in aqueous solution and is commercially available in different molecular weights. The elastic properties of the gel are ensured by the deformable PEG chains, while the mechanical properties are ensured by the structurally stable hyaluronic acid chains.
The influence of the degree of cross-linking on the mechanical properties and degradation behavior of hyaluronic acid gels has been studied. Hyaluronic acid gels are prepared from covalently cross-linked hyaluronic acid and two different molecular weights of polyethylene glycol at various degrees of cross-linking. Experiments have shown that as the theoretical degree of cross-linking of hyaluronic acid gels increases from 0 to 20%, the elastic modulus gradually increases. However, when the theoretical cross-linking degree increased to above 20%, the elastic modulus decreased. When the theoretical cross-linking degree was 20%, the elastic modulus increased, and the molecular weight of the cross-linked molecules decreased. At a theoretical cross-linking degree of 20%, the in vitro degradation rate of hyaluronic acid gels decreased with a decrease in the molecular weight of the cross-linked molecules. As the theoretical crosslinking degree increases from 0 to 20%, the degradation rate of crosslinked hyaluronic acid decreases. However, when the theoretical crosslinking degree rises above 30%, there is no significant difference in degradation rate [15, 16]. Further development of hyaluronic acid gels through in-depth research on their controlled mechanical properties and degradation rates will provide a wide range of medical and biological material applications.
Hyaluronic acid is modified by covalently binding it to polyethylene glycol diamines of different molecular weights. The mechanical properties and degradation rate of crosslinked hyaluronic acid gels can be controlled by varying the molecular weight and degree of crosslinking of the crosslinking molecules. It has been found that crosslinked hyaluronic acid gels have controllable mechanical properties and degradation rates, which can provide a wider range of biomedical applications, such as cell transplantation and drug delivery.
2.2 Cross-linking of hyaluronic acid with polyhydrazide compounds
Hyaluronic acid can be cross-linked with different hydrazide compounds to obtain gels with different physicochemical properties under different cross-linking conditions. Hydrazide cross-linkers make the gel resistant to hyaluronidase. Experiments have shown that gel degradation is independent of the concentration of the cross-linking agent, which indicates that degradation only occurs at the interface of the gel. The stability of hyaluronic acid gels in acidic media and their slow dissolution at pH > 7.0 indicate their potential role in controlling drug delivery in an alkaline environment [15–17].
Hydrazide compounds can be used as cross-linking agents to modify hyaluronic acid hydrogels into more mechanically rigid and brittle gels. Hyaluronic acid can become a stable HA-adipoyl dihydrazide (HA-ADH) derivative in the presence of a large amount of adipic dihydrazide [18]. Paul Bulpitt [19] and others have shown that chemical modification of hyaluronic acid by hydrazide compounds ester intermediates with resistance to hydrolysis and no rearrangement activity can be formed. New hydrogels with good biocompatibility such as HA-hydrazide and HA-amide have been synthesized, and to some extent the water solubility has been reduced to achieve the effect of slow-release drugs [20].
2.3 Hyaluronic acid crosslinked with disulfide
Hyaluronic acid can be crosslinked with disulfide. For example, a certain amount of hydrazinolysis 3,3'-dithiopropionic acid (DTP) can be added to a hyaluronic acid aqueous solution, and the pH of the reaction solution can be adjusted from acid to base using HCl and NaOH. Solid carbodiimide (EDC) is added during the process, and finally, separation, freeze-drying, and purification can be used to obtain a mercapto hyaluronic acid derivative (HA-DTPH) [20].
Experiments have shown that the disulfide-crosslinked mercapto-hyaluronic acid derivative (HA-DTPH) gel degrades slowly both in vivo and in vitro, and the degradation rate can be controlled by changing the degree of disulfide cross-linking. At the same time, hyaluronic acid gels have potential clinical applications in wound healing and tissue repair [21].
2.4 Hyaluronic acid esterification
Carboxyl esterification
The carboxyl group of hyaluronic acid can undergo esterification with fatty alcohols or aromatic alcohols to form esterified derivatives [21]. After esterification, the solution rheological properties of HA are significantly improved, forming a weak colloidal network structure. The solubility of hyaluronic acid esterified derivatives decreases with increasing degree of esterification, and highly esterified derivatives are insoluble in water. In addition, the degree of esterification has a significant effect on the degradation rate. This may be because the hydrophobic fragments of the fully esterified substance make the network of polymer chains more rigid and stable, making it less susceptible to enzymatic degradation. The partially esterified substance is more deformable and more easily combined with water.
Hyaluronic acid esterified derivatives can be made into films and fibers using some conventional process methods, freeze-dried into sponges, or prepared into microspheres by spraying, drying, extracting and evaporating, and can be used as carrier materials for controlled drug release. In addition, this type of hyaluronic acid esterified derivative can be used in the development of artificial skin and artificial cartilage, the culture of mesenchymal stem cells, and also for anti-biofouling and anti-corrosion purposes [20].
Hydroxyesterification
If butyric anhydride and the trimethylpyridine salt of low-molecular-weight hyaluronic acid are reacted in dimethylformamide (DMF) containing dimethylaminopyrimidine, butyric acid can be coupled to hyaluronic acid. As butyric acid can induce cell differentiation and inhibit the growth of tumor cells, hyaluronan butyrate can be used as a new targeted drug delivery system material.
Internal esterification
Internal esterification of hyaluronic acid derivatives is achieved by intramolecular and intermolecular bonding between the hydroxyl and carboxyl groups of hyaluronic acid. Pressato [22] and Belini [23] et al. pretreated a dimethyl sulfoxide (DMSO) solution of hyaluronic acid with triethylamine, converting hyaluronic acid to [R4N] +HA. 2-Chloro-1-methyliodopyridine was then used as a cross-linking agent to cause internal esterification of hyaluronic acid, yielding hyaluronan lactone derivatives with both intramolecular and intermolecular esterification. This method can be used in surgery to reduce adhesions after abdominal surgery and obstetric and gynecological surgery. The internal esterified derivative of hyaluronic acid can also be used as a scaffold for tissue damage repair and regeneration of cartilage and bone.
2.5 Graft modification
Hyaluronic acid can be grafted onto natural or synthetic polymers using cross-linking agents to form new materials with modified biomechanical properties and physicochemical properties [20].
The process is shown in Figure 2. HA can also be grafted onto the surface of liposomes to provide targeting and shielding effects, as shown in Figure 3.
After hyaluronic acid is modified with dihydrazide to form the HA-ADH derivative, drug molecules can be attached to HA-ADH to form HA-bound drugs. Hyaluronic acid can provide novel drug targeting and controlled release. The general process is as follows: after dihydrazide is linked to HA, the remaining NH2 of the hydrazide can be reconnected with other carboxyl groups in the HA molecule, and intra- or intermolecular cross-linking will occur. At the same time, the remaining NH2 can be connected to the active site of the drug to bond the drug to the hyaluronic acid, or the drug can be first connected to the polyhydrazide and then grafted to the HA molecule to obtain a hyaluronic acid-bonded drug system. The structure is shown in Figure 4.
2.6 Composite modification
Hyaluronic acid is a non-antigenic molecule that can be used in combination with other materials without causing inflammation or an immune response. For example, hyaluronic acid can be combined with collagen [20], which is the main structural protein in the extracellular matrix. The combination of hyaluronic acid and collagen gives it good mechanical properties. Hyaluronic acid can also be combined with chitosan (CS) and gelatin [20], to form a CS-Gel-HA composite material (chitosan-gelatin-hyaluronic acid). This composite material can effectively improve the adhesion of cells to the material surface, increase the survival rate of cells on the material surface, and enable cells to enter the normal growth and proliferation cycle as soon as possible. Hyaluronic acid can also be compounded with synthetic polymers such as poly(lactide-co-glycolide) (PLA/PLGA), which is a non-toxic, fully biodegradable synthetic polymer that is easy to process, degradable, and has a controllable degradation rate. Blending PLA or PLGA with HA [24] can reduce the degradation rate of HA and prolong the time HA remains in the tissue.
3 Application of hyaluronic acid in the field of biomaterials
Hyaluronic acid derivatives obtained through modification can improve specific properties as required, which greatly expands the application of hyaluronic acid in the field of biomaterials. At present, hyaluronic acid or its derivatives are used in various fields, including surgical anti-adhesion, arthritis treatment, ophthalmic disease treatment, local drug delivery carriers, tissue engineering, etc. [25-29]. The application of different modified hyaluronic acid products is shown in Table 2.
4 Conclusion
This paper reviews the preparation methods of hyaluronic acid and the modification and compounding of hyaluronic acid. At present, research on the preparation and modification and compounding of hyaluronic acid has made gratifying progress, but there is still some way to go before it can be used in clinical applications. In addition, hyaluronic acid is a kind of bioabsorbable material with high viscoelasticity, plasticity, permeability and unique rheological properties as well as good biocompatibility. Due to its strong moisturizing properties and good biocompatibility, it has also become an important raw material for biomedical applications. It is widely used in ophthalmology, orthopedics, and even extends to surgery, pediatrics, neurology and other fields. In addition, hyaluronic acid can effectively prevent postoperative adhesion without side effects. It can also be used as a drug release carrier and is a popular new biomedical material. However, hyaluronic acid also has shortcomings that need to be compensated for by various chemical modifications to improve its mechanical strength, resistance to hyaluronidase degradation, etc. Common modification methods include cross-linking, esterification, grafting, molecular modification and compounding. In-depth research on hyaluronic acid is therefore continuing. Current research focuses on improving the properties of hyaluronic acid gels and making them smarter, in order to promote the wider use of these materials in the field of biomaterials.
Reference
[1]Weissmann B,Meyer K.The structure of hyalobi- uronic acid and of hyaluronic acid from umbilical cord[J].J Am Chem Soc,1954,76(7):1753-1757.
[2]Saari H. Differential effects of reactive oxygen spe- cies on nativ synovial fluid and purified human um- bilical cord hyaluronate[J].Inflammation,1993,17(4): 403-415.
[3]Jeon O.Mechanical properties and degradation be- haviors of hyaluroni acid hydrogels cross-linked at various cross-linking denseties[J].Carbohydrate Poly- mers,2007,10:1-7.
[4] Pan Hongmei. A review of the current research status of hyaluronic acid [J]. Sichuan Food and Fermentation, 2003, 39(1): 1-5.
[5] J E Scott, C Cummings, A Brass, et al. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation [J]. Bochemial Journal, 1991, 274(3): 699-705.
[6] Evered D, Whelan J. The biology of hyaluronan, ciba foundation symposium [J]. John Wiley & Sons, 1989, 143: 6-15.
[7] Luo Ruiming. The current research status of hyaluronic acid (HA) at home and abroad [J]. Journal of Ningxia Agricultural College, 2002, 22 (1): 62-64.
[8] Weigel P H, Hascall V C, Tammi M. Hyaluronan synthases [J]. Biol Chem 1997, 272: 13997-14000.
[9] Qi Yanrong. Preparation and application of hyaluronic acid [J]. Education and Teaching Forum, 2010, 20: 222-223.
[10] Liang Tianzuo. Research on the production of hyaluronic acid by microbial fermentation [M]. Hebei Agricultural University, 2010: 16-18.
[11] Guo Xueping, Wang Chunxi, Cui Dapeng. Preparation of hyaluronic acid by fermentation [J]. Daily Chemical Industry, 1994 (2): 47-48.
[12] Guo Xueping, Wang Chunxi, Ling Peixue, et al. Overview of hyaluronic acid and its fermentation production [J]. Chinese Journal of Biochemical Drugs, 1998, 19 (4): 209.
[13]Rao Y M,Sureshkumar G K. Improvemen in bio- reactor productivities usin free radicals: HOCI-in- duced overproduction of xanthan gum from Xan-
thomomascampestris and its mechanism[J]. Bio- technol Bioeng,2001,72(1):62-68.
[14] Chen Yonghao, Wang Qiang. Mutagenic breeding of hyaluronic acid producing bacteria [J]. Microbiology Bulletin, 2009, 36(2): 205-210.
[15] Manuskiatti. Hyaluronic acid and skin: wound healing and aging [J]. Int J Dermatol, 1996, 35(8): 539-544.
[16] Laurent T L. Hyaluronan [J]. FASEB J, 1992, 6: 2397-2403.
[17] Koen P Vercruysse, Dale M Marecak, James F Marecek, et al. Synthesis and in vitro Degradation of New Polyvalent Hydrazide Cross-Linked Hy- drogels of Hyaluronic Acid [J]. Bioconjugate Chem, 1997, 8, 686-694.
[18] Ling Peixue, Zhang Tianmin. Hyaluronic acid [M]. Beijing: China Light Industry Press, 2000, 25: 188-194.
[19]Paul Bulpitt,Daniel Aeschlimann. New strategy for chemical modification of hyaluronic acid Prepara- tion of functionalized derivatives and their use in the formation of novel biocompatible hydrogels[J]. J Biomed Mater Res,1999,47(2):152- 169.
[20] Yu Xueli, Wang Chuandong, Li Baolu, et al. Modification of hyaluronic acid and its application [J]. Biomedical Engineering Research, 2005 (1): 61-66.
[21]Borzacchiello A,Ambrosio L.Network formation of low molecular weight hyaluronic acid derivatives [J]. J Biomater Sci Polymer Edn,2001,12(3) : 307- 316.
[22]Pressat o D,Pavesio A. Biomaterials for preventing post-surgical adhesions comprised of hyal- uronic acid derivat ives[P] . WO :9707833,1997.
[23]Belini D,Papare11a A,O Regan M,Callegaro L. Auto crosslinked hyaluronic acid and related phar- maceutical compositions for the treatment of ar- thropathies[P] . WO :9749412,1997.
[24]Lee Sang Young,Jung Hwan Oh,Jae Chan Kim, et al.In vivo conjunctival reconstruction using mod- ified PLGA grafts for decreased scar formation and contraction[J] . Biomaterials,2003,24: 5049- 5059.
[25]Lutolf M P,Raeber G P,Zisch A H,et al.Cell-responsive synthetic hydrogels[J].Adv Mater,2003, 15:888-892.
[26] Huang Jianyan, Bao Lei, Mao Xuan, et al. Agarose-hyaluronic acid copolymer as an insulin carrier [J]. Journal of Materials Science and Engineering, 2009, 27(1): 43-46.
[27] Lee H, Lee K, Park TG. Hyaluronic acid-paclitaxel conjugate micelles: synthesis, characterization, and antitumor activity [J]. Bioconjug Chem, 2008, 19(6): 1319-1325.
[28] Kumar A, Sahoo B, Montpetit A, et al. Development of hyaluronic acid-Fe2O3 hybrid magnetic nanoparticles for targeted delivery of peptides. Nanomedicine, 2007, 3(2): 132-137.
[29] Wang Chao, Zhang Mingchun. Progress in the preparation and application of hyaluronic acid as a medicinal material. China Pharmaceutical Biotechnology, 2009, 4(6): 452-454.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese