Stduy on Ginsenoside Rg1 Rb1 Rg3
Pharmacokinetics is the study of the processes of absorption, distribution, metabolism and excretion of drugs in the body. It is the key to studying the form and mechanism of action of drugs. Only by understanding the pharmacokinetics of a drug can we thoroughly explore the molecular mechanism of its pharmacological action.
Ginsenosides are the main active ingredients in the precious Chinese medicinal herb ginseng. They have the functions of enhancing memory [1], boosting immunity [2-3], improving blood vessels [4], regulating endocrine secretion [5], delaying aging [6] and fighting tumors [7]. In recent years, the pharmacokinetic research of ginsenosides has become a hot topic of concern at home and abroad. A review is now given on the pharmacokinetics of ginsenosides in the body and the factors affecting them.
1 Pharmacokinetics of several ginsenoside monomers
Although ginseng, as a traditional Chinese medicine, has long been proven to have pharmacological effects, research on its in vivo situation is still in its infancy, and most of the current research focuses on the pharmacokinetics of ginsenoside monomers.
1.1 Pharmacokinetics of ginsenoside Rg1
Ginseng saponin Rg1 can easily pass through the intestinal barrier and is absorbed in a time-dependent manner throughout the small intestine [8]. Feng Liang et al. [9] studied the pharmacokinetics and metabolites of ginseng saponin Rg1 in rats after intravenous injection and oral administration. After oral administration to rats, the amount of ginsenoside Rg1 metabolites in the body exceeds that of the parent drug. Four substances, ginsenoside Rg1, Rh1, F1 and protopanaxatriol, were detected in the plasma, and their tmax were 0.92, 3.64, 5.17 and 7.30 h, respectively. and the MRTs were 2.68, 5.06, 6.65 and 5.33 h, respectively, and the AUC0-t values were 2363.5, 4185.5, 3774.3 and 396.2 ng·h·mL-1, respectively. After intravenous administration, ginsenoside Rg1 was mainly in its original form in the rat body, with only a small amount of metabolites. Three substances, ginsenoside Rg1, Rh1 and F1, were detected in its plasma, with t1/2β of 3.12, 5.87 and 6.87 h, respectively, and MRT of 1.92, 5.99 and 7.13 h, respectively, AUC0-t were 1454.7, 597.5 and 805.6 ng·h·mL-1, respectively. Lee et al. [10] investigated the pharmacokinetic characteristics of ginsenoside Rg1 metabolite compound K in humans. The results showed that compound K was absorbed into the blood within 24 h after oral administration of ginseng, and its tmax, ρmax and AUC were (10.76 ± 2.07) h, (27.89 ± 24.46) ng·mL−1 and (221.98 ± 221.42) μg·h·mL−1, respectively. The absorption of compound K by the human body is not affected by the intestinal flora, but its pharmacokinetic parameters are related to the conversion rate of the intestinal flora in each subject and there are individual differences.
1. 2 Pharmacokinetics of ginsenoside Rg2
Ginsenoside Rg2, along with ginsenoside Rg1, is a triterpene ginsenoside and a candidate drug for the treatment of cardiovascular and cerebrovascular diseases. It has two isomers, R and S. The AUC of the different isomers of ginsenoside Rg2 in rats is proportional to the dose (intravenous injection: 10, 20, 50 mg·kg-1) and is consistent with a one-compartment open model. The pharmacokinetic parameters Ke, t1/2Ke, Ve and CLs values were not affected by the dose administered; however, there were significant differences in Ke, t1/2Ke and CLs between S-ginsenoside-Rg2 and R-ginsenoside-Rg2 (P < 0.05) [11]. Gui et al. [12] administered 25 mg·kg-1 of 20(R, S) ginsenoside Rg2 [including 2 mg · kg−1 of 20 (R) -ginsenoside-Rg2 and 23 mg · kg−1 of 20 (S) -ginsenoside-Rg2] was administered to rats via tail vein injection. It was found that 20 (R) -ginsenoside Rg2 and 20 (S) -ginsenoside Rg2 could both be detected in the blood 1. 5 h after injection. and their main pharmacokinetic parameters t1/2α, t1/2β, K12, AUC and CLs were (4.024 6 ± 0.008 7) and (3.724 2 ± 0.045 9) min, (71.1999 ± 3.1586) and (38.4414 ± 1.1134) min, (0.0997 ± 0.157) and (0.0942 ± 0.0358) min-1, (197.7176 ± 5.1766) and (1092.5109 ± 83.0747) μg·min·mL-1, (0. 126 4 ± 0. 000 3) and (0. 023 2 ± 0. 001 3) min−1 , and the pharmacokinetic parameters of ginsenoside Rg2, which contains isomers, conform to a two-compartment model.
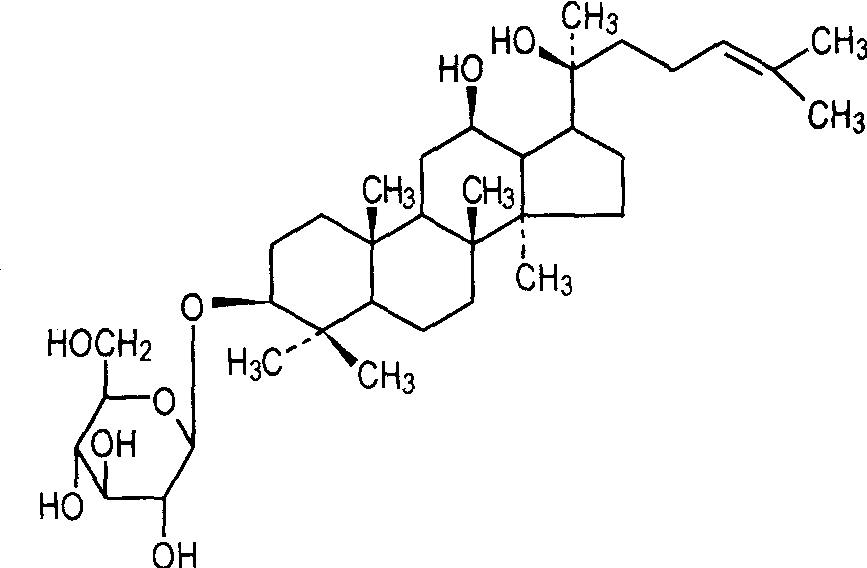
1. 3 Pharmacokinetics of ginsenoside Rg3
After ginsenoside Rg3 was injected intramuscularly (1. 5 mg·kg-1), the content distribution in each organ of the rat was in descending order: lung > spleen > heart > kidney > liver [13]. Cai et al. [14] used LC-ESI-MS to study the pharmacokinetics of ginsenoside Rg3 in rat plasma and its in vitro metabolites. Ginsenoside Rg3 was not detected in rat urine, whether administered orally (50 mg·kg-1) or intravenously (5 mg·kg-1). However, Rg3 can be detected in the blood within 1.5 h after intravenous injection, and is metabolized rapidly (metabolic half-life 14 min). Further studies on the metabolism of ginsenoside Rg3 were carried out in vitro by simulating the gastrointestinal environment. It was found that the main metabolite was protopanaxadiol, which contains a hydroxyl group, while Rh2 and protopanaxatriol, which are recognized metabolites, were not detected. At the same time, it was found that ginsenoside Rg3 mainly undergoes oxidative reactions in the liver. After 16 hours, rat liver microsomal S9 oxidized ginsenoside Rg3 to the aglycone, which was then oxidized by cytochrome P450 to the 24,25-epoxides.
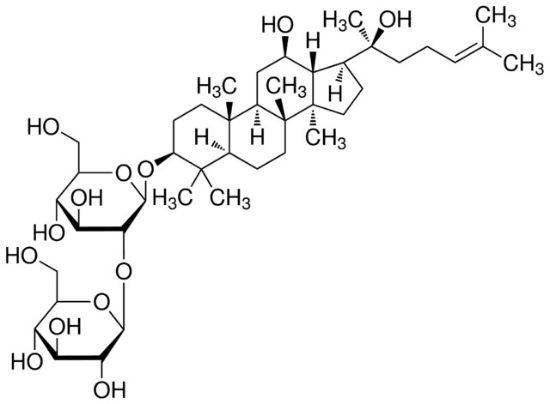
1. 4 Pharmacokinetics of ginsenoside Re
Liu et al. [15] studied the pharmacokinetics of ginsenoside Re in humans. After oral administration at a dose of 200 mg·kg-1, the tmax and t1/2 of ginsenoside Re were (1.19 ± 0.44) and (1.82 ± 0.75) h, AUC0-t and AUC0-∞ were (2. 476 ± 2. 281) and (2. 699 ± 2. 284) μg · h · L-1, respectively, ρmax was (0. 939 ± 0. 549) μg · L-1, CL/F was (124 054 ± 84 725) L·h-1. The metabolites of ginsenoside Re in urine were further studied, and three metabolic pathways of ginsenoside Re were inferred: ① Re→Rg1→F1→PPT, ② Re→Rg2→Rh1→PPT, and ③ Re→Rg1→Rh1→PPT. In addition, Joo et al. [16] found that ginsenoside extract can promote the absorption of ginsenoside Re. Ginsenoside Re was administered orally at doses of 10 mg·kg-1 and 50 mg·kg-1, the absolute bioavailability of ginsenoside Re in ginseng saponin extract in rats (0.33% and 0.75%) was higher than that of its monomer administered orally (0.28% and 0.19%).
1.5 Pharmacokinetics of ginsenoside Rb1
Ginsenoside Rb1 is the main protopanaxadiol saponin. Qian et al. [17] studied the metabolites of ginsenoside Rb1 in rat blood, feces and urine. Intravenous administration (5 mg·kg-1) of ginsenoside Rb1 is mainly metabolized in the rat body by oxidation; while after oral administration (100 mg·kg-1) of ginsenoside Rb1, metabolites such as Rd, Rg3, F2, Rh2, and C-K were detected in the rat feces. and it is speculated that the metabolic pathway of Rb1 in the rat intestine is: Rb1 → Rd → Rg3 or F2 → Rh2 or C-K → ppd. The metabolites in rat urine are similar to those in feces. The difference between the metabolites of oral administration and intravenous administration is that ginsenoside Rb1 is mainly metabolized by removing the sugar group in the gastrointestinal tract.
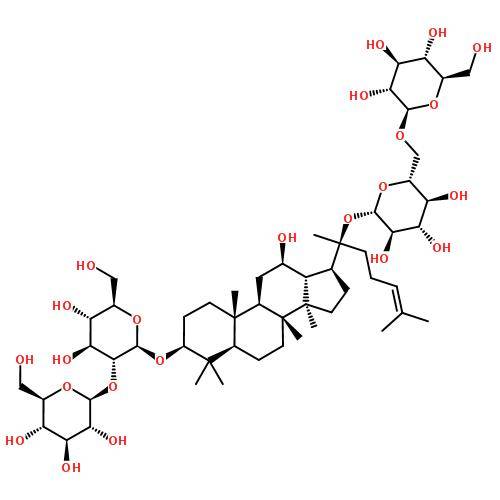
1. 6 Pharmacokinetics of ginsenoside Rh2
Gu et al. [18] investigated the distribution of ginsenoside Rh2 in rats. After a single oral administration of ginsenoside Rh2 (3 mg·kg-1), the highest concentration was found in the liver, while the concentrations in the heart, spleen, stomach and kidney were similar to the blood concentration. A small amount was distributed in the ovaries, adrenal glands and fat, while Rh2 was not detected in the brain, skin, muscles and testes.
1. 7 Pharmacokinetics of ginsenoside Rd
Ginsenoside Rd (10 mg·kg-1) was administered intravenously into the human body, and the peak value was reached 0.5 h later, with a ρmax of (2 841.18 ± 473.03) ng·mL-1 and t1 /2 is ( 19. 29 ± 3. 44) h, AUC0-t is (27 161. 63 ± 8 116. 88) ng · h · mL-1 , and further studies have found that whether ginseng saponin Rd is administered orally or intravenously, metabolites such as Rg3, Rh2, Rd and Rb1 can be detected in rat urine [19]. Wang et al. [20] investigated the pharmacokinetics of ginsenoside Rd in dogs using intravenous (0.2 mg·kg-1) and oral (2 mg·kg-1) administration. The AUC of ginsenoside Rd after intravenous and oral administration in dogs was (76,451.1 ± 15,874.8) and (1,930.3 ± 647.4) ng·h·mL-1, respectively, and the absolute oral bioavailability was 0.26%.
2 Pharmacokinetic study of ginsenosides in traditional Chinese medicine compound
Traditional Chinese medicine compound is the essence of traditional Chinese medicine theory, and is the focus and hotspot of modern traditional Chinese medicine research. In recent years, researchers have also studied the pharmacokinetics of ginsenosides in compound. Jiang Jie et al. [21] studied the pharmacokinetic characteristics of Shengmai Yin (a traditional Chinese medicine compound preparation composed of ginseng, ophiopogon japonicus and schisandra chinensis, with ginseng as the main ingredient) in humans. After a single oral dose of 300 mL of Shengmai Yin was administered to 12 healthy volunteers, the pharmacokinetic profiles of ginsenosides Rg1 and Re both followed a one-compartment model.
The mean peak times tmax for Rg1 and Re were (4. 86 ± 1. 07) and (4. 75 ± 1. 04) h, respectively, and the average peak concentrations ρmax were (26. 33 ± 12. 74) and (43. 32 ± 16. 47) μg · mL-1 , respectively. The elimination half-lives t1/2ke were (7. 99 ± 4. 63) and (7. 91 ± 4. 56) h, respectively, clearance CL was (2. 73 ± 2. 50) and (1. 23 ± 1. 12) mL · h-1, respectively, and apparent volume of distribution V was (31. 10 ± 32. 26) and (11. 96 ± 9. 40) mL, respectively. AUC0-30 were (205. 96 ± 114. 57) and (338. 73 ± 89. 10) μg · h · mL-1, respectively. Ginseng saponins Rg1 and Re are subject to first-order kinetic absorption and elimination in the human body. Meanwhile, Lin Li et al. [22] used a rat intestinal sac model to study the effect of the compound compound ginseng on the absorption of ginsenosides Rg1, Re, Rb1 and Rd. The results showed that compared with a single component, the compound low-dose group (0. 281 mg · mL-1) can promote the absorption of ginsenosides Rg1, Re, Rb1 and Rd, The compound medium-dose group (0. 563 mg · mL-1) and the high-dose group (1. 125 mg · mL-1) showed no significant difference in the absorption of the four ingredients, and the absorption of these four ingredients tended to be inhibited with an increase in the compound dose.

3 Factors affecting the pharmacokinetics of ginsenosides
The absorption and distribution of drugs is a very complex process, and its influencing factors include species differences in subjects, drug dosage forms, routes of administration, and doses administered.
3. 1 Subjects
Due to species differences among the test animals, the pharmacokinetic models of ginsenosides in different test animals are different. Gu et al. [18] studied the pharmacokinetic of ginsenoside Rh2 in dogs and rats. The pharmacokinetic model of ginsenoside Rh2 after intravenous injection in Beagle dogs (0.1 mg·kg-1) was a three-compartment open model, while in rats (0.1 mg·kg-1) it was a two-compartment model.
3. 2 Drug dosage form
The dosage form has a significant effect on the pharmacokinetics of the test drug. Changes in the drug dosage form will lead to changes in the pharmacokinetic parameters. Ginseng saponin preparations are no exception. Rode Feng et al. [23] investigated the in vitro release rate and the absorption characteristics of ginsenoside Rd solid lipid nanoparticles (Rd-SLN) in various intestinal segments of rats, and compared them with ginsenoside Rd monomers. The cumulative drug release rate of Rd-SLN after 120 h was (89.6 ± 1.6) %, while the ginsenoside Rd control solution was almost completely released (97.21 ± 1.19%) in the same medium after 12 h. The absorption rates of Rd-SLN and Rd in the intestinal segments of rats were also different.
There was no significant difference in the absorption of the two in the duodenum and jejunum segments, and the difference in the absorption rates of the two was significant in the ileum and colon segments. Under the same dosage conditions, the peak blood concentration of the Rd-SLN group was significantly higher than that of the Rd group, and t1/2 and MRT were significantly prolonged. The AUMC, AUC0-t and AUC0→∞ of the former were about 2 times, 1.5 times and 2 times those of the latter, respectively. The results show that Rd-SLN can slow the release of the drug, increase the absorption of the drug in the intestine, significantly improve the bioavailability of oral administration, and prolong its half-life in the blood. In addition, Gu et al. [18] investigated the changes in the pharmacokinetic parameters of 20(R)-ginsenoside Rh2 before and after micronization in Beagle dogs. Beagle dogs were orally administered (1 mg·kg-1) 20(R)-ginsenoside Rh2 and its micronized powder, respectively blood drug concentrations were sampled and tested at 0.25, 0.5, 0.75, 1.0, 1.5, 2, 3, 5, 7, 9, 12, 24, and 36 h after administration. The results showed that the ρmax, AUC and bioavailability of micronized 20(R)-ginsenoside Rh2 were about twice those of the original drug, indicating that micronized 20(R)-ginsenoside Rh2 can dissolve better in intestinal fluid and enter the blood more easily.

3. 3 Administration route
Gu et al. [24] investigated the pharmacokinetic changes of ginsenoside 20(R) -Rh2 after oral and intravenous administration in Beagle dogs. The pharmacokinetic curve of ginsenoside 20(R) -Rh2 after intravenous injection (0. 1 mg · kg-1 ) conformed to a three-compartment model, and the main pharmacokinetic parameters t1/2, CL, and AUC0-∞ were (8. 0 ± 2. 8) h, (0.1 ± 0.03) L · kg-1 · h-1, (857.0 ± 209.6) ng · h · mL-1, and a long terminal elimination half-life, suggesting that 20(R) -Rh2 may accumulate to some extent in the body; and the pharmacokinetic model after oral administration (1 mg·kg-1) is a two-compartment model. The main pharmacokinetic parameters tmax, ρmax, t1/2, and AUC0-∞ are (2.6 ± 1.3) h, (371.0 ± 199.6) ng·mL-1, (5.8 ± 2.6) h, (1215.7 ± 598.6) ng·h·mL−1. The slow absorption of 20(R)-Rh2 in Beagle dogs may be related to its poor solubility and dispersion. In addition, due to the efflux of P-glycoprotein and the metabolic action of intestinal flora, the absolute bioavailability of the drug in Beagle dogs is low, only (16.1 ± 11.3)%. Therefore, the development of dosage forms for 20(R) -Rh2 should take into account the administration method to reduce transformation and increase the absorption of the drug.
3. 4 Dosage
Current research has found that for ginsenosides of the same configuration within the set dose range, the pharmacokinetic parameters of each dose group increase with increasing dose concentration, but there is no significant difference (P>0. 05). Deng Yuanhui et al. [25] studied the pharmacokinetic characteristics of ginsenoside-Rd injection after a single intravenous infusion in 12 healthy Chinese volunteers. The results showed that the main pharmacokinetic parameters of the three doses (10, 40, 75 mg·kg-1): ρmax were (2.84 ± 0.47), (10.48 ± 1.74), (19.34 ± 2.62) mg·L-1, and t1/2 were (19.29 ± 3.44), (18.41 ± 2.92), (17.67 ± 2.01) h, and AUC0-t were (27.26 ± 8.12), (112.62 ± 24.08), and (208.36 ± 51.36) mg·h·L-1, respectively. ρmax, AUC0-t and dose have a good linear relationship, increasing proportionally with the increase in the dose of the drug. After dose correction and natural logarithm transformation, there is no significant difference between the three doses of each parameter by analysis of variance. Peng Ying et al. [26] studied the pharmacokinetic characteristics of ginsenoside Re at different doses in rats. After intravenous injection of three different doses (20, 30, 40 mg·kg-1) of ginsenoside Re, the pharmacokinetics of the three groups of rats were all double-compartment models. The t1/2α were 6.505, 6.817, and 4.499 min, t1 /2 β were 28. 96, 30. 49 and 27. 57 min, respectively, and AUC was 599. 31, 1 025. 65 and 1 415. 7 min · mg · L-1, respectively. The main kinetic parameters of the three groups of rats were similar, and the AUC increased proportionally with the increase in dose, indicating that the elimination of Re is linear within this dose range.
4 New ideas for the pharmacokinetic study of ginsenosides
4.1 Pharmacokinetic study of metabolites
Oral administration is currently the main method of administration for most saponin compounds. Studies have found that after oral administration, saponin compounds are easily metabolized by intestinal flora, and the blood concentration of the original drug is low. Therefore, some scholars have studied the pharmacokinetics of ginsenosides by measuring the blood concentration of their metabolites. Ren et al. [27] established an HPLC-APCI-MS method for the determination of 20(S)-protopanaxadiol (PPD) in plasma and studied the pharmacokinetic characteristics of PPD after oral administration in rats. The results showed that the ρmax of PPD in rats was (130.2 ± 41.5) ng·mL-1 and the tmax was (150.0 ± 73.5) min. The absolute bioavailability of PPD was (36.8 ± 12.4)%, 10 times that of its prodrugs ginsenosides Rg3 and Rh2, indicating that PPD is more easily absorbed into the bloodstream. Therefore, measuring the blood concentration of PPD can more accurately reflect the pharmacokinetic characteristics of ginsenosides Rg3 and Rh2.
4.2 Pharmacokinetics of different disease models
In the syndrome model, the pharmacokinetic process of traditional Chinese medicine is different from that of normal physiological animals. Therefore, it is more instructive to study the pharmacokinetics of traditional Chinese medicine on the basis of syndromes. Zhou Wei et al. [28] studied the pharmacokinetic changes of effective components of Qingnao Xuanqiao Fang, including gardenoside, ginsenoside Rg1, Rb1 and notoginsenoside R1, in rats under normal conditions and in the acute and recovery stages of stroke. The results showed that the effective components of Qingnao Xuanqiao Formula: gardenia, ginseng saponin Rg1, and notoginseng saponin R1 were all two-compartment open models in the normal group and the model group, and ginseng saponin Rb1 conformed to a one-compartment model. The absorption of the four components in rats was rapid, reaching ρmax in about 45 minutes. The elimination of gardenoside, ginsenoside Rg1 and notoginsenoside R1 is faster, while ginsenoside Rb1 is slower. For different model animals, the maximum blood concentration and area under the blood concentration-time curve of the four components are, from highest to lowest, acute model group > recovery model > normal group. The bioavailability of the model group to the drug is higher than that of the normal group, indicating that the model animals have an increased absorption of Qingnao Xuanqiao Fang compared to normal animals.
4. 3 Multi-component integrated pharmacokinetics
The effects of traditional Chinese medicine have a mechanism of multi-component and multi-target effects. It is more one-sided to explain the pharmacokinetic process of traditional Chinese medicine and prescriptions by the pharmacokinetic parameters of a single component. Therefore, Li Xiaoyu et al. [29] used panax notoginseng saponins as a model drug to establish an integrated pharmacokinetic research model based on the area under the curve (AUC0-∞) of each component of panax notoginseng saponins with a custom weight coefficient. Rats were administered Panax notoginseng total saponins (300 mg·L-1) by gavage and (10 mg·L-1) intravenously, and the drug concentrations of notoginsenoside R1, ginsenoside Rg1, Rd, Re and Rb1 in rat plasma were determined using LC-ESI-MS. After administration by gavage, total notoginseng saponins are rapidly absorbed in the body of rats, but the t1/2 of the diol saponins Rb1 and Rd is much greater than that of the triol saponins Rg1, Re and R1, and the results of the integrated pharmacokinetic study model established using the percentage area under the curve (AUC0-∞) of each component as a self-defined weighting factor showed that the combined concentration-time curve conformed to the elimination law of drugs administered by gavage or intravenous injection. Panax notoginseng total saponins are rapidly absorbed in rats. The integrated pharmacokinetic t1/2 and AUC after gavage and intravenous administration were 18.88 and 19. 15 h and 25. 33, 84. 83 mg · h · L-1, respectively. This model conforms to the characteristics of classical pharmacokinetic models, and the parameters obtained can characterize the overall disposition of traditional Chinese medicine to the greatest extent. This provides a new research idea and method for establishing pharmacokinetic research on traditional Chinese medicine that conforms to the characteristics of traditional Chinese medicine.
5 Prospects
Ginsenosides have diverse biological activities and significant clinical efficacy. Extensive research has been conducted on their pharmacological effects and pharmacokinetic behavior, and some results have been achieved. However, there are still some areas that need improvement.
On the one hand, ginsenosides are easily metabolized by the gastrointestinal tract and liver, and their blood concentrations in the body are low and not easy to detect. Therefore, modern high-sensitivity analytical techniques (GC-MS/MS, LC-MSn, LC-NMR, etc.) need to be applied to the detection of ginsenosides in vivo. At the same time, since the substances that exert effects on target tissues in vivo may be their metabolites, attention should also be paid to the detection and analysis of ginsenoside metabolites. On the other hand, pharmacokinetic studies of ginsenosides at home and abroad have only studied the relationship between blood concentration and time from a chemical perspective [15, 21], ignoring the connection with efficacy.
Therefore, in future research, the pharmacokinetics of ginsenosides should be combined with pharmacodynamic indicators to explore the three-dimensional relationship between concentration-time-effect and establish a simultaneous pharmacokinetic and pharmacodynamic analysis model, thereby more realistically and objectively reflecting the pharmacokinetic process of ginsenosides in the body and providing a certain basis for clinical efficacy. In addition, there are many types of ginsenosides in the traditional Chinese medicine ginseng, and the pharmacokinetic parameters of each individual are different [30], which cannot be used to characterize the overall pharmacokinetic behavior and characteristics of ginseng. Therefore, we should be guided by traditional Chinese medicine theory and combine modern experimental techniques such as metabolomics, proteomics, and gene chips to explore new methods for pharmacokinetic research on multi-effect components of traditional Chinese medicine from a systems biology perspective [31].
Reference:
[1 ] ZHANG G,LIU A,ZHOU Y,et al.Panax ginseng ginsenoside- Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia [J].J Ethnopharmacol,2008,115 (3) : 441-448 .
[2 ] YU X T,WANG S P.Clinical observation on treatment for postop- erative gastric cancer by ginsenoside Rg3 combined with chemo- therapy [J].Chin J Cancer Prev Treat, 2010,17 ( 10) :779-781 .
[3 ] ZHANG Z ,JIANG B ,ZHENG X ,et al. Immune enhancing effects of Rg3 on peripheral lymphocytes in vitro in cancer patients treated with radiotherapy [J].Chin Pharm J, 2004,39(4) : 261-264 .
[4 ] SUI D Y,YU X F,QU S C,et al.Effects of Panax quinquefoli- um 20S-2protopanaxdiol saponins on experimental ventricular re- modeling in rat [J].Chin Pharm J ,2007,42 (2) : 108-112 .
[5 ] LEE Y,JIN Y,LIM W,et al.A ginsenoside-Rh1 ,a component of ginseng saponin ,activates estrogen receptor in human breast carcinoma MCF-7 cells [J].J Steroid Biochem,2003,84 (4 ) : 463-468 .
[6 ] WILLIAM C S,CHUNG W S,SALLY K W,et al.Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihy- perlipidemic efficacies in streptozotocin-induced diabetic rats [J].Eur J Pharmacol,2006,550( 1-3) : 173-179 .
[7 ] KIM S E,LEE Y H,PARK H J,et al.Ginsenoside-Rs4 ,a new type of ginseng saponin concurrently induces apoptosis and selec- tively elevates protein levels of p53 and p21WAF1 in human hepato- ma SK-HEP-1 cells European [J].Eur J Cancer,1999,35 (3) : 507-511 .
[8 ] LI H,SUN J G,XIE H T,et al.Study on absorption mechanism of ginsenoside Rg1 using rat everted gut sac [J]. Chin J Clin Pharmacol Ther,2004,9 (5 ) : 510-513 .
[9 ] FENG L,HU C J,YU L Y,et al.Pharmacokinetics of ginsen- oside Rg1 and its metabolites in rats [J].Acta Pharm Sin,2010,45 (5) : 636-640 .
[10] LEE J,LEE E,KIM D,et al.Studies on absorption,distribu- tion and metabolism of ginseng in humans after oral administration[J].J Ethnopharmacol,2009,122( 1) : 143-148 .
[11] YANG X W ,GUI F J,TIAN J M ,et al.Pharmacokinetics of ginsenoside-Rg2 in rats [J].Chin Pharmacol Bull,2009,25 (7) : 967-970 .
[12] GUI F J,YANG X W,LI L Y,et al.Simultaneous enantiomer determination of 20 ( R) - and 20 ( S) -ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method [J].J Chromatography B,2007,850( 1-2) : 1-6 .
[13] HUANG Y ,LIU J H.Pharmacokinetic study of ginsenoside 20 ( S) -Rg3 in rats by HPLC-ELSD[J].J Pr Med Theory,2005,12(9) : 2564-2566 .
[14] CAI Z,QIAN T,WONG R,et al.Liquid chromatography-elec- trospray ionization mass spectrometry for metabolism and pharma- cokinetic studies of ginsenoside Rg3[J].Anal Chim Acta,2003, 492( 1-2) : 283-293 .
[15] LIU L,HUANG J,HU X,et al. Simultaneous determination of ginsenoside ( G-Re ,G-Rg1 ,G-Rg2 ,G-F1 ,G-Rh1 ) and proto- panaxatriol in human plasma and urine by LC-MS / MS and its ap- plication in a pharmacokinetics study of G-Re in volunteers [J]. J Chromatography B,2011,22( 15) : 2011-2017 .
[16] JOO K,LEE H J,JEON H Y,et al.Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography / mass spectrometric method [J].J Pharmaceut Biomed,2010,51 ( 1-2) : 278-283 .
[17] QIAN T,JIANG Z H,CAI Z.High-performance liquid chroma- tography coupled with tandem mass spectrometry applied for meta- bolic study of ginsenoside Rb1 on rat [J].Anal Biochem,2006, 352( 1) : 87-96 .
[18] GU Y,WANG G J,SUN J G.Pharmacokinetic characterization of ginsenoside Rh2 ,an anticancer nutrient from ginseng ,in rats and dogs [J].Food Chem Toxicol,2009,47 (9) : 2257-2268 .
[19] YANG L,DENG Y,XU S,et al.In vivo pharmacokinetic and metabolism studies of ginsenoside Rd [J].J Chromatography B, 2007,854( 1-2) :77-84 .
[20] WANG W,WANG G J,XIE H T,et al.Determination of gin- senoside Rd in dog plasma by liquid chromatography-mass spec- trometry after solid-phase extraction and its application in dog pharmacokinetics studies [J].J Chromatography B,2007,852 ( 1-2) : 8-14 .
[21] JIANG J,LI S ,GONG P L,et al.Pharmacokinetics of Shengmaiyin oral solution after a single dose in human [J].Acta Med Univ Sci Technol Huazhong , 2007,36(2) : 272-274 .
[22] LIN L,ZU F Y,ZHANG W W,et al.Effects of compound compatibility on absorption in vitro of ginsenoside saponins[J].Chin J Mod Drug Appl ,2010,4 ( 13 ) : 150- 152 .
[23] LUO D F,YE J T,ZHANGY S,et al.Studies on drug release in vitro and absorption in rat in vivo of ginsenoside Rd solid lipid nanoparticles [J]. Chin Pharmacol Bull, 2009,25 (7) : 923-926 .
[24] GU Y,WANG G J ,SUN J G,et al.Pharmacokinetic study of ginsenoside 20( R) -Rh2 in Beagle dogs by LC-ESI-MS [J].Chin J Clin Pharmacol Ther ,2006,11 (3) : 159-163 .
[25] DENG Y H,ZENG X ,FENG Y,et al.Pharmacokinetics and tolerance of single dose intravenous drip of ginsenoside-Rd injec- tion in Chinese healthy volunteers [J]. Chin J Clin Pharmacol ,2009,25 (2) : 116-120 .
[26] PENG Y,WANG S J,PAN W S,et al.Pharmacokinetics of ginsenoside Re in rat [J].J Shenyang Pharm Univ,2006,23 (4) : 197-200 .
[27] REN H C,SUN J G,WANG G J,et al.Sensitive determination of 20 ( S) -protopanaxadiol in rat plasma using HPLC-APCI-MS : Application of pharmacokinetic study in rats [J].J Pharm Bi- omed,2008,48 (5) : 1476-1480 .
[28] ZHOU W,SHI R B,SUN J N,et al.Pharmacokinetics of effec- tive fractions of Qingnaoxuanqiao Formula in different rat models [J].J Beijing Unit Tradit Chin Med, 2008,31 (4) : 265-268 .
[29] LI X Y,HAO H P,WANG G J,et al.Integrated pharmacokinet- ic study of multiple effective components contained in total panax notoginsenosides[J].Chin J Nat Med,2008, 6(5) : 377-381 .
[30] SONG M,ZHANG S,XU X,et al. Simultaneous determination of three Panax notoginseng saponins at sub-nanograms by LC-MS / MS in dog plasma for pharmacokinetics of compound Danshen tab- lets [J].J Chromatography B,2010,878 (32) : 3331-3337 .
[31] WANG Z ,MA X ,CHEN X ,et al. Current methods and ad- vancesin microbial metabolom ics[J].Prog Chen, 2010,22( 1) : 163-172 .


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese