Your One-Stop Coenzyme Q10 Raw Material Solution: Green Spring Technology
In today's health industry, Coenzyme Q10 (CoQ10) serves as a key factor in cellular energy metabolism and a potent endogenous antioxidant, demonstrating exceptional application value across multiple domains including heart health, skin care, anti-aging, and physical performance enhancement. Its unique multifaceted biological functions provide a robust scientific foundation for developing various health products.
Green Spring Technology: Your One-Stop Solution for Coenzyme Q10 Raw Materials
As a specialized supplier of CoQ10 raw materials, Green Spring Technology deeply understands the practical challenges in product development and is committed to providing customers with comprehensive solutions spanning from raw materials to technology.
Core challenges we address for our clients:
★ Raw material selection difficulties
· Comprehensive product portfolio: High-purity lipid-soluble CoQ10 (≥98%), water-soluble CoQ10 (10%, 20%, 40%), reduced ubiquinol (≥85%), and liposomal CoQ10
· Professional raw material selection guidance tailored to your product positioning and formulation requirements
· Guaranteed consistent raw material quality with high batch-to-batch uniformity
★ Technical Development Bottlenecks
· Dedicated technical service team providing formulation design and process optimization support
· Sharing extensive application expertise to help clients avoid common technical pitfalls
· Customized solutions for specialized dosage form requirements
★ Supply Chain Stability
· Comprehensive quality management system ensuring reliable raw material supply
· Complete technical documentation and regulatory compliance support
· Scalable production capacity to meet diverse client procurement needs
★ Product Differentiation Competitiveness
· Help clients create differentiated products through innovative raw material formulations
· Provide scientific evidence and market insights to support product positioning
· Assist clients in developing end products with distinct competitive advantages
Green Spring Technology is committed to being your reliable strategic partner. We not only supply premium raw materials but also offer comprehensive services including technical consulting, application development, and market insights, empowering your success in major health sectors such as cardiovascular wellness, skincare, and sports nutrition.
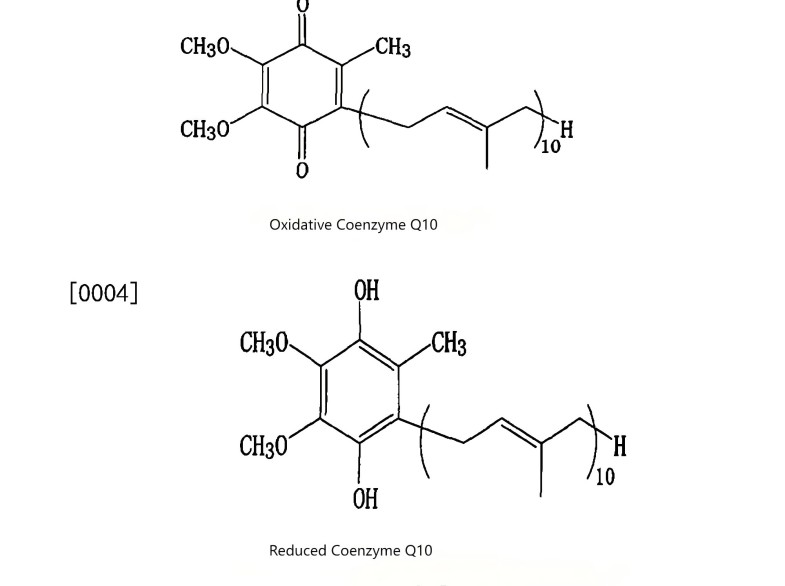
1 Coenzyme Q10: The Scientific Cornerstone of Your Formulations
In today's era of precision nutrition, consumers increasingly demand scientific rationale behind product ingredients. As an endogenous key substance in the human body, Coenzyme Q10 (CoQ10) has become an indispensable scientific cornerstone in premium health product formulations due to its pivotal role in cellular energy metabolism and antioxidant defense.
1.1 Synergistic Dual Forms: Dual Mission of Energy and Protection
Coenzyme Q10 primarily exists in the human body in two mutually convertible forms:
◆ Oxidized Form (Ubiquinone)
Acts as the “spark plug” in the mitochondrial respiratory chain, directly driving cellular energy (ATP) production. It is crucial for approximately 95% of the body's cellular energy generation.
◆ Reduced Form (Ubiquinol)
As the body's sole lipid-soluble antioxidant, it acts as a powerful internal “scavenger,” directly neutralizing free radicals and regenerating other antioxidants (such as Vitamin E), creating a synergistic amplification effect.
These two forms exhibit distinct distributions across organs like the heart and brain, collectively forming the body's robust energy and defense system. This provides a precise scientific pathway for developing products targeting specific health focuses.
1.2 Multiple Biological Functions: Infusing Your Products with Solid Scientific Substance
CoQ10's exceptional value stems from its multiple proven biological functions:
■ Cellular Engine
As an essential cofactor for mitochondrial complexes I, II, and III, it directly drives ATP synthesis, providing the body's fundamental life energy.
■ Universal Antioxidant
Not only does it scavenge free radicals and prevent lipid peroxidation, but it also “regenerates” antioxidants like vitamin E, enhancing the body's overall antioxidant network efficiency.
■ Vascular Guardian
Indirectly supports vascular endothelial health and normal vasodilation by protecting low-density lipoprotein (LDL) from oxidation.
■ DNA Protector
Provides deeper cellular protection by activating DNA repair enzymes while safeguarding cell membranes.
1.3 Green Spring Technology's Raw Material Solutions
We understand that the scientific properties of raw materials can only translate into tangible product efficacy through exceptional quality. Green Spring Technology is committed to providing you with high-purity, high-standard CoQ10 raw materials. Whether in the form of ubiquinone or the more bioavailable ubiquinol, we can meet your customized formulation needs.
Choosing us means gaining not just a raw material, but a solution grounded in deep scientific validation. Let us work together to transform CoQ10's cellular-level energy and protective mechanisms into powerful market competitiveness for your products.
2 The Application Potential of Coenzyme Q10 Across Diverse Health Fields
As a core substance for cellular energy metabolism and antioxidant defense, CoQ10's clinical foundation lies in its broad support for fundamental physiological processes. With advancing research, its application potential across multiple health domains continues to be explored, providing robust scientific backing for innovative health product development.
2.1 Core Mechanisms: From Cellular Function to Systemic Health
CoQ10 provides foundational energy for high-demand organs like the heart and brain by participating in ATP synthesis. Simultaneously, its potent antioxidant activity helps maintain cellular homeostasis. These two core mechanisms underpin its multifaceted value in supporting human health.
2.2 Key Application Areas and Research Advances
2.2.1 Cardiac Health Support
● Scientific Background
As one of the body's most active organs, the heart is highly susceptible to energy metabolism and oxidative stress.
● Research References
Multiple scientific studies indicate that CoQ10 supplementation helps improve myocardial energy metabolism and provides antioxidant support for the cardiovascular system.
2.2.2 Neurological Health Maintenance
▲ Scientific Background
The brain, another high-energy-consuming organ, relies on efficient mitochondrial function to maintain healthy cognitive performance.
▲ Research References
Preliminary studies are exploring CoQ10's potential role in supporting neuronal vitality and defending against oxidative damage, offering insights for brain health product development.
2.2.3 Body Energy and Metabolic Support
★ Scientific Background
Coenzyme Q10 levels are closely associated with overall bodily vitality.
★ Research References
For individuals experiencing reduced energy due to aging, high-intensity exercise, or modern-day stressors, supplementing with CoQ10 is considered an effective nutritional strategy to support cellular energy production and alleviate physiological fatigue.
2.2.4 Adjuvant Drug Intervention
◆ Scientific Background
In specific circumstances, exogenous supplementation aids in maintaining steady-state CoQ10 levels within the body.
◆ Research References
Studies indicate that CoQ10 may provide adjunctive support during certain drug therapies. Additionally, for rare hereditary CoQ10 deficiency disorders, exogenous CoQ10 supplementation remains a primary intervention method.

3 Coenzyme Q10 Raw Material Innovation Trends: Technological Breakthroughs from Basic Nutrition to Precision Health
Against the backdrop of continuous health industry advancement, coenzyme Q10 raw materials are undergoing a profound transformation from basic nutritional supplements to precision health solutions. Raw material technology innovation has become the core driver of product differentiation.
3.1 Industry Challenges and Technological Opportunities
Current coenzyme Q10 raw material development faces two key technical bottlenecks:
· Enhancing Bioavailability: How to improve the bioavailability of raw materials through technological innovation to ensure effective absorption and utilization of active components
· Achieving Form Stability: While reduced CoQ10 (Ubiquinol) offers theoretical advantages, its stability requirements demand higher production standards
These technical challenges point the way for industry innovation. Future product competitiveness will depend on comprehensive solutions encompassing optimized raw material forms, innovative dosage technologies, and refined delivery systems.
3.2 Technological Innovation Pathways and Development Directions
Based on the latest research findings, we have outlined three key technological development pathways:
· Formulation Innovation: Focus on developing more stable reduced ubiquinol raw materials to overcome technical bottlenecks
· Delivery System Upgrades: Significantly enhance raw material bioavailability through advanced technologies like liposomes and microencapsulation
· Comprehensive Solutions: Establish an end-to-end technical support system spanning raw material selection to dosage form design
3.3 Product Portfolio and Technical Advantages of Green Spring Technology
As a specialized CoQ10 raw material supplier, Green Spring Technology has established a comprehensive product line:
Standardized Raw Material Series
· Liposoluble CoQ10: ≥98% purity with excellent bioactivity
· Water-Soluble Coenzyme Q10: 20% content, suitable for aqueous formulation systems
· Reduced Coenzyme Q10: Panthenol content ≥85%, utilizing patented stabilization technology
Innovative Dosage Form Solutions
· Liposomal Coenzyme Q10: Employing advanced encapsulation technology to significantly enhance bioavailability
· Microencapsulated Products: Effectively improving raw material stability and extending product shelf life
Technical Support System
· Comprehensive technical support from formulation design to process optimization
· Established delivery system research platform
· Customized raw material solutions available upon request
Building the Industry's Future Together
We firmly believe that deep collaboration between raw material suppliers and brand owners is key to driving industry innovation. Green Spring Technology looks forward to establishing strategic partnerships with industry peers. Through continuous technological innovation, we will jointly develop high-quality health products that meet market demands.
Cooperation Inquiry
For the following products and services, please contact us:
■ Standardized Raw Material Series (Liposoluble 98%, Water-soluble 20%, Reduced 85%)
■ Innovative Formulation Solutions (Liposomes, Microencapsulated Products)
■ Customized Technical Development Services
■ Comprehensive Formulation Technical Support
Contact Green Spring Technology's professional team at helen@greenspringbio.com or WhatsApp: +86 13649243917 for detailed product information and technical solutions.
References:
[1]Feigin VL,Nichols E,Alam T,et al. Global,regional,and national burden of neurological disorders,1990-2016:a systematic analysis for the Global Burden of Disease Study 2016[J]. Lancet Neurol,2019,18(5):459-480. DOI:10.1016/s1474-4422(18) 30499-x.
[2]Hou Y,Dan X,Babbar M,et al. Ageing as a risk factor for neurodegenerative disease[J]. Nat Rev Neurol,2019,15(10): 565-581. DOI:10.1038/s41582-019-0244-7.
[3] Zeng Z,Li Y,Lu S,et al. Efficacy of CoQ10 as supplementation for migraine:a meta-analysis[J]. Acta Neurol Scand,2019,139 (3):284-293. DOI:10.1111/ane.13051.
[4]RaiznerAE. Coenzyme Q(10)[J]. Methodist Debakey CardiovascJ, 2019,15(3):185-191. DOI:10.14797/mdcj-15-3-185.
[5]Pastor-Maldonado CJ,Suárez-Rivero JM,Povea-Cabello S,et al. Coenzyme Q(10):novel formulations and medical trends[J]. Int J Mol Sci,2020,21(22):8432. DOI:10.3390/ijms21228432.
[6]Hargreaves IP. Ubiquinone:cholesterol 's reclusive cousin[J]. Ann Clin Biochem,2003,40(Pt 3):207-218. DOI:10.1258/ 000456303321610493.
[7]Martínez-Reyes I,Cardona LR,Kong H,et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth[J]. Nature, 2020,585(7824):288-292. DOI:10.1038/s41586-020-2475-6.
[8]Díaz-Casado ME,Quiles JL,Barriocanal-Casado E,et al. The paradox of coenzyme Q(10)in aging[J]. Nutrients,2019,11(9):2221. DOI:10.3390/nu11092221.
[9]Biglan KM,Dorsey ER,Evans RV,et al. Plasma 8-hydroxy-2 '- deoxyguanosine levels in huntington disease and healthy controls treated with coenzyme Q10[J]. J Huntingtons Dis,2012,1(1): 65-69. DOI:10.3233/JHD-2012-120007.
-
Prev
Green Spring Technology's Liposomal CoQ10 Solves Stability Challenge
-
Next
Fermented CoQ10 Ushers in a New Era of Clean-Label Formulation


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese



