How to Prepare Stachyose?
The human gut is a complex microbial ecosystem. There are more than 1,000 species of bacteria, and the ecological balance between beneficial and harmful bacteria directly affects the health of the host [1-2]. The Scientific Consensus on Probiotics (2020 Edition)[3] states that probiotics can improve human intestinal health by regulating the ratio of flora in the gut.
The "food" of probiotics is called prebiotics, which is a widely recognized nutrient with the function of promoting the growth of beneficial intestinal bacteria to form a favorable colony structure [4-6], and the concept of prebiotics was first proposed by Giboson and Robefroid in 1995 [7], and the concept of probiotics was first proposed by Giboson and Robefroid in 1995 [8]. The concept of prebiotics was first proposed by Giboson and Robefroid in 1995 [7], and common prebiotics include oligofructose, soybean oligosaccharide, oligosaccharides, oligosaccharides, oligoisomaltose, oligogalactose, and fructooligosaccharides [8]. Compared with common prebiotics, fructose can more efficiently promote the proliferation of beneficial bacteria such as Bifidobacterium intestinalis [9], which is known as the "super bifidogenic factor", and it is a popular raw material for intestinal functional foods in recent years [10].
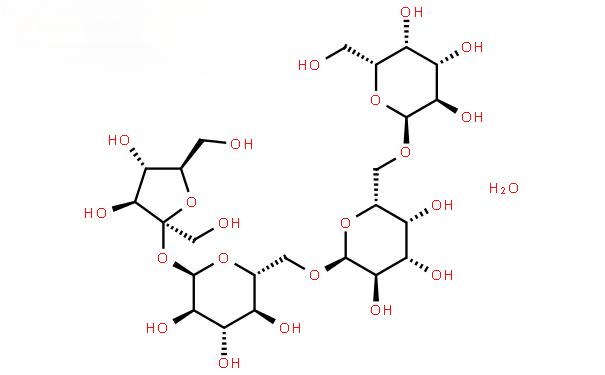
Stachyose is a naturally occurring tetrasaccharide, belonging to the galactoside class of cottonseed glycosides, a non-reducing functional oligosaccharide [11-12], which is a white powder in pure form, slightly sweet, with a sweetness of 22% of that of sucrose [13]. The molecular formula of fructose is C24H42O21, and the molecular structure is galactose-galactose-glucose-fructose (as shown in Figure 1), which is a typical member of the cottonseed oligosaccharide family [14-15] (as shown in Figure 2), and it mainly exists in plants of the genus Fructus of the family Labiatae. In recent years, the physiological functions of fructose have been continuously explored, and the functions reported so far include: immunomodulation, improvement of defecation, prevention of chemical liver injury, synthesis of B vitamins, promotion of intestinal absorption of trace elements, prevention of diabetes mellitus, etc. [16-21]. Sucrose is widely used in pharmaceuticals, food, cosmetics and other fields [22].
The increasing demand of domestic and foreign markets has a broad application prospect, but the lack of efficient production methods has led to the high cost of high-purity threonine, limiting the development of its application, therefore, it is of great significance to explore a fast, efficient and low-cost method to produce high-purity threonine.
The preparation of Stachyose Powder is mainly divided into two stages: extraction and purification. Based on the current development of the preparation process of fructose, this paper reviews the research progress of fructose extraction and purification, and discusses the advantages and disadvantages of different preparation processes, with a view to providing references for the research work related to the industrial production of fructose.
1 Overview of the preparation of stachyose
In recent years, there are more than a thousand research results on the preparation process of fructose, which involve different raw materials, methods and extraction effects. At present, among the more than 10 types of oligosaccharides on the international market, except for soybean oligosaccharides and cottonseed sugars, the rest of the oligosaccharides are mainly prepared enzymatically [23], because the cost of enzyme preparation of fructose is higher, and the yield is lower [24], so the extraction of fructose from natural plants is the mainstream of the preparation of fructose, which is more suitable for the industrialized production. The plant raw materials that can be used as the extraction of fructose include: Dioscorea, Salvia miltiorrhiza, Ginkgo biloba (also known as the herbaceous stoneworm), Zeran, soybean, etc. [25-27]. The process flow of the traditional preparation of fructose is shown in Figure 3.
As shown in Figure 3, the traditional preparation process is to extract the plant materials through maceration, decolorization, desalination, drying and other operations to obtain the fructose extract, based on which, the researchers combined with biological, physical, chemical and other technologies to further optimize the experimental conditions, in order to improve the purity of fructose. The common extraction processes of fructose are divided into three main types: solution extraction, microbial fermentation and enzymatic extraction, and the common purification methods include membrane separation, column chromatography purification and crystallization.
Recently, Gerliani et al [28] found that in the extraction of proteins, soluble carbohydrates and minerals from soybean flour by using electroactivation in combination with phytochemical solutions, a certain amount of fructose and marshmallow sugars were present in the analyte and catalyst samples obtained, and their contents increased with the anode voltage within a certain range of voltage, up to 222.49 mg/g and 34.29 mg/g, respectively. The findings of this experiment may provide a new technical direction for the extraction of fructose.
2 Progress of the extraction process of stachyose powder
2.1 Solution extraction method
Solvent extraction is the most traditional method of extraction and is commonly used to extract active ingredients from natural plants. Based on the principle of similarity and solubility, the solvent with the highest solubility for the extract is chosen to extract it from the solvent with the lowest solubility. The solvents commonly used for the extraction of fructose are water and ethanol. Depending on the purpose of the study, the appropriate extracting solution, raw material, and extraction process should be selected.
2.1.1 Single extraction method
When water is used as the extraction solvent, the cost of extraction is low and the penetration of water into plant cells is strong. Zhang Min et al [29] used deionized water as the solvent to extract fructose from Radix et Rhizoma Dioscoreae, and the best extraction conditions were investigated by one-way and orthogonal tests, and the best extraction conditions were found to be at an extraction temperature of 50 °C, with a material to liquid ratio of 1 : 12 (kg/L), and an extraction time of 60 min, resulting in an extraction rate of 58.84% of fructose. More researchers chose to use the grasshopper as the raw material, because of the high content of fructose in the grasshopper [30], and its brittle texture, high water content of the texture characteristics, Yao Hong et al. [31] used the homogenization method of fresh products, the use of mechanical and hydraulic shear will be fresh grasshopper grinding into pulp, so that the active ingredient outflow, and is more conducive to the extraction of fructose, the final rate of extraction of fructose reached 91.62%. After multi-step purification, 96.10% purity of fructose powder was obtained. The study of high purity and low cost of fructose extraction is expected to provide a reference for the industrial preparation of high-purity fructose, but when water is used as the extracting solution, the water is prone to mildew and deterioration, and it is not easy to be preserved.
When ethanol is used as an extractant, it has the advantages of strong permeability and longer preservation period compared with water extract, but different concentrations of ethanol have a greater impact on the extracted components. Zhong et al [32] used response surface methodology to investigate the influence of different factors on the extraction of fructose in silver bars, and the final results showed that the influence of the size of the material-liquid ratio > ethanol volume ratio > extraction temperature > extraction time, so the key to use ethanol as an extractant is to explore and control the optimal concentration of ethanol extraction. The final results showed that the influence of the material-liquid ratio > ethanol volume ratio > extraction temperature > extraction time, so the key to use ethanol as the extraction solution is to explore and control the optimal concentration of ethanol extraction.

2.1.2 Auxiliary extraction methods
The single water or alcohol extraction method is not efficient and has a lot of impurities, so it usually needs to be combined with some auxiliary techniques. Assisted extraction refers to the method of extracting the solution through physical or biological techniques, such as ultrasonic, microwave, ultrahigh pressure and other assisted techniques. Assisted techniques are based on a single extraction method, assisted in the leaching of extracts, followed by centrifugation, supernatant removal of impurities and refining steps, similar to the process shown in Fig. 3, and the specific operation is based on the nature of the impurities actually produced. The use of assisted extraction techniques can significantly enhance the efflux of active ingredients from the plant [33-35].
Ultrasonic-assisted extraction is actually the use of ultrasound with cavitation effect, mechanical effect and thermal effect, accelerate the speed of molecular movement and increase the penetration of the medium, to accelerate the dissolution of active ingredients [36]. Wang Qi-Wei et al [37] used ultrasonic assisted water extraction method to extract fructose in the grass stoneworm, the results show that the effect on the extraction rate in descending order is the extraction time > ultrasonic power > extraction temperature; Hu Binjie et al [38] found that, in the ultrasonic technology assisted, the extraction time of the water extraction method can be shortened by 3/4, and the extraction rate of polysaccharides to increase by 30.00%. However, ultrasonic technology needs to control the ultrasonic time, too long will destroy the structure of polysaccharides, so that the sugar chain breaks, resulting in a reduction in the extraction rate.
The principle of microwave-assisted extraction is to use the polar substances inside the cell to absorb and convert microwave energy into thermal energy, so that the intracellular temperature rises rapidly, the water vaporizes and the intracellular pressure increases, thus rupturing the cell membrane and the cell wall to form cracks or pores, and then accelerating the entry of the extracting agent from outside the cell to dissolve the effective substances and then flow out of the cell. Chen Chuanyun et al. [39] invented a microwave-assisted extraction method for fructose, and obtained 15 kg of fructose from 50 kg of fresh Bombyx mori, with a purity of 90.02%, which was characterized by short extraction time and high efficiency. This method is characterized by short extraction time and high efficiency. The premise of the microwave-assisted method is that the treated material should have good water absorption and the product should have good thermal stability.
UHP-assisted extraction of bioactive components from natural plants is an emerging technology. This technique can effectively accelerate the mass transfer rate, rupture the plant cells to increase the extraction rate, shorten the processing time and reduce the solvent consumption.Wu et al [40] used high pressure-assisted extraction to extract polysaccharides and β-glucans from the mycelium of Aspergillus mycelium, and the high pressure-treated extract had higher content of polysaccharides and stronger activity compared with that of the conventional shake-soaked extract.
Steam explosion-assisted method is the use of high temperature and high pressure - instantaneous pressure relief method, so that the internal energy of the steam molecules penetrated into the plant tissue into mechanical energy, destroying the cellular layers of biomass tissues, thus accelerating the outflow of cellular contents [41]. Similar to the microwave-assisted method, both methods utilize the physical pressure change to promote the dissolution of extracts, avoiding the secondary contamination caused by chemical treatment, and are characterized by low cost and non-pollution.
Hong Feng et al [41] used steam explosion technology to extract xylooligosaccharides from corn stover, the test showed that the sugars in the steam explosion liquid are mainly oligosaccharides and some soluble polysaccharides, in the 1.60 MPa and 2.00 MPa steam pressure under the dimensional pressure for 5 min, the final oligosaccharides yield of 36.00% to 59.00%. This method is not currently used in the preparation of fructose, but the structure and function of xylooligosaccharides and fructose are similar, comparable, and fructose is thermally stable, steam explosion method may become a new way to assist the extraction of fructose.
2.2 Microbial fermentation
Microbial fermentation is currently the most economical method to purify fructose, and in recent years, studies on the preparation of fructose by microbial fermentation have continued to emerge. Microbial fermentation utilizes microorganisms to selectively consume certain sugars as a source of metabolic reproduction, which is characterized by preferential consumption of non-functional oligosaccharides, thus improving the purity of functional oligosaccharides of the target ingredient. The key to microbial fermentation is to control the factors that affect the growth and metabolism of microorganisms [42], such as the type and number of microorganisms (pure or mixed), enzyme activity, and the fermentation environment (temperature, oxygen, pH, etc.).
2.2.1 Types and numbers of microorganisms
The study and analysis of the fermentation characteristics of certain microorganisms and their rational use can effectively improve the purity of the target products [43]. Wang Zhirong [44] investigated the effect of fermentation of Lactobacillus, three types of yeasts, Aspergillus oryzae and Fusarium. The results showed that Aspergillus oryzae and Saccharomyces cerevisiae were suitable for the fermentation and purification of fructose, and Saccharomyces cerevisiae had the best effect: the retention rate of fructose by Saccharomyces cerevisiae after 48 h of fermentation was 93.31%, which accounted for 87.04% of the total sugar content. Shu Danyang et al. [45] recorded the degradation of mono- and disaccharides and fructose in Aspergillus niger, Aspergillus oryzae, Lactobacillus casei, Lactobacillus swissii, and Lactobacillus rhamnosus by five strains to provide a reference for selection of microorganisms in the field of microbial fermentation for the preparation of fructose.
In the fermented food and enzyme industry, the effect of mixed fermentation is better than pure fermentation [46]. Mixed fermentation has the advantages of multibacterial symbiosis, enzyme complementation and mutual reinforcement, and can overcome the problem of large concentration of pure fermentation intermediate products. The application of mixed fermentation technology to the purification of fructose can effectively improve the efficiency of fructose purification.
Wang Xue et al [47] used Aspergillus japonicus and lactobacillus mixed bacteria fermentation to purify fructose from silver bars, the results showed that after mixed bacteria fermentation, the sucrose and monosaccharide components in the extract were reduced compared with the pure fermentation, the total amount of sucrose and monosaccharides was reduced to 3.00%, and the retention rate of fructose was more than 95.00%, which accounted for 90.00% of the total sugar content, which was combined with the industrial chromatography to further purify fructose, and ultimately obtained the fructose purity. Combined with the industrial chromatographic separation technique, the purity of fructose was 90.00%~95.00% (of the total sugar), and this method has been applied by many famous enterprises in China.
2.2.2 Enzyme activity
Enzymes are one of the important factors affecting microbial fermentation, and enzyme inhibitors that promote mono- and disaccharide consumption or inhibit the degradation of fructose can be added to rapidly improve the purity of fructose and shorten the extraction time. Zhou et al. [48] investigated the effects of two sucrase inhibitors, ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) and ascorbic acid/Vitamin C (VC), on the purification of fructose in Aspergillus niger fermentation with the use of the silkworm Pseudomonas sylvestris as raw material. The effect of two sucrase inhibitors, namely, Aspergillus niger fermentation and ascorbic acid/Vitamin C, on the purification of fructose. The results showed that the addition of appropriate amount of sucrase inhibitors could effectively reduce the probability of sucrase degradation of A. niger during the fermentation process, and thus increase the content of A. niger. The optimal amount of EDTA-2Na was 0.01% of the mass of the extracted liquid, and the purity of fructose was 80.43% under the optimal test conditions.
2.2.3 Fermentation environment
During the growth and metabolism of microorganisms, the nutrients and energy provided by the fermentation environment directly affect the biosynthesis efficiency and quality of the target products, so it is necessary to investigate the optimal culture conditions for the selected strains.
In the study of Xie Jin[49] , the conditions affecting microbial fermentation were investigated more comprehensively. In this study, 0.01% Aspergillus niger and 0.01% Lactobacillus casei were used as the optimal combination of fermentation and fermentation.
The results showed that the retention rate of fructose in the synchronized fermentation group was 10.12% higher than that of the lagged fermentation group after 36 h of fermentation, which is because there is a synergistic effect between microorganisms, and the synchronized fermentation is more beneficial to the improvement of the purity of fructose in the extract than the lagged fermentation. The pH value of the fermentation broth ranged from 5.5 to 7.0, which was favorable for slowing down the degradation of sassafras without affecting the consumption of sucrose by the microorganisms.
Therefore, it is known that microbial fermentation for the purification of fructose is characterized by low production cost and simple process, but the composition of the product is more complex, and the quality of the product is affected by a variety of factors.
2.3 Enzymatic extraction
Enzymatic extraction is a method that uses enzymes to catalyze the decomposition of the cell wall components of plant cells, resulting in the rupture of the cell wall and the leaching of intracellular substances, thus achieving the purpose of extraction. The enzymes commonly used for the extraction of fructose include pectinase and cellulase, as well as complex enzymes.
Zhong Xianfeng et al [50] obtained the authorized invention patent "A method of extracting high-purity fructose from silver bars" in 2019, which used papain and plant composite enzymes combined with alcohol extraction, and obtained fructose with a purity of 95.00%-99.90% after a series of removal of impurities and refining process, which shows that the enzyme extraction method has the advantages of low material cost, process and technology, and the ability to extract fructose.
It can be seen that the enzyme extraction method has the advantages of low material cost, simple process, mild reaction conditions and high extraction rate. However, it also has some limitations, mainly because of more factors affecting enzyme activity, such as temperature and pH value, etc. If the enzyme activity is to reach the highest, the optimal reaction conditions are within a very small range, which need to be precisely controlled, otherwise the enzyme activity will be greatly reduced, affecting the extraction effect. The comparison of various extraction methods is shown in Table 1.
3 Progress in the purification process of fructose
3.1 Membrane Separation
After crude extraction, there are many impurities in the extract, mainly proteins, pigments, salts, amino acids, etc. Membrane separation requires the selection of filter membranes with good impurity removal and recovery according to the nature of the impurities.
The principle of membrane separation is to utilize the selective permeability of membranes to separate and purify different substances. Common separation membranes used in research include ultrafiltration membrane, reverse osmosis membrane, nanofiltration membrane, electrodialysis membrane and so on. Ultrafiltration membrane, nanofiltration membrane and reverse osmosis membrane are all filtration membranes. Ultrafiltration membrane has a pore size of 1 nm-300 nm, which can be used to retain some biomacromolecules and colloidal substances; nanofiltration membrane can retain substances with relative molecular weights of 300-1,000; reverse osmosis membrane is based on the principle that by applying a certain amount of pressure to the high-concentration end of the membrane, a pressure difference is formed between the two sides of the membrane, which makes solvent molecules transfer from the high-concentration to the low-concentration end of the membrane.
The principle of reverse osmosis membrane is that by applying a certain pressure to the high concentration end, a pressure difference is formed on both sides of the membrane, making solvent molecules transfer from the high concentration to the low concentration end. Min Zhang et al. [29] used deionized water as the solvent to extract fructose from Radix et Rhizoma Dioscoreae, and purified it by combining nanofiltration and reverse osmosis membranes. The nanofiltration and reverse osmosis membranes used in the experiment could be reused after cleaning, and their membrane permeability recovery rates were 95.52% and 97.22%, respectively.

An electrodialysis membrane is essentially a charged ion exchange membrane [51]. The membrane is located between the cathode and anode of the solution electric field. When the electric field operates, the anions and cations in the solution move in a directional manner and pass through the ion-selective electrodialysis membrane, thereby removing certain charged ions.
The high desalination efficiency of electrodialysis membranes has led to their use in industrial desalination applications such as seawater desalination [52] and wastewater treatment [53]. In the food industry, it can be used to remove inorganic salts and proteins, etc. Duan Shuran et al. [54] used electrodialysis to purify the extract of cottonseed sugar, in this study, they investigated the effects of changes in the operating voltage and the circulation flow rate on the purification effect and the energy consumption. In this study, they investigated the effects of changes in operating voltage and circulating flow rate on the purification effect and energy consumption. The final results showed that the desalination rate could reach up to 91.20% and the recovery of cottonseed sugar reached 94.50%.
3.2 Column chromatography purification
Column chromatography, also known as chromatography or chromatography, is a classic method for separating multi-component mixtures in scientific experiments, and can be used for qualitative, quantitative, and purification operations. Chromatographic columns usually consist of a stationary phase and a mobile phase, and can be classified into many categories according to the different fillers in the column, such as carbon-calcium chromatography [55], high-performance anion-exchange chromatography (HPAC) [56], and N-phenylphenylamino-β-cyclodextrin-conjugated chiral stationary phase-high-performance liquid chromatography (HPLC) [57], etc.
They all work on the same principle. Their working principles are the same, after the sample enters the column, according to the different partition coefficients between the mobile phase and stationary phase, and the different adsorption capacity of the stationary phase, the components move downward at different speeds, and then flow out and be collected in a certain order, so as to achieve the purpose of separation and purification.
When column chromatography is used for the determination of oligosaccharides, it is often coupled with mass spectrometry, and high-performance liquid chromatography (HPLC) is a more accurate method for the determination of oligosaccharides [58], which has the advantages of shorter analytical time, high selectivity and high sensitivity. Wang et al. [59] developed a method for the simultaneous determination of cyclic allyl ether glycosides and oligosaccharides (sucrose, honey disaccharide, cottonseed sugar, mannose and trehalose) in Dixiandra chinensis. In this study, a rapid separation of seven analytes was performed by hydrophilic interaction liquid chromatography, and the analytes were finally detected by triple quadrupole tandem mass spectrometry (TQ-MS/MS) with sensitivity and selectivity, which showed that the correlation coefficients of the analytes were above 0.99, with good linearity, and the deviation of precision was less than 5.00%, and the recoveries were in the ranges of 93.80%~105.50%.
When column chromatography is used for the purification of fructose, the commonly used stationary phase fillers are resins, activated carbon, etc. Resins are divided into ion exchange resins, macroporous adsorption resins, and gel-type resins. Resins are divided into ion exchange resins, macroporous adsorption resins and gel-type resins, the principle of which is to use the adsorption effect of the resin to retain specific components, and then elute them to achieve the purpose of separating the sample components, which has the advantages of high rate of desalination, good effect of decolorization, automation, and repeated recycling, and so on. Ion exchange resins and macroporous adsorption resins are commonly used for the removal of salts, pigments, proteins, amino acids and other impurities from the extract of fructose.
In the study of Xie Jin [49], in the extraction of fructose from the dried herbaceous silkworm, the elution rate of protein was 93.70%, the elution rate of ash was 97.81%, and the decolorization rate was as high as 99.50% by using the D001 type of macroporous strongly acidic cation exchange resin and the D301 type of macroporous weakly alkaline anion exchange resin with the flow rate of 3.77 BV/h and the temperature at 35 °C. The results of this study were as follows: (1) the elution rate of protein and ash was 97.81%, and the decolorization rate was 99.50%; and (2) the elution rate of protein and ash was 99.50%. Cong Liu et al [60] used HW-40C gel as stationary phase to remove protein and purify cottonseed sugar. Under the optimal process conditions, the purity of cottonseed sugar was 89.10%, and the yield was 64.80%. The gel resin has the advantages of high separation efficiency and easy operation, but it should be noted that the pore space of the gel resin is small, so if the extract contains more pigments, they should be removed first, otherwise the gel resin will be easily clogged by the pigment molecules, resulting in the phenomenon of "poisoning" of the resin.
Activated carbon is a porous medium with a high capacity for adsorption of hydrophobic organics and is often used for decolorization. The principle is to use electrostatic force to physically adsorb small organic molecules or hydrophobic molecules in the micropores of activated carbon to remove impurities from the mobile phase. Bao et al[61] used activated carbon to adsorb and separate cotton seed sugar and sucrose, and through the adsorption-desorption cycle, the purity of cottonseed sugar was greater than 90.00%, with a recovery rate of 79.20%.Bernal et al[62] used Norit powdered acti- vated charcoal (NPAC) at a concentration of 5 g/L, with a trans-membrane pressure of 100 kPa, and an ultrafiltration pressure of 100 kPa. Bernal et al.[62] decolorized beet molasses at a concentration of 5 g/L of norit powdered acti- vated charcoal (NPAC), a transmembrane pressure of 100 kPa, a feed flow rate of 4.24 L/h, and a pH of 3. The color of beet molasses was reduced by more than 96.50%, and the activated charcoal was reconstituted with NaOH, and the loss of its color-removing capacity was less than 10.00%.
3.3 Crystallization
The principle of crystallization is that according to the different crystallization conditions of different substances, the extracted substances are precipitated out of saturated solution and crystallized, while other impurities remain in the solution, so as to achieve the purpose of purification. In the field of preparation of fructose, Zhang Jinze et al[63] purified fructose by using recrystallization and activated carbon adsorption. Recrystallization refers to the secondary crystallization of crystals, i.e., the process of crystallizing crystals from solution or melt after dissolution or melting, and recrystallization can purify the substance to a high degree, and the purity of fructose crystals obtained by this method is higher than 99.00%, which was authorized by a patent of the Chinese Invention Patent in 2016.
This method was authorized by the Chinese invention patent in 2016. Song Jianmin et al[64] invented "a method for the preparation of high purity fructose", in which fructose crystals with purity higher than 99.00% were obtained from the raw material of Fagus sylvatica by multiple extraction methods such as aqueous extraction, microbial fermentation, alcohol extraction, etc., and combined with alkaline precipitation, filtration, activated carbon decolorization, and cooling and crystallization purification methods. The purification of fructose by crystallization method is characterized by high purity, environmentally friendly solvent and simple process, and can be used for the preparation of commercial standard products of fructose, but the period of natural cooling and crystallization is long and the efficiency is low. The comparison of the three purification methods is shown in Table 2.

4 Summary and outlook
Compared with other oligosaccharides, fructose can more effectively promote the growth and reproduction of intestinal probiotics, and has a broad application prospect. The production and preparation of fructose is a popular research direction at present, and this paper summarizes the current status of the technology of fructose preparation in terms of the extraction process and purification process. In terms of economic benefits, microbial fermentation is currently the most economical method for the purification of fructose, and it is more suitable for industrial production, in which the mixed-bacteria fermentation is better than the single-bacteria fermentation, and the simultaneous multibacteria fermentation is better than the lagging fermentation, the pH value of the fermentation environment depends on the fermentation bacteria, and the pH value of 5.5-7.0 is usually suitable, the shaking fermentation is better than the static fermentation, and the slag fermentation is better than the filtration fermentation. In addition, the purification rate can be adjusted by adding some enzyme inhibitors to realize low-cost and high-efficiency mass production of fructose.
However, the microbial fermentation method has the disadvantage of many impurities in the product, which need to be removed in multiple steps in the later refining process. Although the purity of some processes in the purification of fructose has reached international standards and can be used to produce high-purity test standards, there are some limitations.
For example, the membrane separation method and column chromatography purification method have the problems of high cost of consumables and small processing capacity; the time cost of crystallization method is directly proportional to the production capacity and inversely proportional to the evaporation area, which are not suitable for large-scale purification of fructose. Therefore, the establishment of an efficient and low-cost method for the purification of fructose, which can be applied to industrialized mass production, is a direction that needs to be further investigated in the field of fructose processing and application in the future.
References:
[1] TOSCANO M, DE GRANDI R, PASTORELLI L, et al. A consumer's guide for probiotics: 10 golden rules for a correct use[J]. Digestive and Liver Disease, 2017, 49(11): 1177-1184.
[2] Microecology Branch of Chinese Preventive Medicine Association. Consensus on Clinical Application of Digestive tract Microecological regulators in China (2016 Edition)[J]. Chinese Journal of Microeco-logy, 2016, 28(6): 621-631.
[3] Probiotics Society of the Chinese Institute of Food Science and Technology. Scientific consensus on probiotics (2020)[J]. Journal of Chinese Institute of Food Science and Technology, 2020, 20 (5): 303-307.
[4] SCOTT K P, GRIMALDI R, CUNNINGHAM M, et al. Developments in understanding and applying prebiotics in research and practice- an ISAPP conference paper [J]. Journal of Applied Microbiology, 2020, 128(4): 934-949.
[5] HUNGIN A P S, MITCHELL C R, WHORWELL P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms-an updated evidence systematic review: probiotics in the management of lower gastrointestinal symptoms-an updated evidence-based international consensus[J]. Alimentary Pharmacology & Therapeutics, 2018, 47(8): 1054-1070.
[6] BAJURY D M, NASHRI S M, KING JIE HUNG P, et al. Evaluation of potential prebiotics: a review[J]. Food Reviews International, 2018, 34(7): 639-664.
[7] GIBSON G R, ROBERFROID M B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics[J]. The Journal of Nutrition, 1995, 125(6): 1401-1412.
[8] LI C N, WANG X, LEI L, et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice [J]. Phytotherapy Research, 2020, 34(5): 1166-1174.
[9] ZARTL B, SILBERBAUER K, LOEPPERT R, et al. Fermentation of non-digestible raffinose family oligosaccharides and galactoman - nans by probiotics[J] . . Food & Function, 2018, 9(3): 1638-1646.
[10] LIU Y Y, LI T, ALIM A, et al. Regulatory effects of stachyose on colonic and hepatic inflammation, gut microbiota dysbiosis, and pe - ripheral CD4(+) T cell distribution abnormality in high-fat diet-fed mice[J]. Journal of Agricultural and Food Chemistry, 2019, 67(42).
11665-11674.
[11] ZHONG X F, ZHANG Y B, HUANG G D, et al. Proteomic analysis of stachyose contribution to the growth of Lactobacillus acidophilus CICC22162[J]. Food & Function, 2018, 9(5): 2979-2988.
[12] BHATIA L, SHARMA A, BACHHETI R K, et al. Lignocellulose de - rived functional oligosaccharides: production, properties, and health benefits[J]. Preparative Biochemistry & Biotechnology, 2019, 49(8): 744-758.
[13] LIANG Lixin. Functional oligosaccharide-stachyose[J]. China Food Additives, 2004(4): 51-54.
[14] FRENCH D. The raffinose family of oligosaccharides[J]. Advances in Carbohydrate Chemistry, 1954, 9: 149-184.
[15] VAN DEN ENDE W. Multifunctional fructans and raffinose family oligosaccharides[J]. Frontiers in Plant Science, 2013, 4: 247.
[16] HAN Shiwen, GAO Jiatao, SANG Ruojie, et al. The function and mechanism of stachyose on intestinal tract[J]. Food Science and Te- chnology, 2019, 44(4): 281-284.
[17] MA Xuan. Study on extraction and purification process of stachyose in Stachys sieboldii Miq[D]. Shenyang: Shenyang University of Che- mical Technology, 2019.
[18] LI T, LU X S, YANG X B. Evaluation of clinical safety and benefi - cial effects of stachyose -enriched α -galacto -oligosaccharides on gut microbiota and bowel function in humans[J]. Food & Function, 2017, 8(1): 262-269.
[19] CHEN X F, LIAO D Q, QIN Z X, et al. Synergistic interactions of catalpol and stachyose in STZ-HFD induced diabetic mice: syner- gism in regulation of blood glucose, lipids, and hepatic and renal function[J]. Chinese Herbal Medicines, 2019, 11(1): 70-77.
[20] HE L W, ZHANG F, JIAN Z Y, et al. Stachyose modulates gut micro - biota and alleviates dextran sulfate sodium-induced acute colitis in mice[J]. Saudi Journal of Gastroenterology : Official Journal of the Saudi Gastroenterology Association,2020,26(3):153-159.
[21] KENNEDY P J, MURPHY A B, CRYAN J F, et al. Microbiome in brain function and mental health[J]. Trends in Food Science & Te- chnology, 2016, 57: 289-301.
[22] GIACOBBO A, BERNARDES A M, DE PINHO M N. Sequential pressure -driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees[J]. Separation and Purification Technology, 2017, 173: 49-54.
[23] LI Bingxue, NIU Fei, ZHANG Ning. Enzymatic synthesis of func- tional oligosaccharides[J]. Journal of Microbiology, 2017, 37(1): 1 - 6.
[24] NAKATA H, SAKURAI H, NAGURA T, et al. Raffinose production using α-galactosidase from Paraphaeosphaeria sp[J]. Mycoscience, 2013, 54(4): 247-251.
[25] WANG Donghui, LIAO Na, SUN Peng, et al. Changes of transport sugar content in different organs of Rehmannia glutinosa[J]. China Journal of Chinese Materia Medica, 2018, 43(8): 1563-1570.
[26] ZHENG Yunfeng, CHENG Jianming, DING Ning, et al. Method for preparing stachyose by using water-extraction alcohol-precipitation of Salvia miltiorrhiza: CN102229627A[P]. 2011-11-02.
[27] CHEN Yan, ZHONG Xianfeng, HUANG Guidong, et al. Extraction conditions of stachyose from Stachys floridana shuttlw. ex Benth.[J]. Natural Product Research and Development, 2011, 23(1): 123-130.
[28] GERLIANI N, HAMMAMI R, AIDER M. Extraction of protein and carbohydrates from soybean meal using acidic and alkaline solutions produced by electro- activation[J]. Food Science & Nutrition,2020,8
(2):1125-1138.
[29] ZHANG Min, SHI Baoli. application of membrane separation tech - nology on the extraction of stachyose[J]. The Food Industry, 2019, 40(10): 102-106.
[30] YIN J F, YANG G L, WANG S M, et al. Purification and determina - tion of stachyosein Chinese artichoke (Stachyssieboldii Miq.) by high- performance liquid chromatography with evaporative light scattering detection[J]. Talanta, 2006, 70(1): 208-212.
[31] YAO Hong. Stduy on the preparation technology of stachyose from Stachys sieboldii Miq[D]. Wuxi: Jiangnan University, 2010.
[32] ZHONG X F, HUANG G D, CHEN Y, et al. Optimization of extract - ing stachyose from Stachys floridana shuttlw. ex Benth. by response surface methodology [ J]. Journal of Food Science and Technology, 2013, 50(5): 942-949.
[33] CHEMAT F, ROMBAUT N, SICAIRE A G, et al. Ultrasound as- sisted extraction of food and natural products. mechanisms, tech - niques, combinations, protocols and applications. protocols and applications. A review[J]. Ultra- sonics Sonochemistry, 2017, 34: 540-560.
[34] XI J, SHEN D J, LI Y, et al. Ultrahigh pressure extraction as a tool to improve the antioxidant activities of green tea extracts[J]. Food Re - search International, 2011, 44(9): 2783-2787.
[35] MITTAL R, TAVANANDI H A, MANTRI V A, et al. Ultrasound as - sisted methods for enhanced extraction of phycobiliproteins from marine macro-algae. Gelidiumpusillum (Rhodophyta)[J]. Ultrason- ics Sonochemistry, 2017, 38: 92-103.
[36] BHANGU SK, GUPTAS, ASHOKKUMARM. Ultrasonic enhancement of lipase-catalysed transesterification for biodiesel synthesis[J]. Ul - trasonics Sonochemistry, 2017, 34: 305-309.
[37] WANG Qiwei, REN Jianlin. Ultrasonic extraction of stachyose from Stachys sieboldii Miq[J]. Petrochemical Industry Application, 2014, 33(6): 97-99.
[38] HU Binjie, CHEN Jinfeng, WANG Gongnan. Comparative study on extraction of Ganoderma lucidum polysaccharides by ultrasonic method and traditional Comparative study on extraction of Ganoderma lucidum polysaccharides by ultrasonic method and traditional hot water method[J]. Science and Technology of Food Industry, 2007, 28(2): 190-192.
[39] CHEN CHUANYUN, LIANG YUANZHENG, LI SHENGMING. Membrane extraction process for stachyose in Stachys sieboldii: CN106543238A[P]. 2017-03-29.
[40] WU S J, CHEN Y W, WANG C Y, et al. Anti-inflammatory proper- ties of high pressure-assisted extracts of Grifolafrondosa in lipopo- lysaccharide- activated RAW 264.7 macrophages[J]. Food Science & Technology,2017,52(3):671-678.
[41] HONG Feng, SHAN Gu, SUN Weidong, et al. A primary study on xylo-oligosaccharide production with steam explosion[J]. Journal of Chemical Industry of Forest Products, 1999, 33(6): 3-6.
[42] ZHANG Bailiang. Bioenergy technology and engineering [M]. Beijing: Science Press, 2009: 84-103.
[43] LIN T J, LEE Y C. High-content fructooligosaccharides production using two immobilized microorganisms in an internal -loop airlift bioreactor[J]. . Journal of the Chinese Institute of Chemical Engineers, 2008, 39(3): 211-217.
[44] WANG Zhirong. Study on production of high purity stachyose from Stachys sieboldii Miq[D]. Guangzhou: South China University of Technology, 2017.
[45] SHU Danyang, XIE Jin, CUI Chun. study on preparation of high - purity stachyose by microbial fermentation[J]. China Condiment, 2019, 44(5): 1-4.
[46] SHI C Y, ZHANG Y, LU Z Q, et al. Solid -state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococ- cus faecium for degrading antinutritional factors and enhancing nu - tritional value[J]. Journal of Animal Science and Biotechnology, 2017, 8: 50.
[47] WANG Xue, ZHANG Jinze, DUAN Sufang, et al. Study on purifica - tion of stachyose by fermentation[J]. Food and Fermentation Indus - tries, 2010, 36(10): 94-97.
[48] ZHOU Wensi, XIONG Jian, XIE Jin, et al. Study on extraction and purification of stachyose from Stachys seiboibi by fermentation method[J]. China Condiment, 2018, 43(7): 21-25, 32.
[49] XIE Jin. Study on improving purity of stachyose using microorgan - ism fermentation method [D]. Guangzhou: South China University of Technology, 2018.
[50] ZHONG XIANFENG, HUANG GUIDONG, BAI YONGLIANG, et al. Method for extracting high purity stachyose from Stachys flori- dana shuttlw. ex Benth. CN106496287A[P]. 2017-03-15.
[51] ATUNGULU G , KOIDE S , SASAKI S , et al . Ion -exchange membrane mediated electrodialysis of scallop broth: Ion, free amino acid and heavy metal profiles[J]. Journal of Food Engineering, 2007, 78(4): 1285-1290.
[52] GALAMA A H, SAAKES M, BRUNING H, et al. Seawater pre- desalination with electrodialysis[J]. Desalination, 2014, 342: 61-69.
[53] LV Y, YAN H Y, YANG B J, et al. Bipolar membrane electrodialysis for the recycling of ammonium chloride wastewater: Membrane se - lection and process optimization[J]. Chemical Engineering Research and Design, 2018, 138: 105-115.
[54] DUAN Shuran, BAO Zongbi, WEN Guangdong, et al. Electrodialytic desalination in raffinose purification[J]. Food Science, 2016, 37(1): 28-32.
[55] YAMAMORI A, TAKATA Y, FUKUSHI E, et al. Structural analysis of a novel oligosaccharide isolated from fermented beverage of plant extracts[J]. Journal of Applied Glycoscience, 2017, 64(4): 123-127.
[56] MENGY, YIL, CHEN L, et al. Purification, structure characterization and antioxidant activity of polysaccharides from Saposhnikovia di - varicata [J]. Chinese Journal of Natural Medicines, 2019, 17(10): 792- 800.
[57] LI L, CHENG B P, ZHOU R D, et al. Preparation and evaluation of a novel N -benzyl -phenethylamino -β -cyclodextrin -bonded chiral stationary phase for HPLC[J]. Talanta, 2017, 174: 179-191.
[58] ZHOU Yang, LIU Li, GUO Lengqiu, et al. Content determination of stachyose in Radix-rehmanniae pieces by HPLC-ELSD[J]. Asia- Pacific Traditional Medicine, 2019, 15(5): 53-56.
[59] WANG X, WU C T, XU M, et al. Optimization for simultaneous de - termination of iridoid glycosides and oligosaccharides in Radix Rehmannia by microwave assisted extraction and HILIC-UHPLC - TQ-MS/MS[J]. Phytochemical Analysis, 2020, 31(3): 340-348.
[60] LIU Cong, BAO Zongbi, XING Huabin, et al. Separation and purifi - cation of raffinose and proteins by gel permeation chromatography[J]. Journal of Chemical Engineering of Chinese Universities, 2014, 28 (3): 503-509.
[61] BAO Z B, DUAN S R, ZHANG Z G, et al. Adsorption separation of raffinose from sucrose by activated carbon: Equilibrium, kinetics and dynamic breakthrough[J]. Separation Science and Technology, 2016, 51(10): 1636-1644.
[62] BERNAL M, RUIZ M O, GEANTA R M, et al. Colour removal from beet molasses by ultrafiltration with activated charcoal[J]. Chemical Engineering Journal, 2016, 283: 313-322.
[63] ZHANG Jinze, LIN Jing, ZHOU Zhijiao, et al. Method for preparing stachyose crystal: CN103265583A[P]. 2013-08-28.
[64] SONG Jianmin, WANG Dehai, WAN Rongsheng, et al. Preparation method of high-purity stachyose: CN110759956A[P]. 2020-02-07.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese





