Can Stachyose Be Used in Animal Feeding?
Stachyose belongs to α-galactose oligomers and is mainly found in various plants such as the Lamiaceae, Fabaceae, and Scrophulariaceae families, especially in plants in the Lamiaceae family's Osmundaceae genus. Currently, the main methods for preparing stachyose are physical extraction and enzymatic methods. Using plantain, sweet vernal grass, and stachys tuberifera as raw materials, the stachyose content extracted using the physical extraction method is generally 20% to 70% [1]. Using stachys tuberifera as raw material, the purity of stachyose prepared using biological purification and industrial chromatography separation techniques is as high as 90% [2].
The content of stachyose in soy oligosaccharides produced from soybeans and their processed by-products is generally 18% to 71%. In the definition of soy oligosaccharides in GB/T 22491-2008, soy oligosaccharides with a purity of 75% generally contain 18% stachyose, 6% raffinose, and 24% sucrose. Zhang Shuangshuang et al. [3] extracted stachyose with a purity of 90% by fermenting soy protein whey with yeast. Stachyose is also the main functional component of soy oligosaccharides [4]. In recent years, research on stachyose in China has gradually increased. In addition to the original fields of food and medicine, the application of stachyose in animal production has also attracted more and more attention.
1 Molecular structure and physical and chemical properties of stachyose
Stachyose is composed of one molecule of α-glucose, one molecule of β-fructose and two molecules of α-galactose, linked in the manner of galactose (α1→6) -galactose ( α1→6)-glucose (α1→2β) -fructose, and is therefore also known as a tetrose [5]. The molecular formula of stachyose is C24 H42 O21, with a relative molecular mass of 666.59. The molecular structure formula is shown in Figure 1. According to X-ray single crystal diffraction, stachyose belongs to the monoclinic crystal system and forms a three-dimensional layered structure through hydrogen bonding [5-6], as shown in Figure 2.
Figure 1 Molecular structure formula of stachyose
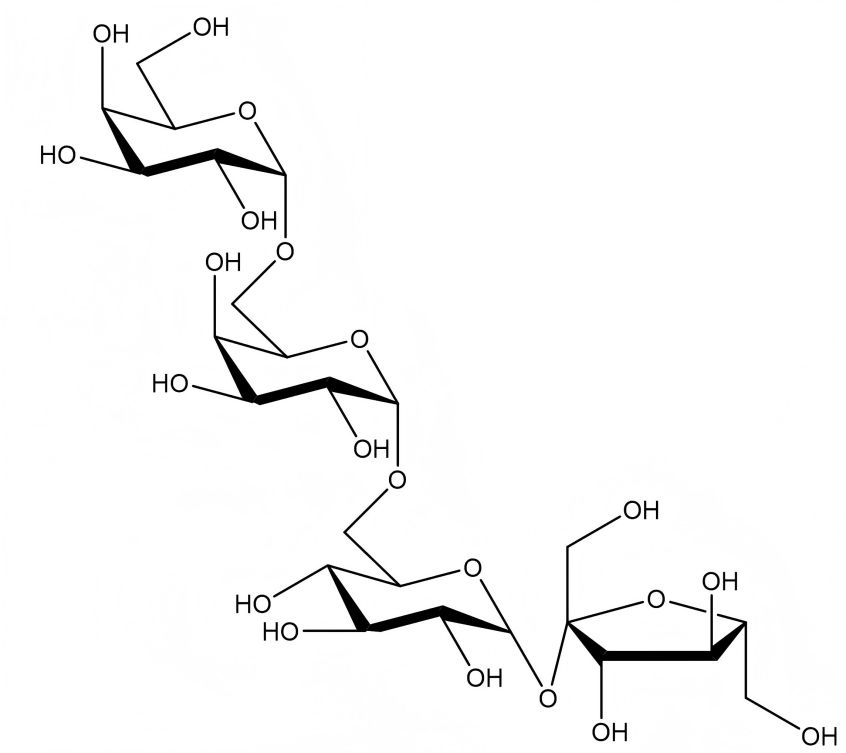
Pure stachyose is a white powder. Its sweetness is 22% that of sucrose, and it has a refreshing taste with no peculiar smell. Stachyose crystallizes into small crystals combined with 4 molecules of water. It is easily soluble in water, with a solubility of 130 g (20 °C). It is insoluble in organic solvents such as ether and ethanol. Its melting point is 101 °C. It can lose its crystal water when heated in a vacuum. The melting point of anhydrous stachyose is 167 ~ 170 ℃, and its moisture retention and moisture absorption are both lower than those of sucrose. It has no reducing properties. Guo Meili et al. [7] proved in a series of toxicological tests that stachyose is safe and non-toxic.
2 Application of stachyose in the food industry
Due to the lack of digestive enzymes that digest α-1,6-glycosidic bonds in the digestive systems of humans and monogastric animals, stachyose can directly enter the hind end of the digestive tract, where it is fermented and utilized by bifidobacteria and a small number of lactobacilli in the intestine. Therefore, non-digestible oligosaccharides such as stachyose are also known as dietary fiber or prebiotics[8]. The development and utilization of fructooligosaccharides has become widespread in developed countries such as the United States, Japan and Europe. With the maturity of fructooligosaccharide production technology and the promotion of its application, its development prospects have gradually attracted the attention and attention of China. Nowadays, there are 153 patents related to fructooligosaccharides in China, including 29 patents on fructooligosaccharide production technology, 2 patents on fructooligosaccharide testing technology, and the rest are all patents on the application of fructooligosaccharides [1].
2.1 Regulating the balance of intestinal flora
Hayakawa et al. [9] showed that soy oligosaccharides containing 23% and 71% raffinose after purification can be effectively utilized by Bifidobacterium in the human intestine, significantly increasing the number of bifidobacteria in the feces. Luo et al. [10] prepared a culture medium with soy oligosaccharides, stachyose, raffinose and sucrose as the base, and inoculated it with Bifidobacterium adolescentis and Escherichia coli from human feces, respectively. They found that stachyose was the main factor promoting the growth of bifidobacteria. Shu Guowei et al. [11] showed that the addition of 0.8% and 1.0% of raffinose to the medium for lactic acid bacteria (MRS) can significantly accelerate the growth of Bifidobacterium bifidum BB01. In addition to preferentially utilizing non-digestible oligosaccharides in the intestine, Bifidobacterium can also preferentially consume xylose under conditions where multiple non-digestible oligosaccharides coexist. Therefore, xylose can directly and selectively proliferate Bifidobacterium [12].
2.2 Improves the body's immune function
Due to the α-1,6-galactose structure in the molecule of raffinose, and based on the molecular mechanism of the sympathetically-activated receptor, it is involved in biological processes such as the adsorption between immune cells and pathogens. This gives raffinose the function of preventing pathogen infection, neutralizing toxins and regulating the immune system [13]. After mice were given a daily dose of 0.2 mL of water-soluble sucrose (per 10 g of body weight) by gavage for 30 consecutive days, it was found that their body immunity and mononuclear macrophage function were both positive [14]. In addition, under the action of bifidobacteria, water-soluble sucrose can be broken down to produce multiple immune function factors that enhance the body's immunity.
2.3 Relieves constipation and prevents diarrhea
Xylooligosaccharides are small, water-soluble dietary fibers that are preferentially utilized by bifidobacteria in the large intestine, producing large amounts of short-chain fatty acids such as acetic acid, propionic acid, and butyric acid [15]. It has been reported that 103 constipated patients who took a daily supplement containing 5 g of xylooligosaccharides experienced improved bowel movements and enhanced intestinal function [16]. In addition, the fructan molecule has a large number of hydroxyl groups, which can absorb water well in the intestine, relieving diarrhea [17]. In addition, fructan also has the functions of protecting the liver, inhibiting cancer, inhibiting atopic dermatitis, anti-arthritis, preventing dental caries, preventing colitis, excreting lead, lowering blood pressure and blood lipids, anti-oxidation and anti-aging [18].
3 Application of stachyose in animal feeding
3.1 Effect of stachyose on the intestinal flora of poultry
Pacifici et al. [19] showed that artificial injection of 5% and 10% raffinose into the amniotic cavity of chicken embryos incubated until day 17 and incubated until day 21 (hatch) showed that raffinose or raffinose had a beneficial effect on the intestinal flora, the biological utilization of iron, and the morphological function of intestinal mucosal tissue in chicken embryos. Xylooligosaccharides can be fermented and utilized by Bifidobacterium and a small number of Lactobacillus in the cecum of broiler chickens, and its fermentation rate is second only to that of raffinose in soybean oligosaccharides[20].
Yi Zhonghua [21] showed that the addition of 1.0% of a xylooligosaccharide preparation to the feed significantly increased the number of bifidobacteria and lactobacilli in the cecal digesta of broiler chickens, and significantly reduced the number of Escherichia coli and Salmonella in the cecal digesta. Lan et al. [22] used 14-day-old broiler cecum bacteria as inoculum, and fermented soybean oligosaccharides in vitro. The results showed that, compared with soybean oligosaccharides and raffinose, stachyose had the largest gas production and gas production rate, and significantly inhibited the growth of Escherichia coli and Clostridium perfringens. Yang Guiqin et al. [23] showed that the addition of 1.0% stachyose significantly increased the richness of the flora in the in vitro cecal contents of broiler chickens and promoted the proliferation of bacteria in the genus Brachysporangium, the genus Bacteroides and the genus Lactobacillus.
Under the action of bifidobacteria fermentation, raffinose produces a large amount of short-chain fatty acids in the intestine, which significantly reduces the pH in the intestine. In this environment, the proliferation of harmful bacteria can be inhibited and the intestinal microecological balance can be adjusted. However, Jiang et al. [24] reported that, except for the significant effect of raffinose on the concentration of butyrate in the cecum of broiler chickens in the initial stage, it did not have a positive effect in other respects. However, Yizhonghua [22] found in his study that the addition of a water sugar preparation to the feed significantly increased the concentration of acetic acid and short-chain fatty acids in the cecal digesta of broiler chickens, as well as the mole percentage of butyric acid in the total amount of short-chain fatty acids. increased, the pH of the cecal and ileal digesta showed a downward trend; in addition, the addition of 0.5% of a xylose preparation was able to effectively reduce the content of volatile basic nitrogen in the intestine, thereby inhibiting the proliferation of early broiler intestinal putrefactive bacteria.
3.2 Effects of raffinose on the morphology of the digestive organs and intestinal mucosa of poultry
Factors such as intestinal polyamines, epidermal growth factor, short-chain fatty acids and pH all affect the morphological structure of the digestive tract of poultry. Most of these factors are regulated by nutrients in the feed, and non-digestible oligosaccharides are one of the important regulators[25]. Yi Zhonghua et al. [26] showed that the addition of different levels of stachyose increased the absolute weight of the digestive organs of broiler chickens, especially the cecum and colon. The addition of 0.5% stachyose significantly increased the villus height of the duodenum, jejunum and ileum of broiler chickens, significantly reduced the crypt depth of the jejunum and ileum, and increased the villus height/crypt depth of the jejunum and ileum.

3.3 Effects of raffinose on poultry performance and nutrient digestibility
Raffinose is a non-digestible oligosaccharide. Due to its unique functionality and non-digestibility, it has an important effect on the digestive physiology and nutrient metabolism of animals, thereby affecting the digestion and absorption of various nutrients and ultimately the performance of animals. Jiang et al. [24] showed that adding 1.2% raffinose to the feed in the feed compared with the normal soybean meal feed, there was no significant difference in the digestibility of nutrients in the feed by broiler chickens, but as the amount of water-soluble sugar added increased, the digestibility of nutrients in the feed by broiler chickens decreased slightly. Yi Zhonghua [21] showed that the addition of 0.5% of a xylooligosaccharide preparation had varying degrees of improvement in the apparent utilization rate of nutrients in the feed, while the addition of 2.0% of a xylooligosaccharide preparation significantly decreased the apparent utilization was significantly reduced. Among them, calcium, phosphorus, neutral detergent fiber and acid detergent fiber were more affected by the amount of xylose added to the feed. The main reason is that a high dose of xylose accelerates the flow rate of the feed in the digestive tract.
Adding stachyose to broiler diets can lead to a decrease in average daily weight gain and feed efficiency. As the amount of stachyose added increases, the growth of broilers shows a linear decrease and a quadratic growth trend [24]. Yi Zhonghua et al. [27] showed that there is a dose-response relationship between the effect of stachyose on the growth performance of broiler chickens. A low dose (0.5%) showed a slight promoting effect, while a high dose (2.0%) showed an inhibitory effect. Moreover, the degree of dose response in the early growth period was stronger than in the later growth period.
3.4 The effect of fucose on the immune function of poultry
Wang Liwan et al. [2, 8] showed that some oligosaccharides, including fucose, can promote the development of immune organs such as the thymus and bursa of Fabricius in egg-laying chicks, and reduce the emission of ammonia (NH3), hydrogen sulfide (H2 S) and other malodorous substances in feces. Adding 0.5% of a fructooligosaccharide preparation to the feed has a tendency to increase the spleen index of 18-day-old broiler chickens, and can significantly increase the serum immunoglobulin A (IgA) content of 18-day-old broiler chickens, but significantly reduce the spleen index of 36-day-old broiler chickens [29]. Therefore, fucose can be used as an immune enhancer to improve the immune function of animals, and its immunomodulatory effect may be achieved mainly by regulating the intestinal microecology of animals [30].
4 Application of fucose in pig feeding
4.1 Effect of fucose on the intestinal flora of pigs
The mechanism of action of xylooligosaccharides in the pig intestine is the same as in broiler chickens, and the effect is also similar. Krause et al. [31] first reported that under in vitro conditions, xylooligosaccharides can be completely fermented by the post-weaning piglet's hindgut microorganisms, and that this fermentation process can be accelerated by the addition of high levels of lactose to the diet. accelerate. Zhang et al. [32] showed that adding 1.0% of raffinose to the diet significantly increased the number of Lactobacillus in the porcine ileum and the number of Bifidobacterium in the cecum and colon, significantly reduced the number of bacteria in the colon, and significantly increased the content of volatile fatty acids in the ileum, cecum and colon. However, high doses of raffinose can reduce the number of lactobacilli and bifidobacteria in the jejunum, ileum, cecum and colon [33].
4.2 Effects of stachyose on pig performance and nutrient digestibility
Raffinose and stachyose in soybean meal are considered to be anti-nutritional factors in monogastric animals, mainly because they ferment in the hindgut of animals and have no positive effect on the provision of metabolic energy [34]. Smiricky et al. [35] also showed that raffinose and stachyose reduced the digestibility of nitrogen and amino acids in pig feed, and that the digestibility of dry matter decreased significantly with increasing addition. It was found that adding α-galactosidase to a diet containing raffinose can eliminate this negative effect [36], because the enzyme can hydrolyze 80% of the raffinose in the small intestine of piglets, thereby significantly increasing the digestibility of dietary α-galactose. Although xylose has a negative effect on nutrient digestibility in pigs, the addition of 1.0% xylose to a soybean-free diet had no significant effect on piglet performance compared to a normal soybean meal diet, but significantly reduced the diarrhea rate of weaned piglets. However, when the addition reached 2.0%, piglet performance was significantly reduced in the first 2 weeks after weaning [32]. This shows that the dose effect of the impact of raffinose on piglet performance is very obvious, and also shows that the anti-nutritional properties of raffinose are mainly manifested in the digestibility of nutrients.

5 Application of stachyose in the production of other animals such as aquatic animals
Cai Yinghua [37] showed that the feed intake rate of flounder increased significantly with the increase of stachyose content in the feed, but the apparent digestibility of dry matter and protein in the feed tended to decrease. After the addition of stachyose, raffinose, or a combination of the two to the feed, the weight and growth rate of Atlantic salmon were between those of a diet containing whole fish meal and a diet containing soybean meal replacing part of the fish meal. It did not have a significant effect on the digestibility of protein and fat [38]. Compared to a diet containing 100% fish meal, the addition of 300 g/kg soybean meal significantly reduced the growth performance of juvenile heteromorphic silver crucian carp, but the addition of a moderate amount of stachyose, raffinose or stachyose + raffinose did not significantly change the growth status and intestinal morphology of the fish during the 8-week trial period [39].
Stachyose also had no significant effect on the body composition and intestinal flora of the hybrid silver crucian carp [40]. In addition, raffinose can improve the non-specific immune function of heterotrophic silver crucian carp to a certain extent [41]. Functional feeds (patented products) containing stachyose have been developed for the prevention and treatment of red skin disease in grass carp [42]. Hu et al. [43] showed that the addition of 1.25% raffinose to the feed significantly improved the growth performance, feed utilization and digestive enzyme activity of juvenile turbot. The addition of 1.25% and 5.00% of stachyose significantly increased the abundance of intestinal cellulolytic bacteria related to digestion and enhanced the barrier function of the intestinal mucosa of juvenile turbot. However, the addition of 2.5% to 5.0% of fructooligosaccharides also resulted in an increase in the feed intake and a decrease in the feed efficiency of turbot. When the addition reached 5.0%, although the number of beneficial bacteria in the turbot intestine increased, the number of some potential pathogenic bacteria also increased[44]. This shows that the effect of fructooligosaccharides on different functions (growth, digestion, intestinal mucosal barrier, etc.) of the same animal varies with the dose.

In addition to chickens, pigs and fish, the positive effects of fructooligosaccharides have also been demonstrated in animals such as silkworms, rhesus monkeys and mice. Chen Chuanjie et al. [45] showed that the addition of 0.50% fructooligosaccharides to the silkworm diet significantly increased the overall life rate of silkworm pupae, the amount of full cocoons, the amount of cocoon layers and the cocoon layer rate. Li Haifang [46] showed that after adding raffinose to the rhesus monkey diet, the number of lactobacilli in the intestine increased and the number of Escherichia coli decreased. After feeding stachyose powder for 6 weeks, the monkeys had normal bowel movements and the faeces were yellow in appearance and in the form of loose strips, indicating that raffinose improved the intestinal function of the monkeys.
In addition, adding stachyose powder also significantly improved the immunity and disease resistance of the macaques. Li et al. [47] found in their study that a soy oligosaccharide preparation containing 55.3% stachyose, 25.8% stachyose and 9.7% stachyose promoted the proliferation of beneficial bacteria in the intestines of mice, inhibited the proliferation of pathogenic bacteria, and significantly promoted intestinal peristalsis and excretion. Wei Yan et al. [48] showed that the prebiotic fructo-oligosaccharide-Lactobacillus plantarum synbiotic can significantly improve the specific cellular immunity, humoral immunity and non-specific immune function of mice with low immunity, increase the levels of some immune factors in the serum, and enhance the immune function of mice. A 4-week trial of feeding rats with type 2 diabetes (model) with stachyose found that stachyose affects the balance of the intestinal flora by changing the expression of the intestinal flora mRNA [49]. A study by Jiang-li Dou et al. [50] showed that the addition of 50 and 100 μg/mL of stachyose significantly increased the activity of neutrophils cultured in vitro from rabbits, and also significantly increased the ability of neutrophils to phagocytize Staphylococcus aureus.
It can be seen that current research on the use of stachyose in animal production mainly focuses on its effects on the intestinal flora, digestion and immunity of animals such as poultry, pigs and fish. 1) Similar to its effect on the human intestinal environment and intestinal flora, stachyose has a positive effect on adjusting the intestinal flora balance of animals such as chickens, pigs, fish, macaques monkeys and rats has a positive effect on the balance of the intestinal flora; 2) Fructooligosaccharides can increase the weight of the digestive organs of animals to varying degrees, improve the morphology of the intestinal villi, and enhance the barrier function of the intestinal mucosa; 3) Fructooligosaccharides have the function of preventing pathogen infection, neutralizing toxins and regulating the immune system. The main research results on the effects of stachyose on the intestinal flora, digestive capacity and immune function of poultry, pigs and aquatic animals are shown in Table 1.
6 Summary
Stachyose powder is derived from natural plants, is highly safe and has a wide range of applications. The rational application of stachyose can significantly improve the balance of the gastrointestinal flora and enhance the body's immune system, but it has little effect on animal production performance, and the dose effect is also very obvious. There are still many problems with its application in animal production, such as cost issues, the impact of the source of xylooligosaccharides, the addition level and the content of xylooligosaccharides in the basal diet on its effect, and the impact of adding xylooligosaccharides to the diet on the quality of animal products, the content of foul-smelling compounds in manure, and other potential physiological functions. Further research is needed, especially on the mechanism of action at the molecular level. Although research on stachyose in China started relatively late, the development and application of stachyose is attracting increasing attention. As the cost of preparing stachyose decreases, it is believed that the scope of stachyose applications will continue to expand with further research, and the market prospects will become even broader.
References:
[1] Zhang Jinzhe. Research overview and application status of the water-soluble dietary fiber stachyose [J]. Food Safety Herald, 2014(13): 62-63.
[2] Zhang Jinzhe, Zhou Zhiqiao, Zeng Ming, et al. Method for preparing high-purity stachyose using industrial chromatographic separation technology: China, CN: 101633676 [P]. 2010-01-27.
[3] Zhang Shuangshuang, Wang Liping, Wang Xichang. Fermentation conditions for the preparation of stachyose using soy protein concentrate whey [J]. Soybean Science, 2008, 27(1): 137-140.
[4] Shi Yun. Study on the purification process and mechanism of oligosaccharides in soybean molasses [D]. Doctoral dissertation. Wuxi: Jiangnan University, 2015.
[ 5 ] WU X Y ,CHAO Z M ,WANG C,et al.Extraction and absolute crystal structure of stachyose [ J] . Chinese Journal of Structural Chemistry ,2014 ,33(1) :65-70.
[6] Ma Yucui, Wang Chun, Wang Wei, et al. Development of a standard sample of stachyose [J]. Chinese Journal of Experimental Pharmacology, 2017, 23(14): 68-73.
[7] Guo Mei, Wanli Fang, Ping, Feng Xuan, et al. Safety toxicology test of stachyose [J]. Journal of Toxicology, 2012, 26(3): 236-238.
[ 8 ] PATEL S , GOYAL A. Functional oligosaccharides :production , properties and applications [ J ] . World Journal of Microbiology and Biotechnology ,2011 ,27 (5) :1119-1128.
[ 9 ] HAYAKAWA K , MIZUTANI J , WADA K , et al.Effects of soybean oligosaccharides on human faecal flora[ J] . Microbial Ecology in Health and Disease , 1990 ,3(6) :293-303.
[10] Luo Yu, Ma Lina, Cai Fangqin. The probiotic effect of soy oligosaccharides on bifidobacteria and enterobacteria in the intestine [J]. Advances in Modern Biomedicine, 2007, 7(3): 399-400.
[11] Shu Guowei, Ji Liyuan, Chen He, et al. Effect of raffinose, oligosaccharide and galactose on the growth of bifidobacteria [J]. Food Science and Technology, 2011, 36 (6): 6-8, 17.
[12] MILANI C, LUGLI G A , DURANTI S , et al.Bifidobacteria exhibit social behavior through carbohy- drate resource sharing in the gut [ J] . Scientific Re-ports ,2015 ,5 :15782.
[13] HASHIMOTO H ,YAMASHITA A ,FUJITA K ,et al.Enzymatic synthesis of α-linked galactooligosaccha- ride ( α-GOS) and its functions[ J] . Journal of Ap- plied Glycoscience ,2004 ,51(2) :169-176.
[14] Liu Xiuying, Hu Yixiu, Hu Yuming, et al. Experimental study on the enhancement of immunity by oral administration of stachyose and jujube extract [J]. Chinese Journal of Tropical Medicine, 2009(3): 571-572, 576.
[15] TUOHY K M ,ROUZAUD G C,BRÜCK W M ,et al.Modulation of the human gut microflora towards im- proved health using prebiotics-assessment of efficacy [J] .Current Pharmaceutical Design ,2005( 11) :75 - 90.
[16] LI T ,LU X S ,YANG X B.Evaluation of clinical safety and beneficial effects of stachyose-enriched α-ga- lacto-oligosaccharides on gut microbiota and bowel function in humans[J] .Food &Function ,2017 ,8(1) : 262-269.
[17] Duan Sufang. Function and application of stachyose [J]. Beverage Industry, 2016, 19(5): 74-78.
[18] Huang Weizhi, Zhong Xianfeng, Peng Jiawei, et al. A brief introduction to the production, function and application of stachyose [J]. Food Industry Science and Technology, 2018(1): 327-332.
[19] PACIFICI S ,SONG J ,ZHANG C,et al.Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut mi- croflora populations[J] .Nutrients ,2017 ,9(3) :304- 313.
[20] GRMANOVÁ M ,RADA V ,SIROTEK K ,et al.Naturally occurring prebiotic oligosaccharides in poultry feed mixtures[J] .Folia Microbiologica ,2010 ,55(4) : 326-328.
[21] Yi Zhonghua. Effects of raffinose on growth performance, intestinal physiology and immune function of broilers [D]. Doctoral dissertation. Beijing: China Agricultural University, 2006.
[22] LAN Y ,WILLIAMS B A ,VERSTEGEN M W A ,et al.Soy oligosaccharides in vitro fermentation charac- teristics and its effect on caecal microorganisms of young broiler chickens[J] .Animal Feed Science and Technology ,2007 ,133(3/4) :286-297.
[23] Yang Guiqin, Yang Hang, Liu Jizhe, et al. Effects of soy oligosaccharides and their functional components on the production of fecal skatole and the composition of the flora in the cecum contents of broiler chicks under in vitro conditions [J]. Journal of Animal Nutrition, 2017, 29(11): 4058-4068.
[24] JIANG H Q ,GONG L M ,MA Y X ,et al. Effect of stachyose supplementation on growth performance ,nutrient digestibility and caecal fermentation characteris- ticsin broils[ J] . British Poultry Science ,2006 ,47(4) :516-522.
[25] Zhang Pei, Yang Guiqin, Liu Haiying. Research progress on the effects and mechanisms of oligosaccharides from soybeans on the intestinal microecology of poultry [J]. Feed Industry, 2016, 37(5): 60-64.
[26] Yi Zhonghua, Ma Qiugang, Wang Xiaoxia, et al. Effects of raffinose on the development of the digestive organs and the morphology of the intestinal mucosa of broiler chickens [J]. Journal of Jiangxi Agricultural University, 2010, 32(3): 566-570, 576.
[27] Yi Zhonghua, Ji Cheng, Ma Qiugang, et al. Effect of adding stachyose to a low oligosaccharide diet on the growth performance of broilers [J]. China Feed, 2008(16): 12-13.
[28] Wang Li, Sun Ning, Su Jun, et al. Effect of oligosaccharides on egg production performance and egg shell quality of laying hens [J]. China Poultry, 2002, 24(3): 11-13.
[29] Yi Zhonghua, Ji Cheng, Wang Xiaoxia, et al. Effect of stachyose on immune organ index and serum immunoglobulin of broiler chickens [J]. Poultry Farming and Prevention of Poultry Diseases, 2009(2) :4-9.
[30] Gao Yuming, Yang Xiaojun. Nutrition and immunity interact [J]. China Animal Husbandry Journal, 2005, 41(5) :3-5, 9.
[31] KRAUSE D O ,EASTER R A ,MACKIE R I.Fermentation of stachyose and raffinose by hind-gut bacteria of the weanling pig[J] .Letters in Applied Microbiolo- gy ,2010 ,18(6) :349-352.
[32] ZHANG L Y , LI D F , QIAO S Y , et al. Effects of stachyose on performance ,diarrhoea incidence and in- testinal bacteria in weanling pigs[J] .Archives of Ani- mal Nutrition ,2003 ,57(1) :1-10.
[33] Pan Baohai, Sun Dongyan. Effect of α-galactosidase and stachyose on growth performance of weaned piglets [J]. Feed Industry, 2011 (Supplement 1): 69-72.
[34] HAGELY K B,PALMQUIST D ,BILYEU K D.Classification of distinct seed carbohydrate profiles in soy- bean[J] .Journal of Agricultural and Food Chemistry , 2013 ,61(5) :1105-1111.
[35] SMIRICKY M R,GRIESHOP C M ,ALBIN D M ,et al.The influence of soy oligosaccharides on apparent and true ileal amino acid digestibilities and fecal con- sistency in growing pigs[J] . Journal of Animal Sci- ence ,2002 ,80(9) :2433-2441.
[36] PAN B H ,LI D F ,PIAO X S ,et al.Effect of dietary supplementation with α-galactosidase preparation and stachyose on growth performance ,nutrient digestibility and intestinal bacterial populations of piglets[J] .Ar- chiv Für Tierernaehrung ,2002 ,56(5) :327-337.
[37] Cai Yinghua. Effects of several soy anti-nutritional factors on the growth and digestive physiology of flounder (Paralichthys olivaceus) [D]. Master's thesis. Qingdao: Ocean University of China, 2006.
[38] SØRENSEN M , PENN M , EL-MOWAFI A , et al. Effect of stachyose ,raffinose and soya-saponins sup-plementation on nutrient digestibility , digestive en- zymes , gut morphology and growth performance in Atlantic salmon ( Salmo salar L. ) [J] . Aquaculture , 2011 ,314(1/2/3/4) :145-152.
[39] KROGDAHL A.The effect of soybean meal (SBM) and its fours chemical factors on growth performance and in- testinal morphology of jovenile allogynogenetic silver crucian carp ( Carassius auratus gibelio ♀ × Cyprinus carpio ) [C]//Program & Abstracts of the,Interna- tional Symposium on Fish Nutrition &Feeding.2010.
[40] CAI C F ,WANG W J ,YE Y T ,et al.Effect of soy bean meal, raffinose and stachyose on the growth , body composition ,intestinal morphology and intestinal microflora of juvenile allogynogenetic silver crucian carp ( Carassius auratus gibelio ♀ × Cyprinus carpio ) [ J] . Aquaculture Research , 2012 , 43 ( 1) : 128 - 138.
[41] Wang Wenjuan, Ye Yuantu, Cai Chunfang, et al. Effect of soybean meal and its anti-nutritional factors on serum biochemistry and nonspecific immune indicators of heterotrophic silver crucian carp [J]. China Feed, 2010(18): 30-33, 41.
[42] Ji Guiju, Huang He, Li Xin. A functional feed for preventing and controlling red skin disease in grass carp and a preparation method thereof. China, CN: 104187171B[P]. 2017-01-04.
[43] HU H B,ZHANG Y J ,MAI K S ,et al.Effects of dietary stachyose on growth performance , digestive en- zyme activities and intestinal morphology of juvenile Turbot (Scophthalmus maximus L. ) [J] .Journal of Ocean University of China ,2015 ,14(5) :905-912.
[44] YANG P ,HU H B,LIU Y ,et al.Dietary stachyose altered the intestinal microbiota profile and improved the intestinal mucosal barrier function of juvenile turbot, Scophthalmus maximus L. [ J] . Aquaculture , 2018 , 486 :98-106.
[45] Chen Chuanjie, Zhang Yaping, Gu Yinyu, et al. The effect of adding fructooligosaccharides to the diet on the life rate of silkworm pupa and cocoon quality [J]. Chinese Sericulture, 2007, 28 (2): 30-32.
[46] Li Haifang. Determination of intestinal bacteria in captive non-human primates and the effect of inulin on intestinal flora [D]. Master's thesis. Fujian: Fujian Agriculture and Forestry University, 2015.
[47] LI T ,LU X S ,YANG X B.Stachyose-enriched α-galacto-oligosaccharides regulate gut microbiota and re- lieve constipation in mice[J] .Journal of Agricultural and Food Chemistry ,2013 ,61(48) :11825-11831.
[48] Wei Yan, Zeng Xiaoqun, Pan Daodong, et al. Effect of Fructo-oligosaccharide-Lactobacillus plantarum synbiotics on immune function in mice [J]. Chinese Journal of Food Science, 2014, 14(1): 14-19.
[49] LIU G ,BEI J ,LIANG L ,et al.Stachyose improves inflammation through modulating gut microbiota of high-fat diet/streptozotocin-induced type 2 diabetes in rats[J] .Molecular Nutrition & Food Research ,2018 , 62(6) :e1700954.
[50] Dou Jiangli, Tan Chengyu, Bai Xuefang, et al. Effects of raffinose and stachyose on the function of neutrophils in rabbit peripheral blood [J]. Fine and Specialty Chemicals, 2008, 16(3/4): 26-27.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese



