How to Prepare Liposomal Astaxanthin?
As the most powerful antioxidant in nature, astaxanthin has antioxidant, anti-inflammatory and anti-photoaging properties, and plays an important role in delaying aging, preventing and resisting inflammation, and improving photoaging of the skin. However, free astaxanthin is unstable, poorly water-soluble and has low bioavailability. The development of a stable astaxanthin carrier with good water solubility and that is safe and non-toxic is of great significance for functional cosmetics and human anti-aging, and is also one of the important future development directions for astaxanthin carriers.
1 Introduction
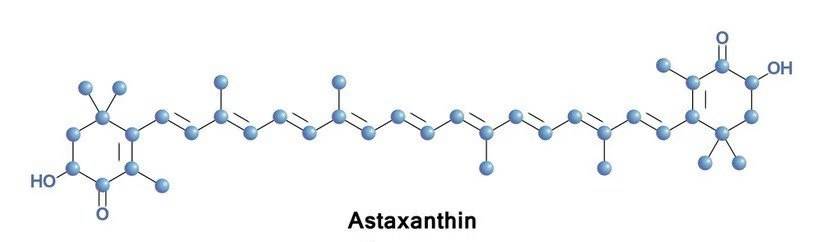
With the improvement of people's living standards and the continuous improvement of skin care concepts, the anti-aging cosmetics market is expanding year by year. Astaxanthin, as a natural and environmentally friendly cosmetic ingredient that can delay skin aging, has become a hot research topic in the international daily chemical industry. Astaxanthin (ASX for short) is a carotenoid (Figure 1) that is found in a variety of aquatic organisms such as shrimp, crab, and algae [1]. Common astaxanthin is a dark pink crystal that is highly fat-soluble, insoluble in water, and easily soluble in organic solvents. Astaxanthin's long-chain conjugated enol structure can effectively quench the activity of reactive oxygen species, so it is by far the strongest natural antioxidant in nature. It has extremely strong antioxidant capacity, with an antioxidant property that is about 500 times that of vitamin E, and is therefore known as the “super vitamin E” [2].
As shown in Figure 2, when the human body is stimulated by sunlight, radiation, make-up, cooking fumes and polluted air, it is prone to producing large amounts of free radicals. When there are excessive free radicals on the surface of the skin, the skin will become slack and saggy, dull and rough, and full of wrinkles. This causes lipid peroxidation, which in turn leads to skin ageing and even disease in severe cases. Although our body has its own antioxidant system to remove free radicals, bad living habits, environmental pollution, ultraviolet rays, work and life pressures, and radiation from electronic products can all accelerate the production of free radicals, which oxidize and damage the skin and body functions, accelerating aging.
Unlike other carotenoids, astaxanthin has a long conjugated double bond and an α-hydroxy ketone, which has a more active electron effect and can provide electrons to free radicals. The hydroxyl groups at both ends of astaxanthin are hydrophilic and can also donate electrons. They can penetrate the blood-brain barrier and enter the middle of the phospholipid bilayer of the cell membrane, where they react with free radicals in the body, preventing further reactions. This removes free radicals from the body, effectively reduces lipid peroxidation, delays aging, and may even be effective in preventing the occurrence of cancer [3-4].
Vitamin C is a water-soluble compound, while carotene and vitamin E are fat-soluble compounds, and their protective effects are relatively single. Compared with the structure of carotene, astaxanthin has more hydroxyl groups, and the hydrophilicity of the hydroxyl groups gives astaxanthin relatively broader cosmetic applications. Therefore, astaxanthin has super high antioxidant capacity, can reduce the damaging effect of light on the skin, and has broad application prospects in the functional cosmetics industry that slows aging. However, due to the presence of many carbon-carbon double bonds in astaxanthin, the carbon-carbon double bonds are very unstable and sensitive to light, oxygen and temperature. Therefore, astaxanthin has physicochemical properties such as easy oxidation and decomposition upon exposure to light, which greatly reduce its bioavailability. This makes it problematic to directly apply free astaxanthin to cosmetics, limiting its application in cosmetics [5]. Therefore, in order to stably apply astaxanthin to cosmetics, researchers must use a highly efficient carrier system for encapsulation.
Among the many carrier systems, astaxanthin encapsulated in nanoemulsions generally has problems such as large particle size, high surfactant content, unstable system, and high cost. Developing a carrier for astaxanthin with high stability, good water solubility, and safety and non-toxicity is of great significance for functional cosmetics and human anti-aging, and is also one of the important future development directions for astaxanthin carriers.
2 Liposome carrier technology
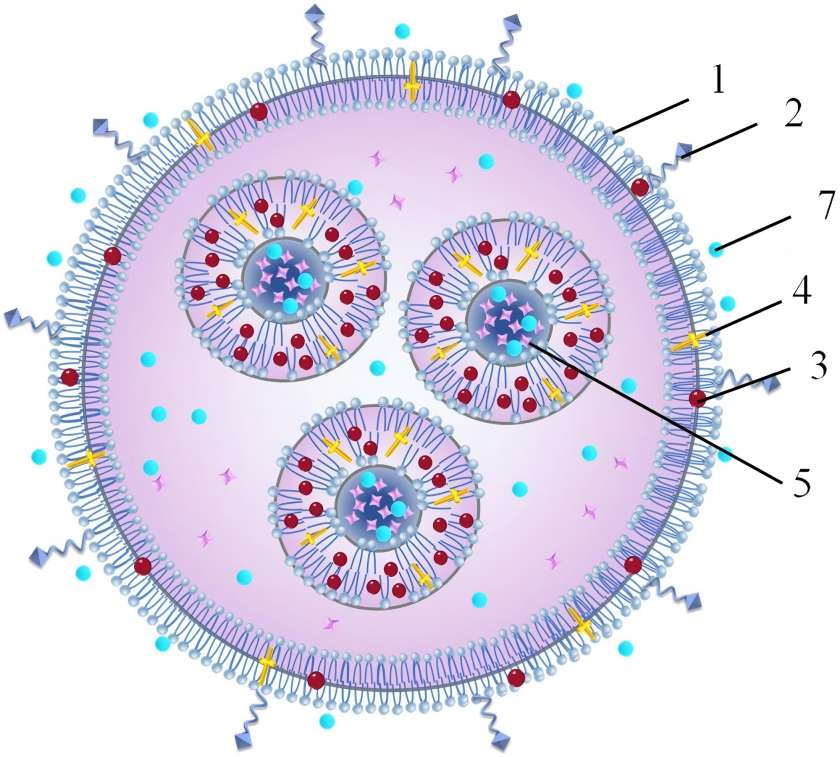
In 1965, Bangham and Standish, two researchers, dispersed phospholipids in water and discovered a kind of nano-spherical vesicles through electron microscopy. The scholars named them liposomes, as shown in Figure 3. The English name is Liposome, which comes from the combination of the Greek words “lipo” (fat) and “soma” (body). Liposomes are composed of a hydrophilic phase and one or more phospholipid bilayers surrounding the hydrophilic phase. Multiple concentric phospholipid bilayers are dispersed in an aqueous phase, and aggregate to form ultra-microspherical, porous particles [6].
Liposomes have the advantages of high bioavailability, targeting, long-acting, good biocompatibility and non-toxicity. The phospholipid bilayer structure of liposomes is similar to that of human skin cells, and they range in diameter from tens of nanometers to hundreds of micrometers. Liposomes can encapsulate many substances within their aqueous phase and phospholipid bilayer membrane. Generally, fat-soluble ingredients are encapsulated between the phospholipid bilayers, water-soluble ingredients can be encapsulated in the innermost aqueous phase, and amphiphilic compounds can be encapsulated at the junction of the aqueous phase and the phospholipids within the membrane. The membrane material of liposomes is generally made from natural raw materials such as lecithin and cholesterol. It has high biocompatibility and safety, high bioavailability, and is well absorbed. It can improve the regeneration capacity of skin cells, maintain the normal function of the skin, and increase the skin's radiance and elasticity.
Liposomal carrier technology was first used in the pharmaceutical industry as a new targeted drug delivery medium. At present, many drugs with liposomes as carriers have been launched on the market one after the other, thanks to their advantages of accurate targeting, long-lasting efficacy and high stability. As research continues to deepen, liposome carrier technology is no longer the “favorite” of the pharmaceutical industry, but has crossed over to become the “ceiling” of the cosmetics industry. In 1986, the world's first liposome cosmetic, Capture, was launched [7]. The active ingredients encapsulated in liposome carriers can be continuously absorbed into the deep layers of the skin. The development of liposome cosmetics has led a “new trend” in skincare technology. Subsequently, liposome cosmetics with anti-aging, whitening, moisturizing and other effects were successively launched, becoming the “stars” of the skincare industry.
As the “cutting edge” of skincare, liposome cosmetics have the following advantages over traditional cosmetics: firstly, liposomes can carry both water-soluble and fat-soluble ingredients, expanding the types of effects they can deliver. Secondly, the liposome coating provides better penetration of the active ingredients into the stratum corneum, allowing more of them to quickly pass through the stratum corneum and improving the bioavailability of the active ingredients. Third, liposome-encapsulated active substances can effectively reduce the oxidation and inactivation of the active substances and improve the stability of the active substances. Fourth, liposome encapsulation can achieve long-term residence in the epidermis and dermis, ensuring long-term sustained release, continuously and slowly exerting its effect, and reducing direct stimulation of the epidermis. Fifth, the membrane material of liposomes is generally made from natural raw materials, which makes them highly safe, with high bioavailability and good absorption.
However, the phospholipid molecular structure of liposomes contains unsaturated acyl groups, which are prone to oxidation and have poor stability, greatly limiting their broad application. The oxidation of phospholipids can generate peroxides and hydroxyl radicals, which accelerate lipid oxidation and are harmful to the human body. The introduction of antioxidants such as vitamin E into the system can protect the phospholipid molecular structure of the liposome, prevent the generation of peroxides and hydroxyl radicals, effectively inhibit lipid peroxidation, and thus greatly improve the stability of the liposome [8].
Liposomal encapsulation can increase the solubility of astaxanthin in an aqueous solution. Astaxanthin can penetrate the membrane of the liposome and interact with the polar groups of the membrane via hydrogen bonds. Therefore, the preparation of astaxanthin liposomes using liposome carrier technology can greatly improve the stability and transdermal rate of astaxanthin, solve the problem of water solubility of astaxanthin, and improve its bioavailability.
3 Preparation method of astaxanthin liposomes
The main preparation processes of liposomes can be divided into two categories: active drug loading and passive drug loading. The active drug loading method involves first forming a blank liposome and then loading the drug, while the passive drug loading method involves simultaneously forming the liposome and loading the drug. Here, we will mainly introduce the passive drug loading method. Passive drug loading technology is relatively simple. It involves dissolving the lipophilic compound together with the phospholipid in an organic solvent, and the water-soluble compound in the aqueous phase, to directly prepare the liposome. The preparation of liposomes requires the selection of suitable lipid materials, taking into account not only their properties and toxicity, but also their purity and the purpose of the liposome application. To date, there have been a large number of reports on the preparation of astaxanthin liposomes. Here, we introduce several typical methods for preparing astaxanthin liposomes.
3.1 Film dispersion method
In the thin-film dispersion method, membrane components such as lecithin and cholesterol are first dissolved in an organic solvent, and after being stirred evenly, the organic solvent is removed by rotary evaporation under reduced pressure to form a uniform thin film of lipids. Then, the aqueous phase is added to hydrate and wash the film to form a liposome suspension. Further sonication, agitation or homogenization is used to obtain a more uniform liposome [9].
When spinning and evaporating for hydration, the temperature should not be too high, as this will cause the unsaturated bonds in the lecithin to denature, making it prone to hydrolytic oxidation, resulting in leakage of the encapsulate and a decrease in the encapsulation rate [10]. Peng et al. [11 used a thin-film dispersion method to prepare astaxanthin liposomes using lecithin and cholesterol as membrane materials and polylactic acid-hydroxyacetic acid copolymer and Tween 80 as surfactants. The astaxanthin liposomes containing surfactants can change shape and penetrate deep into the skin. The liposomes have a particle size of 251±23 nm and contain 89.0±8.6 mg/g astaxanthin. The preparation of astaxanthin liposomes using a combination of film dispersion and ultrasound can effectively reduce the volume of encapsulated astaxanthin.
Pan et al. [12] dissolved astaxanthin, lecithin and cholesterol in chloroform at a ratio of 5:1, removed the chloroform by vacuum distillation, hydrated with 0.05 M phosphate buffer solution, and then sonicated and filtered. The encapsulation rate of the prepared astaxanthin liposomes was 98.68%, the average particle size was about 80 nm, and the potential was 31.80±1.85 mV. Li Qian et al. [13] prepared phytosterol oleate-astaxanthin composite liposomes using a combination of film dispersion and ultrasound. Phytosteryl oleate-encapsulated astaxanthin liposomes have better water solubility and higher stability, with a maximum encapsulation rate of 95.24%. Astaxanthin liposomes modified with chitosan show better results in maintaining the stability of the phospholipid bilayer and inhibiting astaxanthin degradation.
Qiang et al. [14] prepared chitosan-modified astaxanthin liposomes using the thin-film dispersion method. The chitosan-modified astaxanthin liposomes had smaller particle sizes and uniform particles. The encapsulated astaxanthin was not easily oxidized or degraded, and had strong stability, so it could be stored for a long time. Sangsuriyawong et al. [15] prepared astaxanthin liposomes with different lecithin concentrations using the thin film dispersion method. The results showed that, within a certain concentration range, the higher the lecithin concentration, the smaller the liposome particle size and the higher the encapsulation rate. The minimum particle size was 140 nm, and the maximum encapsulation rate could be as high as 97%. Liposomes prepared by the film dispersion method can better encapsulate fat-soluble compounds, but the prepared liposomes have a large particle size and cannot encapsulate a large amount of water-soluble compounds.
3.2 Ethanol injection method
As a skin penetration enhancer, ethanol not only lowers the melting point of lipid molecules in the stratum corneum, effectively promoting the flow and penetration of cell membrane lipids and increasing their transdermal rate, resulting in good skin penetration, but it also effectively reduces the particle size of astaxanthin, changing the net charge of the liposome, thereby greatly improving the stability of astaxanthin. First, the phospholipids and cholesterol are completely dissolved in ethanol, and then the solution is injected into the aqueous phase and placed on a stirrer to hydrate.
Evaporation under reduced pressure removes the organic solvent to obtain nano-liposomes [16]. Yang Anping et al. [17] used the ethanol injection method to prepare astaxanthin liposomes, which is a simple process with good stability. It does not use organic reagents with high toxicity and is suitable for the industrial preparation of astaxanthin liposomes. Although the preparation process is simple, the encapsulation rate of the astaxanthin liposomes obtained is only 35.28%, and the particle size is 143 nm. Ethanol injection combined with ultrasound produces liposomes with a smaller and more uniform particle size, which is conducive to passing through a microporous filter membrane without precipitation and the system is more stable. However, the encapsulation rate of liposomes prepared by the ethanol injection method is low, and there is the problem of reagent residues that are difficult to completely remove.
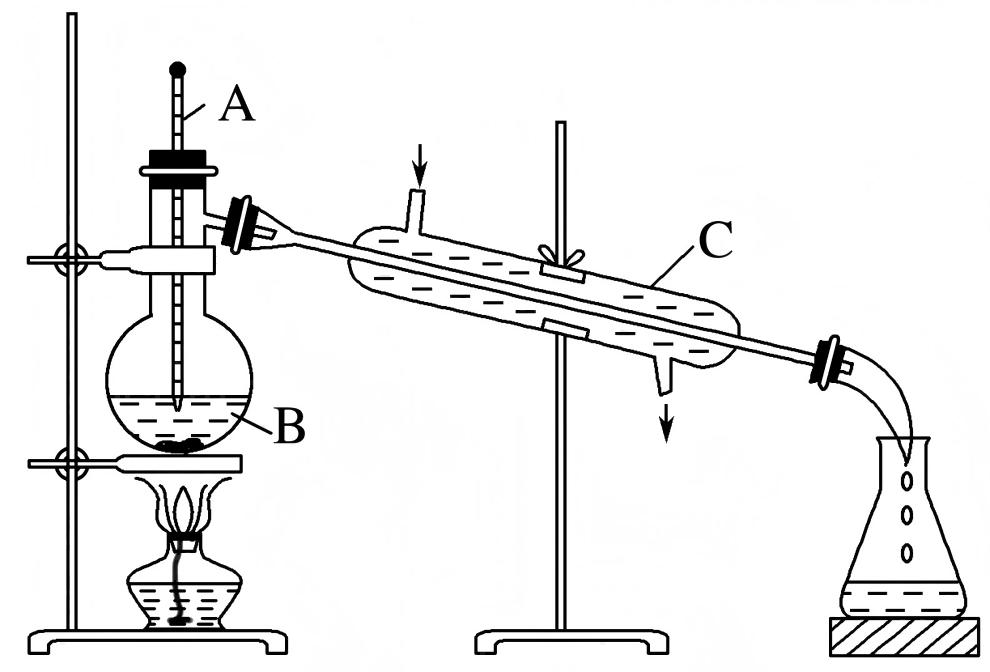
3.3 Reverse evaporation method
The reverse evaporation method is time-saving and has a high encapsulation rate, but it is only suitable for encapsulating water-soluble compounds. Similar to the thin-film dispersion method, the reverse evaporation method first dissolves membrane materials such as lecithin and cholesterol in an organic solvent, then adds a water phase solution, and then sonifies to form a uniform emulsion. The mixture is allowed to stand without stratification, and finally the organic solvent is removed by reduced pressure rotary evaporation to obtain the liposome. Pahila et al. [18] first dissolved a certain amount of astaxanthin and lecithin in chloroform separately, mixed them with an appropriate amount of phosphate buffer solution, and then removed the organic solvent by rotary evaporation under reduced pressure in a water bath at 40 °C. The resulting liposome particle size was 10–25 μm.
3.4 Other methods
Liu Yuan et al. [19-20] used emulsification and evaporation-crystallization at low temperature to prepare astaxanthin liposomes, which were used to reduce skin photodamage and collagen damage. The encapsulation rate of astaxanthin liposomes was about 80%. The preparation method is complex and difficult to industrialize. Jing Yongkang et al. [21] used homogenization emulsification combined with ultrasound to prepare astaxanthin nanoliposomes. The astaxanthin liposomes prepared by this method had a particle size of 128 nm and an encapsulation rate of 55.18%. Lipids with small particle sizes and uniform dispersion can better penetrate the skin barrier and penetrate deep into the skin to achieve a better permeation effect.
4 Conclusion
Astaxanthin, as a super natural antioxidant, has become the focus of attention in the skin care industry. More and more cosmetics use astaxanthin as the main natural active ingredient. The future star of cosmetic science, liposome carrier technology, has played an important role in the stable application of astaxanthin in cosmetics, effectively solving problems such as astaxanthin's low stability and low bioavailability. Astaxanthin liposomes prepared using liposome encapsulation technology have the characteristics of good biocompatibility, stability, low toxicity and high encapsulation rate. In the cosmetics industry, astaxanthin liposomes are a rising star with great potential in the skin care industry in the future.
References
[1] Zhang Weiguo, Luo Hongfu. Food Industry, 2022, 43(12): 88.
[2] Lorenz R, Cysewski G. Trends in Biotechnology, 2000, 18(4): 160.
[3] Zhang Z Y, Hu W L, Qu X F, et al. Journal of Food Safety and Quality, 2020, 11(5): 1431.
[4] Zhu X B, Wu J, Yu L D, et al. Science and Technology Information, 2020, 18(12): 06.
[5] Geng Zhaoyan, Sun Han, Guan Bin, et al. Chinese Journal of Food Science, 2017, 17(7): 86.
[6] Han Xu, Ding Guanyu, Dong Qing, et al. Applied Chemistry, 2018, 35(7): 735.
[7] Dai Xudong, Li Yun, Li Shuangshuang. International Journal of Pharmaceutical Research, 2020, 47(11): 914.
[8] Song Yuan, Huimin Sun, Lixia Ding. Chinese Journal of Pharmaceutical Affairs, 2011, 25(4): 384.
[9] Qiu Y, Gao Y, Hu K. Journal of Controlled Release: Official Journal of the Controlled Release, 2008, 129(2): 144.
[10] Song Y L. Preparation and in vitro transdermal behavior of hydrogel ointment of vinpocetine hydrochloride liposome and liposome [D]. Xi'an, Shaanxi: Northwest University, 2014.
[11] Peng C H, Chang C H, Peng R Y. European Journal of Pharmaceutics and Biopharmaceutics, 2010, 75(2): 154.
[12] Pan L, Zhang S, Gu K. Journal of Food, 2018, 16(1): 607.
[13] Li Q, Liu Y, Pan L, et al. China Oils and Fats, 2023.
[14] Qiang M, Pang X, Ma D, et al. Molecules, 2020, 25(3): 610.
[15] Sangsuriyawong A, Limpawattana M, Siriwan D. Food Science and Biotechnology, 2019, 28(2): 529.
[16] Godin B, Touitou E, Rubinstein E. the Journal of Antimicrobial Chemotherapy, 2005, 55(6): 989.
[17] Yang Anping, Gu Siying, Liang Yijun, et al. Pharmaceutical Guide, 2020, 39(9): 1276.
[18] Pahila J, Ishikawa Y, Ohshima T. Journal of Agricultural and Food Chemistry, 2019, 67(12): 3491.
[19] Li Fumin, Liu Yuan, Liao Jinfen, et al. Journal of Sichuan University (Medical Sciences), 2018, 49(5): 712.
[20] Liu Yuan. A preliminary study on the protective effect of astaxanthin liposomes on UVB-induced skin photodamage in mice [D]. Luzhou: Southwest Medical University, 2016.
[21] Jing Yongkang, Zhang Wei, Gao Hong, et al. China Oils and Fats, 2022.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese