4 Haematococcus Pluvialis Astaxanthin Testing Methods
Astaxanthin, chemically named 3,3'-dihydroxy-beta,beta'-carotene-4,4'-dione, belongs to the keto carotenoids. Studies have shown that astaxanthin is the strongest natural antioxidant [1-2]. Astaxanthin has antioxidant, anti-radiation, anti-aging, anti-tumor, and cardiovascular disease prevention and treatment functions [2-3], and therefore has extremely high economic value. It has been used in health products, medicines, feed additives, cosmetics, functional foods, food additives, and other areas [4].
The main raw materials for the production of astaxanthin come from shrimp and crab shells [3, 5], Haematococcus pluvialis [4] and Rhodotorula glutinis [6]. Among them, Haematococcus pluvialis can accumulate astaxanthin up to 5% of the dry weight under stress conditions. Since the structure of astaxanthin contains two chiral C atoms, the structure in Haematococcus pluvialis is 3S, 3S', and the synthetic form is a chiral mixture, mainly 3R, 3R'. Haematococcus pluvialis is considered to be the best source of natural astaxanthin production.
Haematococcus pluvialis forms a gelatinous shell-like outer wall after stress. When the pigment in the cells is extracted, it is difficult for conventional solvents to enter the interior of the cells to extract the pigment. It is usually necessary to combine a cell wall-breaking method. Secondly, the astaxanthin in Haematococcus pluvialis cells mainly exists in the form of astaxanthin esters, that is, astaxanthin monoester molecules and astaxanthin diester molecules with different acyl chains. HPLC analysis is difficult to achieve baseline separation of all molecules, and the molecular weight is different when calculating the content.
In addition, astaxanthin is unstable and prone to degradation under conditions such as light and heat, so there are certain problems with the accurate determination of astaxanthin. This paper reviews the current research status in terms of the extraction, hydrolysis and detection of astaxanthin from Haematococcus pluvialis, with a view to providing guidance on the selection of appropriate methods for the rapid determination of astaxanthin content in Haematococcus pluvialis for different purposes.
1 Extraction of astaxanthin from Haematococcus pluvialis
The extraction of astaxanthin is the basis for accurate determination of its content and is also one of the uncertain aspects of astaxanthin measurement. Under stress conditions, Haematococcus pluvialis cells accumulate astaxanthin, but cell growth and division stop, forming immobile spores, i.e., gelatinous cells. Mature gelatinous cells have a three-layer thick, hard cell wall, with the outermost layer being algaenan, a material that is highly resistant to acetic acid hydrolysis. The second and third layers are composed of evenly and non-uniformly distributed mannose and cellulose, respectively [7].
For Haematococcus pluvialis with a gelatinous cell wall, conventional dissolution and extraction methods cannot access the interior of the cell to extract the pigment.
At present, in the industry, supercritical CO2 extraction technology [7], high pressure/ultra-high pressure homogenization extraction technology [8], and negative pressure cavitation method [9] are used to extract astaxanthin from Haematococcus pluvialis. The above methods can effectively extract astaxanthin, but they require a large amount of algal powder and are complicated to operate, so they are suitable for large-scale industrial production. The extraction methods used to detect astaxanthin in Haematococcus pluvialis include solvent extraction, mechanical grinding + solvent extraction, dimethyl sulfoxide (DMSO) extraction, and cellulase wall breaking + solvent extraction.
1.1 Solvent extraction method
The solvent extraction method is easy to operate, low-cost, and has low equipment requirements. It only needs to optimize the extraction solvent, liquid-to-solvent ratio, extraction temperature, and extraction time. Commonly used solvents include acetone [10], ethyl acetate, dichloromethane, ethanol, etc. However, due to the outer wall of the gelatinous shell of Haematococcus pluvialis, conventional solvents cannot enter the cells, and the extraction rate of astaxanthin is low. Mendes-Pinto et al. reported that acetone was used as the extraction solvent for astaxanthin from Haematococcus pluvialis, and the extraction rate was only 4 mg ·g-1 (extracted astaxanthin mass per gram of algal powder), while mechanical crushing + acetone extraction was 19 mg ·g-1, indicating that the solvent could not enter the cells to extract astaxanthin [10].
Ruen-ngam compared solvent extraction methods assisted by ultrasound (Ultrasound Assisted Extraction), microwave (Microwave Assisted Extraction), and Soxhlet (Soxhlet Extraction). The extraction rate of astaxanthin reached 74% using microwave assisted extraction at 75 °C for 5 min [11]. In addition, to enhance the extraction efficiency of the solvent, chemical cell wall disruption involves treating Haematococcus pluvialis cells with acids or alkalis. Sarada et al. reported that treating the cells with 2 mol·L-1 hydrochloric acid solution at 70 °C for 2 min, followed by extraction with a solvent, can achieve an astaxanthin extraction rate of 86%–94% [12]. However, it is worth noting that placing astaxanthin in a high concentration of acid or alkali solution can easily cause degradation of astaxanthin. The above results show that due to the special cell wall composition of Haematococcus pluvialis, direct solvent extraction cannot enter the cells, while auxiliary ultrasound, microwave and acid-base treatments are all prone to cause degradation of astaxanthin and are not suitable for astaxanthin extraction.

1.2 Mechanical cell wall disruption + solvent extraction
Mechanical cell wall disruption is the most commonly used method in the laboratory, which uses external forces to break the cell walls of Haematococcus pluvialis. In the national standard for the detection of astaxanthin in Haematococcus pluvialis GB/T 31520-2015 [13], Haematococcus pluvialis is thoroughly ground using a glass homogenizer, and its pigments are extracted using dichloromethane-methanol as the solvent. The mechanical crushing + solvent extraction method is more commonly used in the detection of astaxanthin [10, 14-16]. Mechanical crushing can damage the cell walls, and the pigment can be completely extracted. The method is simple to operate, but this method requires each sample to be ground through a cell homogenizer, which is time-consuming and laborious.
1.3 Cellulase wall disruption + solvent extraction
Since the cell wall of Haematococcus pluvialis is mainly composed of substances such as cellulose, pectin and lipopolysaccharides, cellulase, pectinase and polysaccharidase are used to break the cell wall of Haematococcus pluvialis [8]. Zhou Jinke et al. explored a new enzymatic method for extracting astaxanthin from Haematococcus pluvialis [17]. Cellulase: enzymatic hydrolysis of algal powder, ethanol extraction. The optimal process conditions for enzymatic extraction are: initial pH of the enzymatic solution 4.5, enzymatic hydrolysis temperature 45 °C, enzyme dosage 1.5%, and enzymatic hydrolysis time 15 h. Under these conditions, the astaxanthin extraction rate was 94.6%, which is 61.5% higher than the traditional direct ethanol extraction method. It has the advantages of low operating temperature, less pollution, low cost, and high extraction rate. It is easy to achieve green industrial production, but this method is time-consuming, and high temperatures can also cause degradation of astaxanthin.
1.4 DMSO extraction method
DMSO has good miscibility with various solvents and good permeability to cells. It is often used as a permeation enhancer for drugs or pesticides and as a protective agent in the cryopreservation of cells. Seely first reported that DMSO can be used to extract microalgal chlorophyll and carotenoids [18]. Boussiba et al. used DMSO to extract the pigments from Haematococcus pluvialis. Extraction was carried out in a 70 °C water bath for 10 min, and the algal residue can be made colorless by repeating the extraction 2 to 3 times in a 70 °C water bath [19], which shows that DMSO has good permeability and can penetrate into the cells of Haematococcus pluvialis to completely extract astaxanthin. DMSO extraction does not require treatment of the cell walls of Haematococcus pluvialis, greatly simplifying the astaxanthin extraction process, and has been used in astaxanthin detection [20-21]. In addition, the world's leading microalgae companies, such as Cyanotech, Fuji in Japan, and China's Green A Bioengineering Company [8], have all applied the DMSO method for extracting astaxanthin from Haematococcus pluvialis to astaxanthin detection.
Using DMSO as a swelling agent can increase the permeability of the cell wall, and can be used as an extractant for astaxanthin from Haematococcus pluvialis, simplifying the process of breaking the cell wall required in the detection of Haematococcus pluvialis.
2 Hydrolysis of astaxanthin esters
The astaxanthin accumulated in Haematococcus pluvialis is all-trans 3S, 3S 'configuration, with one hydroxyl group in each endocyclic structure. It is usually esterified with C16, C18 or C20 fatty acids to form astaxanthin esters to stabilize its structure [22]. Most of them are astaxanthin monoesters, accounting for about 75%, astaxanthin diesters accounting for about 20%, and free astaxanthin only accounting for 5% [12, 23]. There are as many as 30 different types of astaxanthin monoesters and diesters in Haematococcus pluvialis [24]. The complexity of astaxanthin esters makes purification and direct and accurate quantification problematic. Therefore, the extracted astaxanthin esters need to be hydrolyzed into free astaxanthin to achieve purification of a single substance and accurate quantification by HPLC. There are generally two methods for hydrolyzing astaxanthin esters: saponification and enzymatic hydrolysis.
2.1 Saponification
Saponification is generally carried out in a NaOH or KOH methanol solution to hydrolyze astaxanthin esters into free astaxanthin. Yuan et al. showed that a high alkali concentration or reaction temperature during saponification is conducive to the hydrolysis of astaxanthin esters, but also accelerates the degradation of astaxanthin [14]. Chen Xingcai et al. also showed that the concentration of free astaxanthin decreased linearly with increasing alkali concentration [25]. Yuan et al. studied the hydrolysis kinetics of astaxanthin under ester saponification and the degradation of astaxanthin under different alkali concentrations. The results showed that in a 22 °C reaction system with a NaOH concentration was 0.0175~0.020 mol · L-1, the astaxanthin ester was completely hydrolyzed and no astaxanthin degradation occurred. However, a higher concentration of NaOH-methanol solution or a higher reaction temperature caused significant astaxanthin degradation [26]. The conditions of the saponification method for astaxanthin esters are harsh. The concentration of the alkali solution, the saponification temperature and time during the saponification process all affect the efficiency of the saponification and the stability of astaxanthin, which is another aspect that affects the accurate determination of astaxanthin.
2.2 Enzyme hydrolysis method
Zhao reported that a water-soluble alkaline esterase from Penicilium cyclopium can convert astaxanthin esters into astaxanthin. The reaction conditions were incubation at 28 °C with stirring for 7 h, and the recovery of astaxanthin reached 63.2% [27]. However, this enzyme has low hydrolysis efficiency and has not been widely used in the determination of astaxanthin content. Jacobs first reported that the lipid-soluble cholesterol esterase can rapidly hydrolyze carotenoid esters [28]. Although there are currently few reports in the literature on this enzymatic method, it is used by domestic and foreign companies producing astaxanthin as a gentle method of hydrolysis. The cholesterol esterase used not only completely hydrolyzes the astaxanthin ester, but also does not easily cause oxidation of astaxanthin, which can more accurately determine the astaxanthin content.
The use of cholesterol esterase on Haematococcus pluvialis extract containing astaxanthin for a short period of time greatly improves the detection efficiency of astaxanthin in Haematococcus pluvialis.
3 Quantitative detection of astaxanthin in Haematococcus pluvialis
The main methods for detecting astaxanthin are spectrophotometry, hydrolysis-HPLC, and HPLC-MS.
3.1 Spectrophotometry
Boussiba reported that chlorophyll in Haematococcus pluvialis was destroyed by heating with 5% KOH-30% methanol solution for about 10 minutes, and then the astaxanthin was extracted with DMSO. The absorbance was measured at a wavelength of 475 nm to calculate the astaxanthin concentration. This method can quickly estimate the astaxanthin content and is widely used in cultivation and production [12, 29]. However, some reports have shown that the treatment of the alkali solution to destroy the chlorophyll in this method causes 25% to 40% degradation of astaxanthin [16].
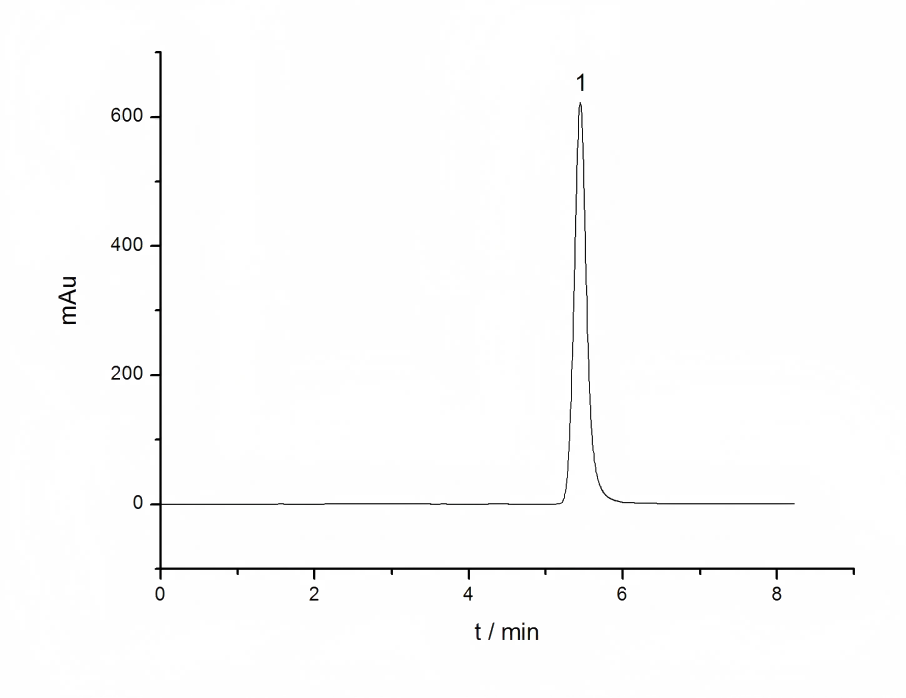
Therefore, Li et al. improved Boussiba's method by extracting directly from DMSO without alkali treatment, and detecting at a visible light wavelength of 530 nm to avoid interference from other carotenoids and chlorophyll. This method was used for the rapid detection of astaxanthin content in Haematococcus pluvialis [16]. Geng Jinfeng's research shows that the content of carotenoids and astaxanthin in Haematococcus pluvialis during the cultivation process is in a stable linear relationship. Using a method that directly and easily measures carotenoids and indirectly obtains the astaxanthin content, the carotenoid content can be quickly determined, and then based on the correlation between the obtained carotenoids and astaxanthin, the astaxanthin content in Haematococcus pluvialis can be quickly calculated. The data from this laboratory also show that there is a good linear relationship between the carotenoid content measured by spectrophotometry and the astaxanthin content measured by enzymatic hydrolysis-HPLC (Figure 1). Therefore, the DMSO extraction method can be directly used to determine the astaxanthin content using the spectrophotometric method at 475 nm and the relationship in Figure 1.
3.2 Hydrolysis-HPLC method
After the Haematococcus pluvialis pigment is pretreated by saponification or enzymatic hydrolysis, HPLC can be used to accurately determine the free astaxanthin. At present, the national standard for astaxanthin determination GB/T 31520-2015 uses the method of HPLC after saponification for determination [13]. The separation column is a reverse-phase C30 column, and the detection of all-trans-free astaxanthin, 9-cis- astaxanthin, and 13-cis-astaxanthin can be detected in 20 min. Yuan used a C18 column to analyze astaxanthin esterified with saponin. Methanol/dichloromethane/acetonitrile/water was used as the mobile phase for gradient elution, and a single sample was detected in 16 min [30]. Cyanotech's method for determining the content of Haematococcus pluvialis is to extract the pigment and then hydrolyze it for HPLC determination. This pretreatment by hydrolysis converts astaxanthin esters into astaxanthin, which converts the mixed components containing astaxanthin compounds into the detection of a single astaxanthin component, making HPLC analysis simpler, allowing for accurate quantification, and with high repeatability. More astaxanthin reversed-phase chromatography separation conditions are summarized in Table 1.
3.3 HPLC-MS method
Due to the diversity and complexity of extracting astaxanthin and its ester derivatives, conventional spectrophotometry and HPLC methods cannot identify the differences between different astaxanthin ester molecules. Mass spectrometry can better distinguish between different molecules by using the differences in mass and fragment information between astaxanthin ester molecules, and then carry out qualitative and quantitative analysis. The method of directly measuring the extracted pigment sample by HPLC/MS without treatment avoids the degradation of astaxanthin during the saponification process, and can simultaneously measure astaxanthin, astaxanthin esters, other carotenoids and chlorophyll.
It has certain applications in the determination of astaxanthin composition and the study of astaxanthin metabolic mechanisms. Holtin et al. used LC-(APCI)MS to qualitatively analyze free astaxanthin isomers, astaxanthin monoesters, astaxanthin diesters, and lutein in Haematococcus pluvialis [24]. Weesepoel et al. used ESI-IT and MADIL-TOF/TOF to perform a more detailed analysis of astaxanthin esters in Haematococcus pluvialis, including the determination of the astaxanthin ester acyl chain and the distinction between cis and trans isomers [32]. More chromatographic separations of astaxanthin and its ester derivatives are summarized in Table 1. It is worth noting that the low polarity of carotenoids and astaxanthin esters makes it difficult to ionize them using the commonly used soft ionization ESI ion source. The APCI ion source is more commonly used for the analysis of astaxanthin esters.
This paper reviews and evaluates the current methods for the extraction, hydrolysis and detection of astaxanthin in Haematococcus pluvialis. The astaxanthin content is directly measured by spectrophotometry at a wavelength of 475 nm after extraction with DMSO. The astaxanthin content is quickly calculated based on the linear relationship between the carotenoid content measured by spectrophotometry and the astaxanthin content measured by HPLC. This method is more conducive to quickly obtaining important parameters during research. After DMSO extraction, cholesterol esterase is used for hydrolysis, and HPLC is used to determine the content of free astaxanthin, which can be used as a method for accurately determining the astaxanthin content. For different detection purposes, different methods can be used for analysis, and data comparison between different laboratories or methods should be corrected to the results after hydrolysis treatment of the extracted sample and HPLC analysis.
Reference:
[1]Kobayashi M,Sakamoto Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis[J].Biotechnology Letters,1999,21(4):265-269.
[2]Naguib Y M A. Antioxidant activities of astaxanthin and related carotenoids[J]. Journal of Agricultural and Food Chemistry,2000,48(4):1150-1154.
[3]Higuera-Ciapara I,Félix-Valenzuela L,Goycoolea F M. Astaxanthin:A review of its chemistry and applications[J]. Critical Reviews in Food Science and Nutrition,2006,46(2):185-196.
[4]Cuellar-Bermudez S P,Aguilar-Hernandez I,Cardenas-Chavez D L,et al.Extraction and purification of high-value metabolites
from microalgae:essential lipids,astaxanthin and phycobiliproteins[J].Microbial Biotechnology,2015,8(2):190-209.
[5]Lin W C,Chien J T,Chen B H.Determination of Carotenoids in spear shrimp shells(Parapenaeopsis hardwickii)by liquid chromatography[J].Journal of Agricultural and Food Chemistry,2005,53(13):5144-5149.
[6] Ni Hui, Hong Qinglin, Xiao Anfeng, et al. Production performance of a high-yield strain of astaxanthin from Pichia pastoris [J]. Chinese Journal of Bioengineering, 2011, 27(7): 1065-1075.
[7]Kim D-Y,Vijayan D,Praveenkumar R,et al. Cell-wall disruption and lipid/astaxanthin extraction from microalgae:Chlorella and Haematococcus[J].Bioresource Technology,2016,199:300-310.
[8] Yu Shaolei, Du Weichun, Yao Qiao, et al. Process research on the enzymatic hydrolysis combined with physical method for the wall-breaking treatment of Haematococcus pluvialis [J]. Food Engineering, 2016 (4): 38-40.
[9] Zu Yuangang, Liu Lina, Xue Yanhua, et al. Extraction of astaxanthin by negative pressure cavitation method [J]. Journal of Northeast Forestry University, 2007, 35(2): 59-60.
[10]Mendes-Pinto M M,Raposo M F J,Bowen Jet al. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis:effects on astaxanthin recovery and implications for bio-availability[J]. Journal of Applied Phycology,2001,13(1):19-24.
[11]Ruen-ngam D ,Shotipruk A ,Pavasant P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis[J].Separation Science and Technology,2011,46(1):64-70.
[12]Sarada R,Vidhyavathi R,Usha D,et al.An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis[J].Journal of Agricultural and Food Chemistry,2006,54(20):7585-7588.
[13] GB/T 31520-2015. Determination of astaxanthin in Haematococcus pluvialis - Liquid chromatography method [P]. China, 2015.
[14] Yuan J P,Chen F. Chromatographic separation and purification of trans-astaxanthin from the extracts of Haematococcus pluvialis[J].Journal of Agricultural and Food Chemistry,1998,46(8):3371-3375.
[15] Sun Weihong, Xiao Ronghui, Leng Kailiang, et al. C30-reversed-phase high performance liquid chromatography method for the determination of astaxanthin in Haematococcus pluvialis [J]. Journal of Analytical Testing, 2010, 29 (8): 841-845.
[16] [16]Li Y,Miao F,Geng Yet al. Accurate quantification of astaxanthin from Haematococcus crude extract spectrophoto-metrically[J].Chinese Journal of Oceanology and Limnology,2012,30(4):627-637.
[17] Zhou Jinke, Li Jinhua, Ge Fahuan, et al. Research on a new enzymatic method for extracting astaxanthin from Haematococcus pluvialis [J]. Chinese Materia Medica, 2008, 31(9): 1423-1425.
[18] Seely G R,Vidaver W E,Duncan M J.Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide[J].Marine Biology,1972,12(2):184-188.
[19]Boussiba S,Vonshak A. Astaxanthin accumulation in the green alga Haematococcus pluvialis[J]. Plant and Cell Physiology,1991,32(7):1077-1082.
[20]Orosa M,Franqueira D,Cid A,et al.Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis [J].Bioresource Technology,2005,96(3):373-378.
[21]Boussiba S,Bing W,Yuan J P,et al. Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses[J].Biotechnology Letters,1999,21(7):601-604.
[22]Breithaupt D E.Identification and quantification of astaxanthin esters in shrimp(Pandalus borealis)and in a microalga (Haematococcus pluvialis)by liquid chromatography mass spectrometry using negative ion atmospheric pressure chemical ionization[J].Journal of Agricultural and Food Chemistry,2004,52(12):3870-3875.
[23]Miao F P,Lu D Y,Li Y G,et al.Characterization of astaxanthinesters in Haematococcus pluvialis by liquid chromatography-
atmospheric pressure chemical ionization mass spectrometry[J].Analytical Biochemistry,2006,352(2):176-181.
[24]Holtin K,Kuehnle M,Rehbein J,et al. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy [J].Analytical and Bioanalytical Chemistry,2009,395(6):1613-1622.
[25] Chen Xingcai, Huang Weiguang, Ouyang Qin. Saponification of astaxanthin esters and purification and separation of free astaxanthin from Haematococcus pluvialis [J]. Journal of Fuzhou University (Natural Science Edition), 2005, 33 (2): 264-268.
[26] Yuan J P,Chen F.Hydrolysis kineticsofastaxanthinesters and stability of astaxanthin of Haematococcus pluvialis during saponification[J].Journal of Agricultural and Food Chemistry,1999,47(1):31-35.
[27]Zhao Y,Guan F,Wang G,et al.Astaxanthin preparation by lipase-catalyzed hydrolysis of its esters from Haematococcus pluvialis algal extracts[J].Journal of Food Science,2011,76(4):C643-C650.
[28]Jacobs P B,Leboeuf R D McCommas S A,et al.The cleavage of carotenoid esters by cholesterol esterase[J].Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology,1982,72(1):157-160.
[29] Geng Jinfeng, Zhang Huimin, Yang Jianqiang, et al. A rapid method for the determination of astaxanthin content in Haematococcus pluvialis [J]. Food Research and Development, 2016, 37(12): 125-128.
[30] Yuan J P,Chen F.Identification of astaxanthin isomers in Haematococcus lacustris by HPLC-photodiode array detection [J].Biotechnology Techniques,1997,11(7):455-459.
[31]Peng J,Xiang W,Tang Q,et al.Comparative analysis of astaxanthin and its esters in the mutant E1 of Haematococcus pluvialis and other green algae by HPLC with a C30 column[J].Science in China Series C-Life Sciences,2008,51(12):1108-1115.
[32]Weesepoel Y,Vincken J-P,Pop R M,et al.Sodiation as a tool for enhancing the diagnostic value of MALDI-TOF/TOF-MS spectra of complex astaxanthin ester mixtures from Haematococcus pluvialis[J].Journal of Mass Spectrometry,2013, 48(7):862-874.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese