How About the Stability of Natural Pigment Phycocyanin Powder?
Phycocyanin is a photosynthetic pigment protein complex obtained by isolating and purifying it from microalgae cells [1]. It is a secondary pigment of chlorophyll [2]. Phycocyanin powder has been widely used in many fields. It can be used as a natural coloring agent in food [3], as an additive in the cosmetics industry, and as a nutritional supplement in the health care industry [4]. Due to its high sensitivity to fluorescent reagents, it can also be used in immunoassay experiments as a marker for antibodies and receptors [5]. In addition, phycobiliproteins have biological activities such as anti-tumor, anti-inflammatory, antioxidant, and immune regulation [6], and can also be used in the pharmaceutical industry as anti-inflammatory agents and antioxidants.

However, due to the different aggregation states of the protein in phycocyanin, it is prone to degradation. This property may be related to many factors, such as light, temperature, pH and protein concentration [7–8]. The sensitivity of phycobiliproteins to heat and light is manifested in the loss of color and the appearance of precipitation. Moreover, with the increase of light intensity and the increase of temperature, the activity of phycobiliproteins also decreases or even inactivates, which greatly restricts the application of phycobiliproteins [9]. Some studies have shown that the degradation rate of phycobiliproteins can be slowed down by adding stabilizers, structural modification, immobilization and microencapsulation, etc. [10], thereby improving the stability of phycobiliproteins. This paper reviews the structural characteristics of phycobiliproteins, the factors affecting stability and the methods to improve stability, with a view to providing theoretical reference for improving the stability of phycobiliproteins and expanding their scope of application.
1 Structural characteristics of Phycocyanin
Phycobilisomes (PBS) are light-harvesting complexes with an absorption range of 500–660 nm [11]. Each phycobilisome is composed of colored proteins called phycobiliproteins (PBP). These molecules are arranged in an antenna-like manner; the absorbed energy is transported to the reaction center of photosystem II with an efficiency of over 95%. Therefore, cyanobacteria can use red, yellow, green and, to a lesser extent, blue light [12]. It contains the colored proteins phycocyanin (PC, λmax=610–620 nm), phcoerythrin (PE, PE, λmax=540~570 nm) and allophycocyanin (APC, λmax=650~655 nm) (Fig. 1 [13]). As shown in Figure 1, different phycobiliproteins are assembled in a specific order, so that energy can be efficiently transferred to the reaction center in one direction. The order is phycocyanin, then phycobilin, and finally allophycocyanin, which ultimately transfers to the reaction center photosystem I (photosystem I, PS I) and II (photosystem II, PS II) [14].
Phycocyanins are composed of a chain-opening tetrapyrrole chromophore, phycobilin, covalently attached to the protein molecule. The type and number of phycobilin chromophores causes phycobiliproteins to appear in different colors [15]. Changes in the structure of the chromophore cause phycobiliproteins to lose their color and antioxidant activity. Phycobiliproteins are located at the ends of peripheral rods and are adjacent to a core cylinder composed of allophycocyanin. Its basic structure is composed of monomers with two homologous subunits, α and β [11]. These monomers aggregate into (αβ)3 trimers with C3 facing each other to form a symmetry, and every two (αβ)3 form a (αβ)6 hexamer with triple symmetry [16‒17]. The hexamer further assembles into a rod or core cylinder, and the non-pigment linking protein is isolated in the large pore at the center of the trimer, hexamer, rod or core cylinder.
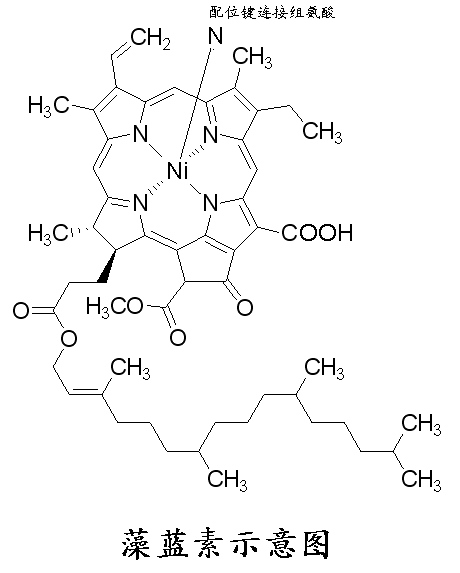
The protein structure is highly correlated with the stability of phycocyanin and promotes the protection of chromophores. Therefore, any factor that affects the stability and structure of the protein may prevent or accelerate the degradation of phycocyanin. Compared to trimers and monomers, the hexameric structure is more stable and provides higher protection for phycobiliproteins [18]. The maintenance of the linear conformation of phycobiliproteins is also important for preventing their degradation [19]; when the protein is denatured, the reduction in the hydrogen bond network causes the phycobiliprotein molecule to rearrange from the linear conformation to the cyclic conformation, resulting in color fading [20].
2 Stability studies of Phycocyanin
Phycocyanins are water-soluble protein-pigment complexes. The rate of phycobiliprotein degradation depends on the aggregation state of the protein, which is affected by light, pH, temperature and other factors.
2.1 Effect of pH on the stability of phycobiliproteins
pH is the main factor affecting the aggregation and decomposition of phycobiliproteins in solution, such as monomers, trimers, hexamers and other oligomers. When pH changes, the charge and dissociation of phycocyanin also change, which affects stability. When the pH is close to 7.0, hexamers predominate, which is the most stable structure and prevents the denaturation of phycobiliproteins. However, at higher or lower pH, this structure is prone to dissociation, and its stability is reduced [21]. When the pH is acidic or alkaline, the conformation of the phycobiliprotein chromophore is changed, which changes its color and affects its stability [15, 22]. Ren Shuncheng et al. [23] found that the conformation of the phycocyanin chromophore remained stable and displayed bright blue at pH 4.0–7.0, while at pH<4.0 or pH>7.0 the blue color changed to green and precipitated at pH 2.5–3.0.
2.2 Effect of light on the stability of phycobiliproteins
Light can damage the structure of phycocyanins, which destabilizes the conformation of the chromophores attached to them and reduces the stability of the phycocyanins. The stability of phycobiliproteins gradually stabilizes as the light intensity decreases. Wu et al. [24] found that the degradation of phycobiliproteins under a light intensity of 100 μmol m−2 s−1 is higher than that under 50 μmol m−2 s−1 . When phycobiliproteins are exposed to light for a long time, they often lose their chromophores, thereby losing their color and stability [25]. Liang Xiao et al. [26] selected a light condition of 1500 Lx to irradiate phycobiliproteins. As the light time and intensity increased, the color of phycobiliproteins gradually lightened, and their retention rate decreased, indicating that not only the light intensity but also the light time will affect the stability of phycocyanins.
2.3 Effect of temperature on the stability of phycocyanins
An increase in temperature can lead to an extension reaction of the phycobiliprotein-phycocyanin structure, causing a conformational change from a linear to a cyclic form, thereby affecting the three-dimensional structure of phycobiliproteins. The determinants of the thermal stability of phycobiliproteins mainly include: the number of hydrogen bonds, the fraction of polar surface, the content of secondary structure and the difference in the ratio of surface area to volume [27]. MUNAWARO et al. [28] found that phycocyanins have the potential to maintain spectral intensity at 60 °C, but begin to decrease at 70 °C and higher temperatures, indicating that the pigment protein is thermally unstable during heating. Studies have found that the structure of phycocyanin is destroyed above 40 °C [29‒30]. Bcker et al. [31] determined the intermediate point transition temperatures of phycocyanin trimers and hexamers to be 58.4 °C and 60.9 °C. At 40 °C, there is no change in the absorbance or fluorescence spectrum of phycocyanin, When the temperature is greater than 50 °C, the spectrum changes with the increase of temperature. Not only is phycobiliprotein sensitive to high temperatures, but its stability is also not high under low temperature conditions. CHOI et al. [32] stored phycobiliprotein at 4 °C, and the content of phycobiliprotein decreased by 10.39%, and its stability decreased.

2.4 Other factors
In addition to the above factors that affect the stability of phycocyanins, metal ions, additives, etc. can also affect the conformation of phycocyanin chromophores. The addition of metal ions affects the stability of phycobiliproteins, while the addition of emulsifiers or foaming agents can form bubbles around phycobiliproteins to protect them, thereby maintaining the stability of phycocyanin [22]. Zhang Yanyan et al. [33] found that the stability of phycocyanin was better in solutions with low concentrations of Mn2+, Al3+, Zn2+, and Cu2+. The stability of phycobiliproteins was not significantly affected by changes in the concentration of Na+ and Mg2+, while the higher the concentration of Fe3+, the more beneficial it was for the stability of phycobiliproteins. Some organic reagents can reduce the stability of phycobiliproteins. Zhao Bingbing et al. [34] added different concentrations of additives to phycocyanin and found that their stability decreased with the increase of ethanol, sodium benzoate, and citric acid. Among them, citric acid had a greater effect.
3 Methods for improving the stability of Phycocyanin
3.1 Adding stabilizers
Adding stabilizers is the simplest way to improve the stability of phycocyanin. This method is easy to apply, does not require complicated or expensive equipment, but does require the stabilizer to be highly safe, low in toxicity and harmless, and the amount of additive to be large. At present, the main stabilizers commonly used are sugar, sorbitol, benzoic acid, sodium azide and dithiothreitol. CHENTIR et al. [35] added polyethylene glycol-4000, sucrose and sorbitol to a 0.5 mg/mL phycobiliprotein solution. Polyethylene glycol-4000 had the best thermal stabilizing effect on phycobiliprotein, followed by sorbitol, and the protective effect on the algin protein increases with the concentration of the stabilizer. FAIETA et al. [36] studied the effects of thermal effects and equivalent thermal effects on the discoloration of algin protein in water and solutions of different concentrations of sucrose and trehalose. At a constant temperature, the loss of color increases with time, while the concentration of the solute increases. This shows that the concentration of the stabilizer is positively correlated with the stability of phycobiliproteins, but prolonged heating will lead to a decrease in the stability of phycocyanin.
Under food processing conditions, the stability of phycocyanins can be improved by adding proteins. Proteins can wrap up phycocyanins, thereby improving their stability [37]. ZHANG et al. [38] treated whey protein and phycocyanins at pH 3.0 and 80 °C for 1–20 min. A 10% whey protein solution was found to prevent phycobiliprotein aggregation, and natural whey protein was more effective than denatured hydrolyzed whey protein.
3.2 Chemical modification
Chemical modification is a technique that uses bifunctional reagents to covalently bind two chemical groups on the surface of a protein to strengthen the folded structure of phycocyanins and improve their stability. Formaldehyde, methylglyoxal, and propionates can be used to cross-link phycobiliproteins and stabilize their tertiary and quaternary structures, thereby improving their stability [39–41]. The stability of the pigment can also be maintained by covalently linking the protein to polysaccharides to preserve the extended tetrapyrrole structure. SELIG et al. [42] evaluated the stabilizing effect of beet pectin, guar gum and soluble soy polysaccharides on phycocyanin. The results showed that beet pectin can stabilize phycocyanin, maintain its color, and reduce the ability of enzymes (such as alkaline protease, papain and bromelain) to degrade the protein.
3.3 Encapsulation technology
3.3.1 Microcapsule encapsulation
Microencapsulation technology involves using a core material of solid, liquid or gas, and then using a natural or synthetic polymeric material as a wall material to form a semi-permeable or sealed microparticle [43]. The wall material used for encapsulation must be biocompatible, biodegradable, low-toxicity and low-cost. Microencapsulation technology can effectively improve the stability, solubility and bioavailability of the core material. Microcapsules of phycocyanin can be prepared using a variety of methods, such as freeze-drying, spray drying, and extrusion encapsulation. Microcapsules of phycocyanin prepared using these methods have good heat resistance and high antioxidant activity [44‒45]. Different coating materials also affect the stability of phycocyanin, with maltodextrin and carrageenan as the best coating materials [46]. Lv Xiaoling et al. [47] prepared microcapsules of phycocyanin using air suspension coating. It was measured that under the optimal conditions (inlet air temperature 80 °C, core wall material ratio 1:1.5, atomization pressure 0.15 MPa, gelatin content in the wall material 20%), the stability of phycocyanin was increased by 26.21%, and the storage stability increased by 75.1%. SCHMATZD et al. [48] used polyvinyl alcohol to encapsulate phycocyanin by electro-spraying technology. The phycocyanin-polyvinyl alcohol ultrafine particles have high heat resistance, with a heat resistance temperature of up to 216 °C, and maintain the antioxidant activity of phycocyanin.
3.3.2 Liposome encapsulation
Liposomes are formed from phospholipid molecules that have a tendency to form stable lipid bilayers in aqueous phases. They can not only effectively improve the stability and solution dispersibility of the substances encapsulated in the inner layer, but also play a role in the delivery system, enhancing the functional role of the active substances. They have great potential as drug carriers for targeted drug delivery in the treatment of diseases.
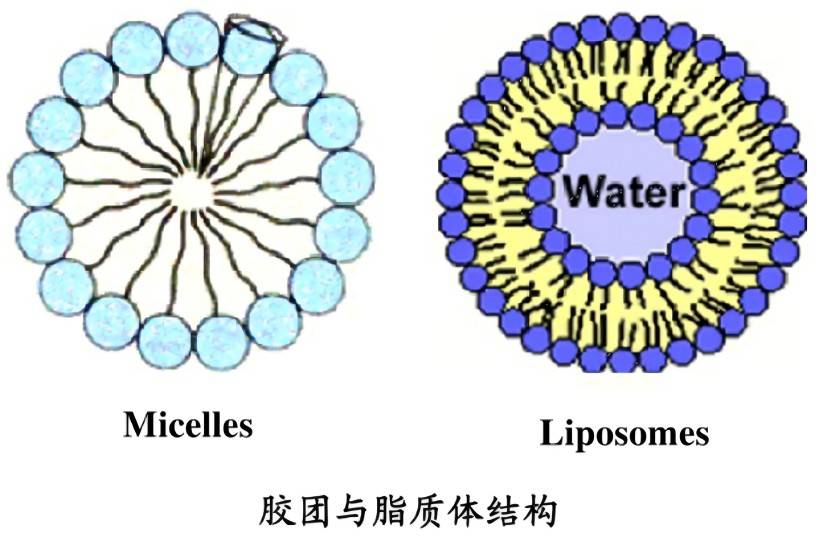
Algin protein can be embedded in different materials to improve its stability. CHUNG et al. [49] prepared algin protein liposomes using chitosan. The hydrophilicity of chitosan was used to disperse the liposomes evenly in solution and improve the thermal stability of algin protein. NOGUEIRA et al. [50] hydrated a chloroform solution of soy lectin with trimethylglycine, magnesium chloride, and phycocyanin were hydrated to form phycocyanin liposomes, which improved the stability and specificity of the protein, as well as its antioxidant, anti-inflammatory, and neuroprotective activities. SEYED et al. [51] prepared phycocyanin liposomes using diethylene glycol at 70 °C and found that the resulting liposomes had high stability in terms of sedimentation and suspended particles.
3.4 Other methods
The stability of phycocyanins can also be improved by methods such as high pressure. High pressure causes phycobiliproteins to form a more compact protein structure and aggregate with changes in secondary structure. ZHANG et al. [52] studied the effect of high pressure treatment on the structure and color stability of phycobiliproteins, phycobiliprotein whey protein and phycocyanin-carrageenan mixtures. High-pressure treatment of phycocyanin-whey protein and phycocyanin-carrageenan formed beneficial aggregates at pH 5.0, which reduced the corresponding phycocyanin loss. This provides a new way to improve the storage stability of phycocyanin under light conditions.
4 Applications
Phycocyanin powder is widely used as a natural water-soluble pigment in the food, cosmetics and pharmaceutical industries. Phycocyanin not only improves the color of food, but also increases its functional ingredients. It also has the effect of stimulating erythrocyte colony formation, replenishing blood and improving lymphatic activity.
4.1 Application in food
Since phycocyanin powder is unstable to light and heat, its application in baked goods is limited. At present, its application is mainly concentrated in dairy products, jelly candies and other foods. MG et al. [53] added phycocyanin to yogurt at pH 4.5. At 4 °C, as the concentration of phycocyanin increased, the viscosity of the yogurt increased accordingly, resulting in a significant decrease in Streptococcus thermophilus and Lactobacillus bulgaricus at 14th and 21st day, respectively. Alginin can increase the dehydration rate of yogurt and enhance the texture of yogurt. DEWI et al. [54] prepared gel sugar using alginin microcapsules. Maltodextrin and sodium alginate were used as coating materials to prepare alginin microcapsules. then added 0, 1%, 3% and 5% of the microcapsules to the gel sugar at a temperature of 40 °C. Adding 5% of the microcapsules produced a bright blue color, and the microcapsules had a certain degree of persistence during the processing of the gel sugar.

4.2 Applications in the medical field
Alginin powder is biologically sensitive, biocompatible, bioabsorbable, and has low toxicity to the human body. It can be used as an antioxidant, neuroprotective agent, pancreatic anticancer agent, and can also promote skin wound healing. MADHYASTHA et al. [55] used alginin to conjugate silver nanoparticles to significantly reduce the toxicity of silver nanoparticles, enhanced the movement of red blood cells to the wound surface, and reduced cell stress at the wound edge. MADHYASTHA et al. [56] isolated cyanopeptide β2 from the β-chain of phycocyanin, which can scavenge free radicals in plasma, enhance iron reduction capacity, and inhibit damage to DNA by reactive oxygen species, thereby maintaining the integrity of the DNA. FERNÁNDEZ-ROJAS et al. [57] studied the preventive effect of phycocyanin on cisplatin (CP)-induced mitochondrial dysfunction in male CD-1 mice. The study showed that phycoerythrin can reduce abnormal mitochondrial reactions. LIAO et al. [58] studied the therapeutic potential of phycocyanin as an anti-pancreatic cancer drug in vitro and in vivo. The study showed that phycocyanin exerts anti-pancreatic cancer activity by inducing apoptosis and autophagy cell death, thus confirming that phycocyanin is a promising anti-pancreatic cancer drug.
4.3 Other applications
Phycocyanin powder has the advantages of high fluorescence quantum yield, high molar extinction coefficient and large Stokes shift, which are superior to many synthetic dyes currently in use. Phycoerythrin is now combined with immunoglobulin, protein A and antibiotic proteins to form fluorescent probes. ZHENG et al. [59] developed a relatively sensitive new fluorescence detection method for detecting phycoerythrin by making the purified phycoerythrin fluorescent probe compatible with the light emitting diode (LED)-charge coupled device (CCD) fluorescence density bar qualitative detection system. This method solves the problem of traditional purification methods LED)-charge coupled device (CCD) fluorescence density bar qualitative detection system, developed a relatively sensitive new fluorescence detection method for detecting phycobiliproteins. This method solves the disadvantages of the traditional purification method, which is complex, has a low collection rate and insufficient quantity. It can provide convenient quantitative information and shows great potential for rapid detection in environmental and food safety research.
5 Outlook
As a natural pigment, phycocyanin powder can be used as a coloring agent in the cosmetics industry, as an additive in food, and as an anti-inflammatory agent, antioxidant, anticancer agent, immunomodulator and fluorescent detection probe in medicine. As people's understanding of the properties and functions of phycocyanin continues to deepen, its application prospects are becoming more and more extensive. However, stability has become a bottleneck that limits its application, so solving the problem of stability will greatly promote the scope and scale of its application. At present, although the stability of phycobiliproteins can be improved in various ways, such as adjusting the pH, adding stabilizers, cross-linking agents or encapsulating phycocyanins, the chemical interactions between phycobiliproteins and stabilizers and food substrates need to be further explored. It remains to be seen whether the stabilizers of phycobiliproteins will affect the nutrition and sensory properties of foods, and whether they can be used under food processing and production conditions. In addition, during the application and promotion of phycocyanin, it is necessary to solve the problems of efficient extraction and purification technology, and to thoroughly explore its bioavailability and the mechanism of its biological activity and effect. It is believed that with the continuous deepening of research on phycocyanin, the market application of phycocyanin will become even more extensive.
Reference:
[1] YU LY. Research progress on the properties and extraction technology of phycocyanin [J]. Fujian Texti, 2020, (5): 46‒51.
[2] MARKOU G, NERANTZIS E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions [J]. BiotechnolAdv, 2013, 31(8): 1532‒1542.
[3] NICCOLAI A, VENTURI M, GALLI V, et al. Vegetable oils protect phycocyanin from thermal degradation during cooking of spirulina-based “crostini” [J]. LWT-Food Sci Technol, 2020, 138: 110776.
[4] KUMAR D, DHAR DW, PABBI S, et al. Extraction and purification of C-phycocyan in from Spirulina platensis (CCC540) [J]. Indian J Plant Physiol, 2014, 19(2): 184‒188.
[5] MOGANY T, KUMARI S, SWALAHA FM, et al. Extraction and characterisation of analytical grade C-phycocyanin from Euhalothece sp. [J]. J Appl Phycol, 2019, 31(3): 1661‒1674.
[6] HAO S, QIN Y, WANG CT. Research progress on the physiological activity of functional food phycocyanin [J]. J Food Sci Biotechnol, 2017, 36(12): 1233‒1240.
[7] LU YN, ZHANG FY, HU SH, et al. Experimental study on the stability of phycocyanin in Chaohu cyanobacteria [J]. J Hefei Univ Technol (Nat Sci Ed), 2017, 40(11): 1557‒1562.
[8] HOU YH, YAN MH, WANG QF, et al. C-phycocyanin from Spirulina maxima as a green fluorescent probe for the highly selective detection of mercury(II) in seafood [J]. Food Anal Methods, 2016, 10(6): 1931‒1939.
[9] FRATELLI C, BURCK M, AMARANTE M, et al. Antioxidant potential of nature's “something blue”: Something new in the marriage of biological activity and extraction methods applied to C-phycocyanin [J]. Trends Food Sci Technol, 2020, 107: 309‒323.
[10] PAN-UTAI W, KAHAPANA W, IAMTHAM S. Extraction of C-phycocyanin from Arthrospira (Spirulina) and its thermal stability with citric acid [J]. J Appl Phycol, 2018, 30(1): 231‒242.
[11] SCHULZE P, BARREIRA LA, PEREIRA H, et al. Light emitting diodes (LEDs) applied to microalgal production [J]. Trends Biotechnol, 2014, 32(8): 422‒430.
[12] GAO KH. Preparation and properties of phycobiliprotein microcapsules [D]. Xiamen: Jimei University, 2014.
[13] MING HL, CASTILLO G, OCHOA-BECERRA MA, et al. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability [J]. Algal Res, 2019, 42: 101600.
[14] FERNÁNDEZ-ROJAS B, HERNÁNDEZ-JUÁREZ J, PEDRAZA -CHAVERRI J. Nutraceutical properties of phycocyanin [J]. J Funct Foods, 2014, 11: 375‒392.
[15] LIANG X. Study on the stability of optical properties and biological activity of C-phycocyanin [D]. Nanning: Guangxi University, 2020.
[16] REN SC, CAO Y, LI LZ, et al. Research progress of natural food pigment phycocyanin [J]. Food Res Dev, 2021, 42(7): 203‒208.
[17] SUN L, WANG S, QIAO Z. Chemical stabilization of the phycocyanin from cyanoba-cterium Spirulina platensis [J]. J Biotechnol, 2006, 121(4): 563‒569.
[18] KHANDUAL S, SANCHEZ E, ANDREWS HE, et al. Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: Efficient extraction process and stability evaluation of phycocyanin [J]. BMC Chem, 2021, 15(1). DOI: 10.1186/s13065-021-00746-1
[19] ZHENG JX, YIN H, SHEN CC, et al. Functional and structural properties of Spirulina phycocyanin modified by ultra-high-pressure composite glycation [J]. Food Chem, 2019, 306: 125615.
[20] BERNS DS, MACCOLL R. Phycocyanin in physical chemical studies [J]. Chem Rev, 1989, 89(4): 807‒825.
[21] XU R. Research of the storage stability of phycocyanin from Spirulina platensis [D]. Tianjin: Tianjin University of Science and Technology, 2017.
[22] LI Y, ZHANG Z, PACIULLI M, et al. Extraction of phycocyanin-A natural blue colorant from dried spirulina biomass: Influence of processing parameters and extraction techniques [J]. J Food Sci, 2020, 85(3). DOI: 10.1111/1750-3841.14842
[23] REN SC, WANG FW, CAO Y, et al. Study on the stability of algae blue pigment [J]. J Henan Univ Technol (Nat Sci Ed), 2020, 41(6): 1‒7.
[24] WU HL, WANG GH, XIANG WZ, et al. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis [J]. Int J Food Prop, 2016, 19(9-12): 2349‒2362.
[25] MATHILDE M, SÉBASTIEN J, ALVA W, et al. Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum [J]. Food Chem, 2014, 150: 400‒407.
[26] LIANG X, WANG CH, LU X, et al. Stability and fluorescence characteristics of C-phycocyanin in Spirulina platensis [J]. Mod Food Sci Technol, 2020, 36(6): 89‒96.
[27] FERRARO G, IMBIMBO P, MARSEGLIA A, et al. X-ray structure of C-phycocyanin from Galdieria phlegrea: Determinants of thermostability and comparison with a C-phycocyanin in the entire phycobilisome [J]. BBA-Bioenergetics, 2020, 1861(9): 148236.
[28] MUNAWAROH H, DAROJATUN K, GUMILAR GG, et al. Characterization of phycocyanin from Spirulina fusiformis and its thermal stability [J]. J Phys Conf, 2018, 1013(1): 012205.
[29] CHAIKLAHAN R, CHIRASUWAN N, BUNNAG B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives [J]. Process Biochem, 2012, 47(4): 659‒664.
[30] MARTELLI G, FOLLI C, VISAI L, et al. Thermal stability improvement of blue colorant C-phycocyanin from Spirulina platensis for food industry applications [J]. Process Biochem, 2014, 49(1): 154‒159.
[31] BCKER L, HOSTETTLERR T, DIENER M, et al. Time- temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina [J]. Food Chem, 2020. DOI: 10.1016/j.foodchem.2020.126374
[32] CHOI WY, LEE HY. Kinetic analysis of stabilizing C-phycocyanin in the Spirulina platensis extracts from ultrasonic process associated with effects of light and temperature [J]. Appl Sci, 2018, 8(9): 1662.
[33] ZHANG YY, CHEN BL, CHEN ZJ. Study on the stability of rose phycocyanin [J]. Subtrop Plant Sci, 2014, 43(2): 103‒107.
[34] ZHAO BB, ZHANG FY, CHEN Y, et al. Four-step salting-out extraction of phycocyanin from fresh cyanobacteria in Chaohu Lake and its stability [J]. J Environ Eng, 2016, 10(5): 2302‒2308.
[35] CHENTIR I, HAMDI M, LI S, et al. Stability, bio-functionality and bio-activity of crude phycocyanin from a two-phase cultured Saharian arthrospira sp. Strain [J]. Algal Res, 2018, 35: 395‒406.
[36] FAIETA M, NERI L, SACCHETTI G, et al. Role of saccharides on thermal stability of phycocyanin in aqueous solutions [J]. Food Res Int, 2020, 132: 109093.
[37] ZHANG S, ZHANG Z, DADMOHAMMADII Y, et al. Whey protein improves the stability of C-phycocyanin in acidified conditions during light storage [J]. Food Chem, 2021, 344. DOI: 10.1016/j.foodchem.2020.128642
[38] ZHANG Z, LI Y, ABBASPOURRAD A. Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein- phycocyanin interactions [J]. Food Hydrocolloid, 2020, 105. DOI: 10.1016 / j.foodhyd.2020.105747
[39] MUNAWAROH H, GUMILAR GG, ALIFIA CR, et al. Photostabilization of phycocyanin from Spirulina platensis modified by formaldehyde [J]. Process Biochem, 2020, 94: 297‒304.
[40] MARTELLI G, FOLLI C, VISAI L, et al. Thermal stability improvement of blue colorant C-phycocyanin from Spirulina platensis for food industry applications [J]. Process Biochem, 2014, 49(1): 154‒159.
[41] FUKUI K. Relationship between color development and protein conformation in the phycocyanin molecule [J]. Dyes Pigments, 2004, 63(1): 89‒94.
[42] SELIG MJ, MALCHIONE N, GAMALELDIN S, et al. Protection of blue color in a spirulina derived phycocyanin extract from proteolytic and thermal degradation via complexation with beet-pectin[J].Food Hydrocolloid, 2018, 74: 46‒52.
[43] YOU YI, CHENG SHASHA, ZHANG LI, et al. Rational modulation of the luminescence of upconversion nanomaterials with phycocyanin for the sensing and imaging of myeloperoxidase during an inflammatory process [J]. Anal Chem, 2020, 92(7): 5091‒5099.
[44] PURNAMA FNW, AGUSTINI TW, KURNIASIH RA. The effect of different temperature on the stability of phycocyanin on microcapsule Spirulina platensis [C]. IOP Conference Series: Earth and Environmental Science. IOP Publishing, 2020.
[45] PURNAMAYATI L, DEWI EN, KURNIASIH RA. Phycocyanin stability in microcapsules processed by spray drying method using different inlet temperature [J]. Iop Conf, 2018, 116: 012076.
[46] PRADEEP HN, NAYAK CA. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation [J]. J Food Sci Technol, 2019, 56(10):4526‒4534.
[47] LV XL, XU LR, CHEN ZH, et al. Preparation of phycocyanin microcapsules by air suspension coating method [J]. Food Technol, 2013, 38(2): 260‒263.
[48] SCHMATZD A, DASILVEIRAMASTRANTONIOD J, COSTAJ AV, et al. Encapsulation of phycocyanin by electrospraying: A promising approach for the protection of sensitive compounds [J]. Food Bioprod Process, 2020, 119: 206‒215.
[49] CHUNG GY, SHIM KH, KIM HJ, et al. Chitosan-coated C-phycocyanin liposome for extending the neuroprotective time window against ischemic brain stroke [J]. Curr Pharm Design, 2018, 24(17): 1859‒1864.
[50] NOGUEIRA A, KOKUSZI L, CORDEIRO AP, et al. Spirulina sp. LEB 18-extracted phycocyanin: Effects on liposomes’ physicochemical parameters and correlation with antiradical/antioxidant properties [J]. Chem Phys Lipid, 2021: 105064. DOI: 10.1016/j.chemphyslip. 2021.105064
[51] SEYED YA, SHAHIDI F, MOHEBBI M, et al. Preparation, characteri zation and evaluation of physicochemical properties of phycocyanin -loaded solid lipid nanoparticles and nanostructured lipid carriers[J]. J Food Meas Charact, 2018, 12(1): 378‒385.
[52] ZHANG Z, CHO S, DADMOHAMMADI Y, et al. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing [J]. Food Hydrocolloid, 2021, 110: 106055.
[53] MG A, SZ A, MG B. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage [J]. LWT, 2019, 102: 230‒236.
[54] DEWI EN, KURNIASIH RA, PURNAMAYATI L. The application of microencapsulated phycocyanin as a blue natural colorant to the quality of jelly candy [C]. IOP Conference Series: Earth and Environmental Science. IOP Publishing, 2018.
[55] MADHYASTHA H,MADHYASTHA R, THAKUR A, et al. C-phycocyanin primed silver nano conjugates: Studies on red blood cell stress resilience mechanism [J]. Colloids Surf B Biointerfaces, 2020, 194: 111211.
[56] MADHYASTHA H, VATSALA TM. Cysteine-rich cyanopeptide beta2 from Spirulina fusiformis exhibits plasmid DNA pBR322 scission prevention and cellular antioxidant activity [J]. Indian J Exp Biol, 2010, 48: 486‒493.
[57] FERNÁNDEZ-ROJAS B, RODRÍGUEZ-RANGEL DS, GRANADOS -CASTRO LF, et al. C-phycocyanin prevents cisplatin-induced mitochondrial dysfunction and oxidative stress [J]. Mol Cell Biochem, 2015, 406(1): 183‒197.
[52] ZHANG Z, CHO S, DADMOHAMMADI Y, et al. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing [J]. Food Hydrocolloid, 2021, 110: 106055.
[53] MG A, SZ A, MG B. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage [J]. LWT, 2019, 102: 230‒236.
[54] DEWI EN, KURNIASIH RA, PURNAMAYATI L. The application of microencapsulated phycocyanin as a blue natural colorant to the quality of jelly candy [C]. IOP Conference Series: Earth and Environmental Science. IOP Publishing, 2018.
[55] MADHYASTHA H, MADHYASTHA R, THAKUR A,et al. C-phycocyanin primed silver nano conjugates: Studies on red blood cell stress resilience mechanism [J]. Colloids Surf B Biointerfaces, 2020, 194: 111211.
[56] MADHYASTHA H, VATSALA TM. Cysteine-rich cyanopeptide beta2 from Spirulina fusiformis exhibits plasmid DNA pBR322 scission prevention and cellular antioxidant activity [J]. Indian J Exp Biol, 2010, 48: 486‒493.
[57] FERNÁNDEZ-ROJAS B, RODRÍGUEZ-RANGEL DS, GRANADOS -CASTRO LF, et al. C-phycocyanin prevents cisplatin-induced mitochondrial dysfunction and oxidative stress [J]. Mol Cell Biochem, 2015, 406(1): 183‒197.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






