What Is Vanillin Made From?
Vanillin is also known as vanillin, vanillin, etc., the chemical name is 3-methoxy 4-hydroxybenzaldehyde, relative molecular mass 152.15, white to slightly yellow needle or powder crystals. 15, white to slightly yellow needle-like or powdery crystals. Vanillin is widely found in nature in the form of free form and glucoside, and the mass content of vanillin in the pods of Vanilla planifolia is 1.5%~3% [1]. The mass content of vanillin in vanilla pods is 1.5%-3% [1]. Pure vanillin has a strong milky aroma and no odor. As a flavor additive, it is favored for its small amount of addition and unique aroma. Widely used as a fixative, flavoring agent, used in cosmetics, soaps, cigarettes, pastries, confectionery, beverages and baked goods and other industries in the production of the world's largest production of synthetic fragrances, with an annual output of nearly 10,000 tons [2].
Vanillin is also an important raw material and intermediate in the pharmaceutical industry, from which 3,4,5-trimethoxybenzaldehyde (TMB) can be synthesized [3], and TMB is an important intermediate in the synthesis of sulfonamide potentiator methoxybenzylaminopyrimidine (TMP), cough and expectorant asthma and antiepileptic 3,4,5-trimethoxycinnamoyl isopropylamine, etc. It is also an important intermediate in the synthesis of biphenyl dibenzoate, lisinopril, piperazole and other drugs [4]. Vanillin is an important intermediate in the synthesis of biphenyl dibenzoate, lisdexamfetamine, piperazole and other drugs [4]. Vanillin can also be used as electroplating polish, plant growth promoter, ripening agent, etc., has become a variety of uses of organic compounds, and its demand increased by 10% per year [5].
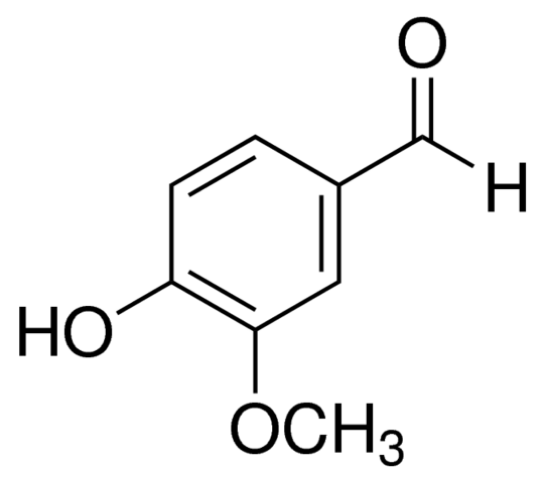
Due to the limitation of natural resources, it is no longer possible to satisfy the increasing demand for vanillin by extracting it from plants alone. Since Tiemann and Haarmann determined the molecular structure of vanillin in 1874, with the development of production and in-depth research, the diversification of synthetic raw materials to provide conditions for the preparation of vanillin, there are four production routes with more economic value [6].
1 Process route for vanillin production
1 . 1 Eugenol as a raw material
The synthesis of vanillin by this method was started by Reimer and Tiemann at the end of the 19th century and has been continuously improved. There are currently three process routes for this method.
Firstly, the isomerization of eugenol (from clove oil) in the presence of alkali converts the allyl group in the molecule of eugenol to propenyl group, resulting in sodium isoeugenol, which is then oxidized by an oxidizing agent to the sodium salt of vanillin, which is then acidified to obtain vanillin. The isomerization can be carried out by high temperature method with concentrated alkali, and the oxidation process conditions include protection of hydroxyl group and direct oxidation.
The second is indirect oxidation, the isomerization of eugenol to produce sodium isoeugenol, and (CH3 CO)2 O action, isoeugenol acetate, after oxidation in acidic media hydrolyzed to vanillin [7].
Thirdly, after isomerization of eugenol to sodium isoeugenol, vanillin was produced by electrolysis in a diaphragm electrolysis cell with Mn3+/Mn2+ as redox intermediates, PBO2 (Ph-based) anode and Ni as anode at a current of 15A/dm2 and a temperature of 50-60°C, with an average yield of 50% [8]. The aroma of vanillin obtained by this method is pure, but the cost is more than 20 times higher than that of the lignin method, which was used in the Netherlands and the United Kingdom.
1 .2 Based on safrole
In 1927, Japan Takasago synthesized vanillin by ozonation of safrole, and three process routes were formed after continuous improvement. First, safrole (from camphor oil) was treated with alkali, converted to isosafrole by double-chain migration, oxidized to piperonal, interacted with PCl5 to obtain protocatechuic aldehyde, and then methylated with (CH3)2 SO4 to obtain a mixture of vanillin and isovanillin. Secondly, the isomerized ring-opening mixture is oxidized by heating with an aromatic nitro compound (nitrobenzene) in an alkaline solution. The 4-OH isomers in the mixture do not react and can be separated.
The former was decomposed with dilute acid to obtain protocatechualdehyde, and the latter was methylated and treated with dilute acid to recover isoeugenol. Thirdly, the pepper aldehyde obtained by oxidizing isosafrole is dissolved in nitrobenzene solution, and then added with aluminum boride or aluminum trioxide to oxidize protocatechuic aldehyde at 0~20℃, the yield is up to 83%, and then methylated to get vanillin. This method was used to produce vanillin in Japan, the product has good aroma, but the isovanillin in the side reaction is difficult to remove, and the cost is more than 10 times higher than that of lignin method.
1 .3 Lignosulfonates as raw materials
In 1938, the salvo company in the United States began to use lignin to produce vanillin, using sulfite pulp method of paper mills in the discharge of sulfite cooking waste, about 50% (to solids) for lignosulfonate, lignosulfonate as a raw material for the preparation of vanillin reaction equation is as follows:
Since K. Freudenberg first proposed the production of vanillin by alkaline-nitrobenzene oxidation of lignin, several plants have been built in Canada, the USA and the former Soviet Union. Since K. Freudenberg first proposed that vanillin can be produced by alkaline-nitrobenzene oxidation of lignin, a number of plants have been built in Canada, the United States, and the former Soviet Union for the production of vanillin from sulfite pulp waste streams, which has the lowest cost of raw materials and the yield of vanillin is generally about 15%. The production process includes concentration, neutralization, oxidation, acidification, extraction and refining, etc. The alkaline-nitrobenzene oxidation has been changed to air-catalyzed oxidation. For the concentration of raw material liquid, the new process of ultrafiltration technology is also being studied to replace the method of heating concentration, and for the post-treatment process of extracting vanillin from the oxidized liquid, there are advanced processes such as alkaline extraction, ion exchange extraction and carbon dioxide extraction to replace the backward acidic extraction method [9]. At present, most of the domestic factories follow the guaiacol route, and some paper mills adopt the lignin route in order to treat paper waste liquid. The product quality of this route is low, and the vanillin produced contains a large number of heavy metals, which can not be used in food and pharmaceutical industry, and most of them have been discontinued.
1 .4 Guaiacol as raw material
This method has been used in foreign countries for more than 90 years, and is also used in China to produce vanillin in large quantities. Guaiacol is condensed with p-nitroso N,N-dimethylaniline hydrochloride, formaldehyde or hexamethylenetetetramine as raw materials to prepare vanillin. Advantages of this method: the vanillin alcohol generated by side reaction interacts with p-hydroxy amine group N , N-dimethylaniline hydrochloride to form cifuroxyl, and after decomposition, the alcohol participating in the reaction can be transformed into vanillin, so the total yield is higher, about 60% (in terms of guaiacol). The process of guaiacol reaction with formaldehyde is the main process of vanillin production in China.
This method does not rely on any natural raw materials and is a fully synthetic method. Although the total yield of this method has been improved (about 60% of the total yield), the whole process is more complicated, the emission of three wastes is large, and the cost is more than 6 times higher than that of lignin. Guaiacol-formaldehyde process has been used in China for decades, and it is still the main process route for the production of vanillin in China. This process uses 4-nitroso-N,N-dimethylaniline as oxidizing agent, and the by-product of the reaction, 4-amino-N,N-dimethylaniline, is more difficult to deal with the pollution and has many steps of refining, so in foreign developed countries, this process has been replaced by a new guaiacol method in the early 1980s [10]. In this method, guaiacol is condensed with glyoxalic acid under alkaline condition, and the condensation reaction solution recovers guaiacol, and then oxidized by copper salt catalytic oxidation, which oxidizes the condensation product substituting phenylglycolic acid (substituting mandelic acid) into corresponding keto acid. The condensation product is oxidized to the corresponding keto acid by decarboxylation.
The industrial production yield of this method can reach 70%, and this method and lignin method are the main methods for producing vanillin in foreign countries. However, the domestic production capacity of this method has not yet been formed. Since the mid-80s, some units in Tianjin, Liaoning, Jiangsu and Beijing have developed this process and conducted pilot tests, and the yield of vanillin reached 48% ~ 60%, but it could not be industrialized due to technical reasons [11]. At present, foreign manufacturers with large production capacity of vanillin include Ontario Paper Mills of Canada, Monsanto Chemical Company of the United States, Rhône-Poulenc of France, and Norwegian Bole Gede Company. The main production plants of vanillin in China are Jilin Chemical Industry Company and Shanghai Fragrance Factory, in which the White Bear brand vanillin produced by JICC enjoys a high reputation in the international market.
2 Progress of vanillin synthesis route
Synthetic fragrances, which now dominate the flavor industry, are not subject to natural conditions, are of consistent quality, can be produced on a large or small scale, and are much cheaper than their natural counterparts. With the increase in the demand for spices (sales of spices have increased about 10 times in the last 20 years and are forecast to increase by 8.2% per year in the future), the demand for synthetic spices is increasing. 2% per year in the future), the development and exploitation of new synthetic fragrances is becoming increasingly important. Recently, a number of articles have been published on the production of vanillin by fully synthetic methods, but no large-scale industrial production has been reported, and these methods are briefly described below.
2 . 1 P-hydroxybenzaldehyde as raw material
Vanillin is synthesized from p-hydroxybenzaldehyde by bromination and methoxylation:
The total yield of vanillin reported by this method is 50% 85% [12, 13], which is difficult to be generalized due to the use of more reagents, complex operation and the need for a pressure reaction kettle [14]. Recently, more studies on this process have been conducted. The key to the industrialization of this route lies in the solution of the cheap synthesis method of p-hydroxybenzaldehyde. The northeast region of China is very rich in p-cresol as a by-product of forestry, so the method of preparing vanillin from p-hydroxybenzaldehyde should be actively developed.
2 .2 Preparation of vanillin by reaction of guaiacol with trichloroacetaldehyde
Guaiacol is condensed with trichloroacetaldehyde in K2 CO3 or Na2 CO3 solution, and the resulting hydroxyphenyltrichloromethylmethanol is heated in the presence of oxidizing agents such as nitrobenzene or CU(OH)2 and Na2 CO3 or NaOH to produce vanillin.
This process was developed by India, Poland and other countries, the yield is about 60%, in the condensation of trichloroacetaldehyde and guaiacol, there are a lot of resinous substances generated, it is very difficult to filter, it is difficult to be used in the industry.

2 .3 Preparation of vanillin by reaction of guaiacol with chloroform [15]
Guaiacol and chloroform are used as raw materials to produce dichlorocarbin adducts in the presence of NaOH, which are then hydrolyzed to produce vanillin with the following reaction formula:
The synthesis of vanillin has been reported in 39.2% yield using PEG-600 as phase transfer catalyst under ultrasonic radiation [16]. 2% [16]. This method is still in the exploratory stage and no industrial production has been reported.
In conclusion, there are many chemical synthesis routes for vanillin, but some raw materials are expensive, some routes have low yields, and some impurities and odors are difficult to be eliminated, so it is necessary to carry out in-depth research on how to find out a process route with simple technology, high yield, low cost, easy management of three wastes, and suitable for industrialized production.
3 Research progress of vanillin production process
The route of guaiacol as raw material for the preparation of vanillin is a mature process with a wide source of raw materials, and it is the only fully synthetic process among the four economically valuable production routes at present, which has a great prospect for development. Among them, the guaiacol-glyoxylate route has been adopted by the French company Rhône-Poulenc and produced vanillin and ethyl-vanillin in large quantities. The company's annual output of methyl vanillin is more than 6000 tons [17]. The process is simple, the reaction conditions are easy to control, and the reaction yield is high, which can reach 70%. Currently, most of the research progress on vanillin production process focuses on the improvement of this route.
When using high-temperature chemical oxidation method to oxidize the condensation reaction solution, it is necessary to add a large amount of copper sulfate as a catalyst, and after oxidizing it for several hours at 100~105℃ with oxygen, the copper sulfate itself will be reduced into fine-grained cuprous oxide, which is very difficult to filter. Moreover, it is necessary to add a set of oxidizing device in order to oxidize these cuprous oxide into copper sulfate for recycling. For this reason, Shenyang Institute of Pharmacy, Beijing Institute of Technology and other units proposed a new process of electrolytic oxidation, the process is still guaiacol and acetaldehyde condensation reaction to produce vanillin, but the second step of the oxidation reaction catalyzed by copper sulfate oxygen oxidation reaction to electrolytic oxidation, at the same time, the original process to make the corresponding changes [18, 19].
The method of implementation is as follows: Guaiacol and glyoxalic acid for liquid condensation reaction, recovery of unreacted guaiacol, the reaction solution was added to the electrolysis tank, electrolysis in accordance with certain electrolysis conditions, electrolysis, adding strong acid to adjust the reaction solution for weak acidity, decarboxylation reaction will occur to release CO2, extracted with organic solvents, recovery of solvents, the yellow crude, and then recrystallization and refinement can be obtained from the high-purity product. After extracting vanillin with organic solvent, the solvent can be recovered and the yellow crude product can be obtained. This method has the following advantages over chemical oxidation.
(1) The electrolysis reaction is carried out in a one-compartment electrolyzer, which makes the process simple and smooth, and is conducive to industrialized production. The organic electrolysis reaction is highly selective and the reaction products are of high purity.
(2) Electrolysis and oxidation reaction takes place in the anode chamber, so there is no need to add oxidizer and catalyst, which saves raw materials and low cost. Since no chemicals are added, the post-treatment process of reaction liquid is eliminated, thus reducing the pollution of waste liquid.
(3) The whole electrolysis reaction is carried out at low current density and low tank voltage, with low power consumption.
In the research of condensation reaction, the reaction temperature has been increased and the reaction time has been greatly shortened from the original 24 h to more than one hour [20], and catalysts that can increase the yield and reduce the by-products are also being searched for, such as Al2 O3, znO, and so on. According to a Japanese patent, the addition of 1/1000 of β-cycloalkyl dextrin or starch to the reaction solution significantly increased the yield of the intermediate 4-hydroxy-3-methoxyphenylglycolic acid (mandelic acid) [21], and the use of phosphoric acid or phosphite also had the same effect [22].
In order to increase the yield of the decarboxylation reaction and shorten the decarboxylation time, the oxidized reaction solution was acidified and then decarboxylated by pressurization. In the refining process, the traditional decompression distillation process has also been improved, due to the P-vanillin and "-vanillin and their methylated products boiling point is very similar, such as in 0.53-0.53-0.53, the boiling point is very similar, such as in 0.53-0.53, the boiling point is very similar, such as in 0.53-0.53, the boiling point is very similar. For example, at 0.53-0.66kPa, P-vanillin and "-vanillin and their methylated products have very similar boiling points. For example, at 0.53-0.66kPa, the boiling point of P-vanillin is 149~151℃, while that of its methylated product is 154~156℃. Refining vanillin requires a very high vacuum and takes a long time, which may lead to deterioration and decomposition of the vanillin. This has led to the development of processes that avoid the use of decompression distillation, and the electrolytic oxidation process, as described earlier, has the potential to avoid decompression distillation and yield a product of higher purity. According to a Japanese patent report, recrystallization with an appropriate amount of hot water can also yield a qualified product with a purity of more than 99% [23].
4 Prospects for the development of the vanillin production process
At present, most of the vanillin powder in the market is produced by guaiacol method, and a small part of it comes from lignosulfonate of papermaking waste liquid, and other methods are decreasing day by day, and the synthesis of vanillin with guaiacol as the basic raw material is becoming more and more dominant. Among the production processes using guaiacol as raw material, glyoxalic acid method is characterized by simple process, easy control of reaction conditions and high yield, and there are mature production processes in foreign countries, so it is the development direction of vanillin production process in China. With the improvement of the production technology of glyoxalic acid, the production cost of glyoxalic acid has been greatly reduced, which makes this synthesis route more economic. Research on the improvement of the process route should be strengthened, mainly to improve the yield of the condensation reaction and the efficiency of the separation process.
In the separation technology of vanillin, supercritical CO2 extraction will be an important topic in the future research of this process. Germany and France have carried out research in this area, and the extraction yield is more than 90%. France Rhone-Poulenc extracted vanillin with supercritical CO2 under 50-100℃ and 7.5 ~ 40MPa, and the yield is 96%. France Rhone-Poulenc company in 50-100 ℃, pressure 7.5 ~ 40MPa under the use of supercritical CO2 extraction of vanillin, the yield reached 96.8%. The yield was 96.8%.
Another promising route is the route of p-hydroxybenzaldehyde, which has simple steps and high reaction yield, and it is reported that the yield of the two-step reaction is above 90%. However, the reaction conditions of this method are harsh, and the research on the reaction under normal temperature and pressure should be strengthened, and a new catalytic system should be developed to make this process more suitable for industrialized production. In conclusion, among the various production routes of vanillin, guaiacol-glyoxylate route is the most promising route for industrialization in China. At present, the main factor affecting the price of vanillin is the price of guaiacol. With the development of guaiacol synthesis from phenol, the price of guaiacol is expected to decrease significantly, which will make this route more advantageous.
References:
[ 1 ] Liu YM, Liu H, Xu M. Hebei Chemical Industry, 1997(4): 40 ~ 42
[ 2 ] Pan Xiandao. Chinese and Foreign Science and Technology Intelligence, 1995(12): 29 ~ 30
[ 3 ] Ji Yafei, Wei Xianyong. Modern Chemical Industry, 1999, 19(8): 28 ~ 29
[ 4 ] Song Guoan. Shanghai Chemical Industry, 1998, 23(6): 31 ~ 35
[ 5 ] Song Guoan, Li Rui, Fan Weixing et al. Shaanxi Chemical Industry, 1998(3):5,6,15
[ 6 ] Chen Huangqiang, Liu Youjun. Spice product development and application . Shanghai: Shanghai Science and Technology Press, 1994
[ 7 ] Cheng Chu-sheng . Chemistry of fine chemicals . Shanghai: East China University of Science and Technology Press, 1996 . 306 ~ 312
[ 8 ] CHENG Hua, YUAN Boqing. Fine Chemical Industry, 1993, 10(3): 16 ~ 20
[ 9 ] Tang Li-Ling, Tu Li-Xing, Lin Zhen-Wu et al. Guangdong Chemical Industry, 1993(3): 21, 22 ~ 24
[10] kirk-Othmer. EnCyClopedia of ChemiCal TeChnology. 1983, 23(3):704 ~ 717
[11] Li J S, Zhang S Y . Jiangsu Chemical Industry, 1993, 21(3): 13 ~ 16
[12] Zhang Zhangfu, Chen Jiwei. Guangxi Chemical Industry, 1989(2): 40 ~ 41
[13] Yuan Cubing, Ding Yong. Modern Chemical Industry, 1990(1): 33 ~ 35
[14] Zhou Ningzhang, Yuan Cubing, Dai Yongchuan. Chemical Engineer, 1998(4): 14 ~ 15
[15] Li Zhonggui, Zou Ying. Chemistry World, 1991(1): 18 ~ 20
[16] Jiang YR, Xu JH. Journal of Central South College of Mining and Metallurgy, 199425(1): 132 ~ 136
[17] CI Dapeng, ZHOU Yahui, ZHANG Jia. Chemistry World, 1998(9): 475 ~ 478
[18] Li Qiyun, Pang Kaiqi. Shenyang Chemical Industry, 1993(2): 1 ~ 2
[19] Chen L. Research on the synthesis method of vanillin: [Dissertation]. Beijing: Beijing Institute of Technology, 1994
[20] sChouteeten Alair, Christidis yani. EP, 0023459. 1980
[21] Junkuro Umemura, Nagamine Takamitsu, et al. JP Showa 54-61142 [22] Junro Umemura, Taishi Shiraishi, et al. JP Showa 57-112346 [23] Junro Umemura, Fumio Iwata, et al. JP Showa 55-122731


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






