How to Test Vanillin in Food?
Abstract: Vanillin and ethyl vanillin can bring unique milk flavor to food and have been used in food and beverage, among which they are more used in dairy products, bakery products, beverages and edible oils. However, the long-term intake of vanillin and ethyl vanillin may cause dizziness, nausea and other adverse reactions, which are hazardous to human health. Therefore, it is necessary to analyze the content of vanillin and ethyl-vanillin in food and regulate them.
This paper reviews the methods for the determination of vanillin and ethyl vanillin in food in recent years, such as liquid chromatography, liquid chromatography-tandem mass spectrometry, gas chromatography, gas chromatography-tandem mass spectrometry, etc. It compares the characteristics and differences of different methods, with a view to providing theoretical bases for the future development of simple, sensitive and fast detection methods for the determination of vanillin and ethyl vanillin, and it has an important significance to the supervision of the food industry. It is of great significance for the supervision of food industry.
Vanillin is a naturally occurring natural flavor with the scientific name of 4-hydroxy-3-methoxybenzaldehyde (Figure 1A), also known as vanillin and vanillin [1], which can be added to food products to give them a distinctive milky flavor and thus enhance appetite. Ethyl vanillin is a synthetic flavor named 3-ethoxy-4-hydroxybenzaldehyde (Figure 1B), which is 3-4 times more aromatic than vanillin and has a longer lasting aroma.

With the development and progress of science and technology, the production of vanillin and ethyl vanillin has been increasing year by year, and they have been widely used in food, beverage, spice and pharmaceutical industries, etc. However, the accumulation of "quantity" will eventually lead to "quality" changes. As food additives, vanillin and ethyl-vanillin are permitted to be used reasonably under the regulations of international organizations and some countries. However, the accumulation of "quantity" will eventually lead to "quality" changes, and the overuse or overconsumption of vanillin and ethyl-vanillin will affect the health of human beings. Health. Experimental studies have found that the use of vanillin and ethyl-vanillin in large doses can lead to dizziness, nausea, vomiting, respiratory difficulties, and even damage to liver and kidney functions, which is very harmful to human body [2-5].
Relevant experiments have shown that the non-harmful dose of vanillin is 500 mg per kg body weight, and according to the current China National Standard for Food Additives Use in Food Safety (GB2760-2014), the maximum use level of vanillin in larger infant and toddler formulas is 5 mg/100 mL, and that in cereal supplementary foods for infants and toddlers is 7 mg/100 g [6]. The maximum use level of vanillin in cereal-based food supplements for infants and young children is 7 mg/100 g [6]. In recent years, incidents of "flavored milk powder" and "essential oils" have aroused widespread public concern, and therefore, the use of vanillin and ethyl-vanillin in food must be strictly regulated, and the use of vanillin and ethyl-vanillin in dairy products, bakery products, beverages, and edible oils is the most important food products that need to be monitored for the use of vanillin and ethyl-vanillin. Dairy products, bakery products, beverages and edible oils are the main focuses for the monitoring of vanillin and ethyl-vanillin levels.
The methods for the determination of vanillin and ethyl-vanillin in foodstuffs have been reported in the literature, including high performance liquid chromatography (HPLC), high performance liquid-tandem mass spectrometry (HPLC-MS/MS), spectrophotometry, electrochemistry, gas chromatography, gas chromatography-tandem mass spectrometry (GC-MS/MS), capillary electrophoresis, etc. [7-15]. In this paper, the methods for the determination of vanillin and ethyl-vanillin in foods in recent years are summarized, and the advantages and disadvantages of different methods are compared, so as to provide references for the future development of easy-to-operate, widely applicable and sensitive methods for the determination of vanillin and provide theoretical support for the supervision of food safety.
1 Detection methods
1.1 High performance liquid chromatography
The principle of high performance liquid chromatography (HPLC) is that the sample is extracted, separated by liquid chromatography and detected by a detector, commonly used ultraviolet detector, diode array detector, etc. The quantification of vanillin and ethyl-vanillin is usually done by external standard method[7] . The advantages of high performance liquid chromatography (HPLC) are rapid and high efficiency, and it is suitable for the determination of vanillin and ethyl-vanillin in foods or beverages with simple matrices.
Shanshan Xiao et al. [16] developed a method for the determination of vanillin and ethyl vanillin in liquid soft drinks by high performance liquid chromatography (HPLC) through optimizing the extraction method and mobile phases. The method was simple and rapid, and the average recoveries of vanillin and ethyl vanillin were 96.7%~100.4%, with relative standard deviations (RSDs) ranging from 3.53% to 4.70%, and the limits of detection (LODs) were both 0.5mg-kg-1 . The limits of detection were 0.5mg-kg-1. For samples with complex matrix, the high performance liquid chromatography (HPLC) method may have poor separation effect and strong interference, which requires the exploration of suitable purification methods.
In the determination of vanillin and ethyl-vanillin in powdered formulae for infants and young children, Tao Bohua et al. [17] used zinc acetate to precipitate the egg white, and then cleaned up the samples with MAX-SPE columns, which removed most of the impurities in the samples, and greatly reduced the interference of the substrate in the process of the determination. However, the method recoveries of vanillin and ethyl vanillin were low, ranging from 82.1% to 91.2%, which may be related to the large number of sample pretreatment steps.
Studies have shown that the pretreatment method for protein precipitation may cause partial adsorption of the components to be measured, which may affect the recovery, and it is necessary to adjust the amount of protein precipitant used according to the actual sample volume[18] . Chen Jing et al.[19] compared n-hexane and HLB solid-phase extraction columns for the determination of vanillin and ethyl-vanillin in milk and dairy products, and finally chose n-hexane for the defatted cleanup and established a reversed-phase high performance liquid chromatographic (HPLC) method with a limit of quantification (LOQ) of 0.2 mg kg-1 and a limit of detection (LOD) of 0.06 mg kg-1 . It can be seen that for the determination of vanillin and ethyl-vanillin by high performance liquid chromatography (HPLC), it is necessary to choose suitable extraction and purification methods for different sample matrices to ensure the accuracy of the determination.
1.2 Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
High performance liquid chromatography-tandem mass spectrometry (HPLC-MS) is a liquid chromatographic separation followed by mass spectrometry as a detector for qualitative and quantitative analysis[20] . HPLC-MS is characterized by high sensitivity, short analysis time, high selectivity and high qualitative accuracy, and has been widely used in food safety testing. Yang Huamei et al.[4] used ultra performance liquid chromatography-electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) to determine the levels of vanillin and ethyl-vanillin in samples such as milk, ham, melon seeds and rice, and the detected levels of vanillin and ethyl-vanillin were in the ranges of 0.082-53 mg-kg-1 and 0.10-6.8 mg-kg-1 , respectively.
The method is based on electrospray ionization with positive ion multiple reaction monitoring (MRM) mode for mass spectrometry, and the recoveries were in the range of 75.8%~116% with the relative standard deviations (RSDs) of 1.58~4.01. The limits of detection for vanillin and ethyl-vanillin were 0.025 mg kg-1 and 0.015 mg kg-1, respectively, and the injection and analysis time for each needle was 6 min. The experimental data showed that the method has a wide detection range, fast analytical speed, sensitivity and accuracy. Tao Bohua et al.[17] used liquid chromatography and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to separate and analyze powdered infant formula samples, and compared the detection limit and detection time of the two methods, which showed that although the detection limit of the LC-MS method was 5-10 times higher than that of the LC-MS method, the detection time of the LC-MS method was about 40% shorter.
In the determination of vanillin and ethyl-vanillin in vegetable oils by liquid chromatography-tandem mass spectrometry (LC-MS/MS), Liu Qiangxin et al. [21] used EMR-Lipid purification to reduce the interference of lipids in the samples, and the limit of detection (LOD) of this method was significantly lower than that of the national standard method, BJS 201705, "Determination of Vanillin, Methyl-vanillin and Ethyl-vanillin in Foods". It can be seen that the method of liquid chromatography-tandem mass spectrometry (LC-MS/MS) can meet the requirements of rapid qualitative and quantitative analysis of vanillin and ethyl-vanillin in most of the foods.
1.3 Gas Chromatography
Gas chromatography (GC) is a chromatographic separation and analysis method using gas as the mobile phase, which is characterized by simplicity, stability, high sensitivity and high selectivity, and is suitable for the quantitative and qualitative analysis of highly volatile and thermally stable organic compounds[22] . Meng Qingshun et al.[23] determined the contents of vanillin and ethyl-vanillin in rice flour by gas chromatography internal standard method, and the results showed that the detection limits of vanillin and ethyl-vanillin were 2.2 mg kg-1, and the average recoveries of vanillin and ethyl-vanillin were 92.64%~98.62% and 92.56%~99.36%, with the RSDs of 2.21% and 2.48%, which satisfied the national standard GB/T274-1, and met the standard of GB/T274-2, with the RSDs of 2.21 and 2.48%, respectively. The average recoveries of spiked vanillin and ethyl vanillin were 2.21% and 2.48%, respectively, which meet the requirements of the national standard GB/T 27417-2017 "Guidelines for Confirmation and Verification of Conformity Assessment Chemical Analysis Methods".
Nie Kun [24] established a gas chromatographic method for the determination of vanillin and ethyl-vanillin in coconut water. The diatomaceous earth solid-phase extraction method precipitates the proteins in the samples, which simplifies the pre-treatment process and ensures a better purification effect, and it is suitable for the determination of batch samples, and the average recoveries of vanillin and ethyl-vanillin in the method ranged from 95.3% to 110.5%, with the RSDs ranging from 1.2% to 2.5%, and the limits of detection (LOD) were 0.2~0.5 mg -kg-1, which can meet the daily detection of vanillin and ethyl-vanillin in coconut water. The average recoveries of vanillin and ethyl-vanillin were 95.3%~110.5% with RSD% of 1.2~2.5, and the limits of detection were 0.2~0.5 mg kg-1 , which can satisfy the daily detection of vanillin and ethyl-vanillin in coconut milk. Shan Zhichu et al.[25] determined the vanillin content in yellow wine by gas chromatography. After removing the ethanol in yellow wine by nitrogen blowing, the scholars extracted the vanillin in the sample with ether as the extraction solvent, and the average recoveries of the method ranged from 99.37% to 103.32%, the RSDs ranged from 2.22 to 3.67, and the limit of detection was 0.2 mg-L-1 .
1.4 Gas Chromatography-Tandem Mass Spectrometry (GC-MS)
Gas chromatography-tandem mass spectrometry (GC-MS/MS) has been widely used in the analysis of food samples with complex matrices and trace target components due to its advantages of high sensitivity and high immunity to interference[26,27] . Xu et al.[28] determined vanillin and ethyl-vanillin in commercially available solid and liquid milk teas by gas chromatography-mass spectrometry (GC-MS), and extracted the target compounds by headspace solid-phase microextraction (HS-SPME) based on the chemical structure and volatility of vanillin and ethyl-vanillin, and quantified them by using 3,4-methylenedioxyacetophenone (3,4-methylenedioxyphenyl ketone) as an internal standard.
The results showed that the linearity of vanillin (R2 = 0.9974) and ethyl-vanillin (R2 = 0.9987) was good in the detection range of 0.5-25.0 µg. The limits of detection for vanillin and ethyl-vanillin were lower than those for solid matrix (0.5 mg kg-1 and 0.3 mg kg-1) in liquid samples (0.05 mg kg-1 and 0.03 mg kg-1). The average recoveries of vanillin and ethyl vanillin ranged from 88.99% to 105.15% with the RSDs ranging from 2.35 to 9.26. This method is simple, accurate and reliable, and no organic solvent is needed in the extraction process, which is basically harmless to the environment and operators, and it is suitable for the determination of vanillin and ethyl vanillin in commercially available milk tea. Gao Haiyan [29] used a gas chromatograph to determine vanillin in cow's milk in multiple reaction monitoring (MRM) mode, and the linear range was 0.1-4.0 mg-L-1 with good linearity (R2=0.9998), and the spiked concentration was 0.2 mg kg-1 with the recoveries ranging from 79.1% to 86.2% and the RSD% of 3.3, and the limit of quantification (LOQ) was 0.02 mg kg-1 . The limit of quantification was 0.02 mg -kg-1 .
Peng Feijin et al. [30] used GC and GC-MS for the determination of vanillin and ethyl-vanillin in beverages, and the experimental results showed that the detection results of GC and GC-MS were similar in the same sample, but the detection limit of GC-MS was 4 times lower than that of GC (2 mg kg-1), which was convenient for the determination of samples at low concentrations and for the detection of false-positive samples. In the determination of vanillin in milk powder by gas chromatography-mass spectrometry (GC-MS) developed by Zhang Jianhui et al. [31], a new extraction and clean-up method of liquid-liquid extraction (LLE)-acceptance phase solidification (APSC) and back-extraction (BEX) was designed to simplify the operation procedure and improve the accuracy and sensitivity of the method. The linear range was 0.2~10.0 µg-mL-1 with good linearity (R2=0.9995) and the limit of detection was 0.004 mg-kg-1 (S/N = 3).
Compared with GC, GC-MS greatly reduces matrix interference, improves sensitivity and accuracy, and at the same time provides richer information about the target, which makes the characterization more accurate and more suitable for the determination of samples at micro-, trace- or lower concentration levels, and better meets the requirements of the national standards on the limits of vanillin and ethyl-vanillin.
1.5 Electrochemical methods
Electrochemical methods are analytical methods based on the relationship between the concentration of the substance to be measured and the conductivity or potential or current of a chemical cell[32] . Depending on the electrical parameters to be measured, electrochemical methods can be categorized into voltammetry, electrolysis, conductivity and potential analysis. Electrochemical methods are widely used in food quality testing because of their wide measurement range, simple instrumentation, low cost, high sensitivity and accuracy.
Wang et al. [33] used a Nafion-graphene-modified electrode for the determination of ethyl vanillin in chocolate by differential pulsed dissolution voltammetry (DPSV). An anodic dissolution peak was obtained by enriching the chocolate sample solution with stirring for 60 s in a B-R buffer solution of pH 1.98 at 0.83 V. The peak current was linearly related to the mass concentration of the sample (R2=0.9995). The peak current of the peak was linear with the mass concentration of the sample (R2=0.9995) in the range of 2.4-8.2 µg-mL-1 , and the RSD% of the results was 1.60 (n=15). The detection limit of ethyl vanillin was 0.076 µg-mL-1 and the spiked recoveries were 98.60%-101.38%. Si et al. [34] developed a graphene/cobalt tetraoxide (Co3O4/GR/GCE) electrochemical sensor for the determination of vanillin in cookies, and the average recoveries of the spiked samples were 102.4% with an RSD of 1.47%.
It was found that the oxidation current value (Ip) of vanillin increased with the increase of its concentration, and the electrode showed a good linear response in the concentration range of 0.1-80 µmol-L-1, with the linear equation of Ip(µmol-L-1)=0.1518C+0.5103 (R2=0.997), and the method limit of detection was 0.033 µmol-L-1 (S/N=3). L-1 (S/N=3). Because of the correlation between the electrical signals measured by the sensor and the concentration of vanillin, the method can realize the qualitative and quantitative analysis of vanillin in samples. Lv Yu et al. [35] found that the glassy carbon electrode pretreated with electrochemical oxidation had a good electrochemical response to vanillin, and there were two pairs of redox peaks. Therefore, cyclic voltammetry and linear scanning voltammetry were used to determine the content of vanillin in chocolate, and the concentration of vanillin showed a good linearity with the oxidation peak currents within the range of 1.0 × 10-6-2.0 × 10-4 mol-L-1 , with the limit of detection (LOD) of 6.7 × 10-7 mol-L-1 . The limit of detection was 6.7 × 10-7 mol-L-1 (S/N=3).
Compared with chromatographic methods, electrochemical methods have certain advantages, such as high sensitivity, good stability, no need for tedious pre-treatment, and low cost of instrumentation, which are promising for the application in the detection of real samples. However, these methods have some limitations, such as the short sensor life, frequent replacement, and the tedious process of electrode modification.
1.6 Capillary electrophoresis
Capillary electrophoresis (CE), also known as high-efficiency capillary electrophoresis (HECE), is an electrophoretic technique in which charged particles are separated efficiently and rapidly by their fullness or distribution coefficients under the action of an applied electric field with the capillary tube as the separation channel[36] . Capillary electrophoresis has higher resolution and lower solvent consumption than HPLC, and requires relatively low sample pretreatment, which is suitable for many food and drug products with complex matrices.
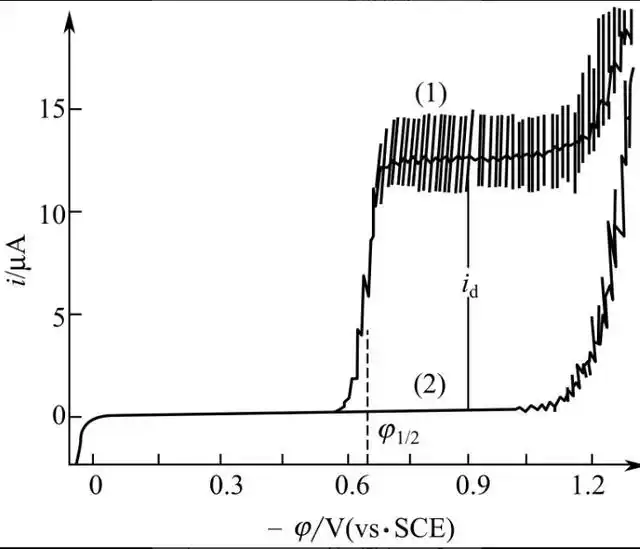
Zhao Jianfen et al [37] measured the content of vanillin in Great White Rabbit milk candy by capillary electrophoresis to be 0.0254 mg - g-1 , which was much lower than the maximum allowable use level of 200 mg - kg-1 in confectionery as stated in the GB2760-2014 Hygienic Standard for the Use of Food Additives. Xing Xiaoping [38] showed a good response of copper electrode to vanillin in 30 mmoL-L-1 of borax (pH = 9.24) running buffer with an applied separation voltage of 15 kV and an electrode potential of +0.65 V (vs. SCE). A good linear relationship was found for vanillin in the range of 5.0 × 10-6~1.0 × 10-3 g-mL-1 , and the detection limit was 3.87 × 10-7 g-mL-1 . The recoveries of the actual samples were 96.5%~108.2%, which were satisfactory.
Yang Guijun et al.[39] used a phosphoric acid-borax buffer system as the running buffer and a capillary zone electrophoresis instrument to detect vanillin in beverage, jelly and candied fruit samples, and the analysis could be completed within 8 min with one-shot injection. The linear range of the method was 2.5-1000 µg-mL-1 with good linearity (R2 range of 0.9986-0.9998), and the average recoveries of the spiked samples ranged from 85.2% to 100.3% with the RSD% ≤ 6.98 (n=5), and the limits of detection of the method were 0.25-10 µg-mL-1. Elbashir et al.[40] developed a rapid and simple capillary electrophoresis method for the determination of vanillin in beverage, jellies and candied fruit samples. Elbashir et al. [40] developed a rapid and simple capillary electrophoresis method for the simultaneous analysis of caffeine, vanillin and ethyl-vanillin in beverages, which was detected at 200 nm, and the three substances were well separated within 3 min. The linearity of the three analytes was good in the concentration range of 5 µg-mL-1 (R2 > 0.9986), and the limits of detection and quantification were 2.96 and 5.78 µg-mL-1 , respectively. The limits of detection (LOD) and limits of quantification (LOQ) were 2.96 and 5.78 µg-mL-1 , respectively, and the recoveries of the three analytes were in the range of 85%~108% (n=3).
1.7 Other detection methods
A review of the literature shows that in addition to the above six commonly used detection methods, the methods for vanillin and ethyl-vanillin in foodstuffs also involve ultraviolet spectrophotometry, surface-enhanced Ramanscattering (SERS), Fourier transform infrared spectrometer (FT-IR), and so on. spectrometry (SERS), Fourier transform infrared spectrometer (FT-IR), and so on.
The spectrophotometric method is mainly based on the fact that the substance to be measured has a certain absorbance value at or within a specific wavelength, for example, vanillin has the maximum absorption wavelength at 436.0 nm, which is characterized by simplicity and rapidity. According to the experimental results of Zhang Y et al. [41] on the determination of vanillin in cereals by UV spectrophotometry, the detection limit of the spectrophotometric method is higher, and it is more suitable for the samples with relatively high vanillin content.
In addition, due to the interference between vanillin and ethyl-vanillin, it is not possible to distinguish the two when using spectrophotometry, which is rarely used in food testing. Surface-enhanced Raman spectroscopy is characterized by easy portability, simple operation, no damage to the sample, low dosage and high sensitivity, and it can realize on-site rapid detection and provide the fingerprints of the target substances, which has a promising future for the application in the fields of food safety and medical and health care.
Wang Shi et al. [42] developed a rapid method for the determination of vanillin and beta-vanillin in larger infant milk powder by surface-enhanced Raman spectrometry. The qualitative method was based on the Raman shifts at 1149, 1497, and 1575 cm-1, and the quantitative method was based on the peak intensity of 1531 cm-1, and the peak intensity of vanillin at 1497 cm-1 was normalized. The linear range was 30~300 µg-mL-1 with R2= 0.9913 and the limit of detection was 10 µg-mL-1 . The results showed that the method is simple in sample processing, short in analytical time (about 5 min), and credible, which is suitable for the rapid on-site determination of vanillin in milk powder for older infants and young children.
Fourier transform infrared (FTIR) spectroscopy has the advantages of high resolution, good reproducibility and fast scanning speed, and has been applied to the qualitative and quantitative analysis of substances in different fields. Chen D et al. [43] utilized long-range FTIR spectroscopy to detect the doped vanillin in the milk powder system, which reduced the interference of the complex matrix of the milk powder on the analysis of vanillin, and realized the detection of vanillin with high sensitivity, and the method is expected to be a powerful tool for the rapid detection of vanillin in dairy products. This method is expected to be a powerful tool for the rapid detection of vanillin in dairy products.
In this paper, the limits of detection, recoveries, RSDs and time for single needle on-line analysis of vanillin and ethyl-vanillin in food were summarized in Table 1.
Table 1 Determination of vanillin and ethyl-vanillin in foods
Sample matrix |
Purification method |
Detection Methods | Method detection limit (mg- kg-1) | Recovery rate (%) | RSD (%) | Single-needle loading time (min) | References |
soft drink |
Modified QuEChERS |
HPLC-UV |
0.5 |
96.7 to 100.4 |
3.53~4.70 |
12 | [16] |
powdered infant formula | Oasis MAX Solid Phase Extraction Column Cleanup |
UPLC-UV |
0.1 |
84.0~93.3 |
5.2 to 6.2 |
10 | [17] |
soy milk | / | HPLC-UV | 0.04~0.18 | 63.39~88.73 | 0.9~4.5 | 42 | [18] |
dairy products | Hexane Degreasing Purification | HPLC-UV | 0.06 0.2 | 80.4 to 110.0 | 1.66~9.52 | 35 | [19] |
Milk, ham, melon Rice, rice, drinks. and pastries | STYRE- SCREENRSSH2P Solid Phase Extraction column cleanup | UPLC- MS/MS |
0.075~0.090 |
75.8~116 |
1.58~6.01 |
6 |
[4] |
vegetable oil | EMR-Lipid Purification | UPLC- MS/MS | 0.035~0.0376 | 72.8~91.8 | 1.6 to 8.6 | 8 | [21] |
vermicelli | / | GC | 2.2 | 92.56~99.36 | 2.21~2.48 | 30 | [23] |
coconut milk | Diatomaceous earth solid phase extraction column purification | GC | 0.2~0.5 | 95.3 to 110.5 | 1.2~2.5 | 25 | [24] |
"yellow wine" (mulled rice wine, usually served warm) | / | GC | 0.2 | 99.37~103.32 | 2.22 to 3.67 | 25 | [25] |
milk tea | / | GC /MS | 0.03~0.05 | 88.99~105.15 | 2.40~9.26 | 27 | [28] |
cow's milk | / | GC /MS | 0.02 | 79.1 to 86.2 | 3.3 | 60 | [29] |
drinks | Graphitized carbon black column purification | GC /MS | 0.5 | 81~110 | 2.4 to 5.6 | 16.5 | [30] |
infant formula | Acceptance Phase Curing - Reverse Extraction | GC /MS | 0.004 | 82.0~98.0 | 2.83~6.83 | 24 | [31] |
chocolates | / | electrochemical method | 0.076 µg- mL-1 | 98.60 to 101.38 | 1.6 | 1 to 2 | [33] |
chocolates | / | electrochemical method | 6.7 × 10-7 mol- L-1 | 99.3~102.1 | 3.2 | / | [34] |
cookies | / | electrochemical method | 0.033 µmol- L-1 | 100.8 to 103.8 | 1.47 | / | [35] |
toffee | / | CE | / | >95 | <2.8 | 20 | [37] |
chocolates | / | CE | 3.87 × 10-7 g - mL-1 | 96.5~108.2 | 1.7~2.8 | 10 | [38] |
Beverages, jellies, preserves | / | CE | 1 µg - mL-1 | 90.3~95.9 | 2.85~4.43 | 8 | [39] |
rolled oats | / | spectrophotometry | 2.2206 × 10-2 g - L-1 | 101.8 | 0.44 | / | [41] |
infant formula | / | SERS | 10 µg- mL-1 | 80.5~86.9 | <8.6 | 5 | [42] |
2 Conclusion
At present, the detection method of vanillin and ethyl-vanillin in foodstuffs is chromatography, which is more common. According to Table 1, it can be seen that the detection limits of ordinary chromatographic methods are relatively high, which can easily lead to the misjudgment of over-range added samples, and with the development of detection technology, liquid-liquid-mass spectrometry (LC-MS) and gas-mass spectrometry (GC-MS) have been widely used in foodstuffs analysis. The coupling technology utilizes the complementary functions of chromatography and mass spectrometry, combining the high separation ability of chromatography for complex samples with the advantages of MS in terms of high selectivity, high sensitivity and the ability to provide relative molecular mass and structural information, which significantly reduces the detection limit, and this type of detection method is not only technically mature, but also achieves accurate detection results. However, the sample pretreatment of chromatography is more complicated than that of electrochemistry and capillary electrophoresis, and most of the samples need to be purified, which may affect the accuracy of the results to a certain extent.
In addition, from the perspective of the analysis time of single injection samples, the analysis time of the liquid method was significantly lower than that of the gas phase method and the gas method, which shows that the liquid method has a certain advantage in the rapid and efficient determination of vanillin and ethyl-vanillin in foodstuffs.
The advantages of electrochemical detection methods include high sensitivity, good stability, and no need for tedious pre-treatment, etc. However, electrochemical sensors do not have a long life span and are frequently replaced, and the modification of the electrodes in the actual detection process is also tedious. Surface-enhanced Raman spectroscopy, with simple sample pre-treatment, has a wide range of applications in rapid, non-destructive and trace detection. In conclusion, different detection methods have different scope of application and their own advantages and disadvantages. When analyzing and detecting vanillin and ethyl-vanillin in food, the inspection agency can choose the appropriate detection method according to the actual experimental conditions and substrates.
References:
[1] Walton N J , Mayer M J , Narbad A . Vanillin[J]. Phytochemistry, 2003, 63(5):505-515.
[2] LIU Xiangping , HUANG Wei. Simultaneous determination of methyl vanillin and ethyl vanillin in foods by high performance liquid chromatography[J]. Chinese Journal of Preventive Medicine , 2006, 40(004):296-297.
[3] NI Y on g ni an , ZHANG Gu ow en , KOKO T S . Simultaneous spectrophotometric determination of maltol, ethyl maltol, vanillin and ethyl vanillin in foods by multivariate calibration and artifcial neuralnetworks[J]. Food Chemistry, 2005, 89: 465-473
[4] YANG Huamei , HANG Li. Simultaneous determination of four commonly used flavors in foods by ultra performance liquid chromatography-tandem mass spectrometry[J]. Chromatography , 2015, 33(3):250-255.
[5] Xuemei Sun , Qiang Xu , Xiaomeng Sun , et al. Simultaneous determination of vanillin and ethyl-vanillin in milky beverages by high performance liquid chromatography[J]. Journal of Food Safety and Quality Testing , 2020, 11(17):6023-6027.
[6] National Health and Family Planning Commission of the People's Republic of China, State Food and Drug Administration. National standard for food safety: GB 2760-2014 [S]. Beijing: China Standard Press , 2014.
[7] Richard, D, Thompson, et al. Determination of coumarin as an adulterant in vanilla flavoring products by high-performance liquid chromatography[J ]. Journal of Chromatography A, 1988, 438(1):369-382.
[8] He Jiang , Sheng Yueheng . Establishment of UPLC method for the determination of vanillin in milk powder[J]. Food Industry ,2018, 39(5): 327-330.
[9] Qu Baocheng , Dai Xuedong , Zhang Jingbo , et al. Determination of vanillin, methyl vanillin and ethyl vanillin in vegetable oils by high performance liquid chromatography tandem mass spectrometry[J]. Journal of Food Safety and Quality Testing , 2018,9(4): 935-941.
[10] Ou Jufang , Gu Xiuying , Bao Zhongding , et al. Determination of vanillin and ethyl-vanillin in infant formula by gas chromatography[J]. Jiangxi Science , 2011, 29(1): 23-25.
[11] ZHENG Xiaoyan. Determination of vanillin and ethyl vanillin in formula milk powder by gel permeation chromatography-gas chromatography-triple quadrupole mass spectrometry[J]. Fujian Analytical Testing , 2018, 27(4): 12-17.
[12] Feng Caiting, Yang Lixia, Li Shujing. Determination of vanillin in milk powder by ultraviolet-visible spectrophotometry[J]. Hebei Chemical Industry , 2012, 35( 6): 78-80.
[13] Lv Yuanqi , Sheng Yong , Zhou Lianwen , et al. Determination of vanillin, ferulic acid and vanillic acid by zonal capillary electrophoresis[J]. Chemical Engineer , 2005(10): 25-26.
[14] Jiang L, Ding Y P, Jiang F, et al. Electrodeposited nitrogendoped graphene/carbon nanotubes nanocomposite as enhancer for simultaneous and sensitive voltammetric determination of caffeine and vanillin[J]. Analytica Chimica Acta, 2014, 833(6):22-28.
[15] Yazana Z, Erden S, Dinc E. A comparative application of twoway and three-way analysis to threedimensional voltammetricdataset for the pKa determination of vanillin[J]. Journal of Electroanalytical Chemistry, 2018,826(8):133-141
[16] XIAO Shanshan , SUN Xingquan , LI Yichen ,et al. Determination of vanillin and ethyl vanillin in soft drinks by high performance liquid chromatography[J]. Journal of Food Safety and Quality Testing , 2015(1):152-158.
[17] TAO Bao-Hua , CHU Xiao-Jun , LAI Shi-Yun ,et al. Comparison of vanillin and ethyl vanillin in powdered infant formula[J]. Journal of Food Safety and Quality Testing , 2013(2):421-426.
[18] Chi Qiuchi, Li Xiaowen, He Jiajin, et al. Simultaneous determination of five flavorings, maltol, ethyl maltol, vanillin, methyl vanillin and ethyl vanillin in soymilk by high performance liquid chromatography[J] . Journal of Food Safety and Quality Testing , 2016(7):2690-2695.
[19] CHEN Jing , DUAN Guoxia , LIU Lijun ,et al. Rapid determination of four vanillin compounds in milk and dairy products by high performance liquid chromatography[J]. Dairy Science and Technology , 2020(23):19-24.
[20] WANG Zheng , ZHENG Juanmei , WANG Haibo ,et al. Simultaneous determination of 54 food additives in beverages by high performance liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety and Quality Testing , 2020, 11(6):1909-1919.
[21] LIU Qiangxin , ZHANG Hong , HU Yunqiang ,et al. Simultaneous determination of vanillin, methyl vanillin and ethyl vanillin in vegetable oils by modified QuEChERS-high performance liquid chromatography-tandem mass spectrometry[J]. Anhui Agricultural Science , 2020, 48(11):198-201.
[22] Cai Peidian , Bai Weidong , Qian Min. Advances in analytical techniques for milk flavors[J]. China Food Additives , 2010(3):180-184.
[23] MENG Qingshun , BU Yuanyuan , CHEN Changyi ,et al. Rapid determination of vanillin and ethyl vanillin in rice flour by gas chromatography[J]. China Food Additives , 2021(5):90-95.
[24] Nie Kun. Determination of four flavors in coconut milk by solid phase extraction column cleanup-gas chromatography[J]. Food Science and Technology , 2016(11):266-268.
[25] Shan Zhichu , Yu Hongbo , Shen Xiang , et al. Determination of vanillin in yellow wine by gas chromatography[J]. Brewing Science and Technology , 2015(9):125-127.
[26] COSTA BRB, PADILHA MC, RODRIGUES LML, et al. Analysis of anabolic agents in whey protein by gas chromatography coupled to triple quadrupole mass spectrometry [J]. Food Anal Method, 2020, 13(11): 2003-2013.
[27] Jager L , Perfetti G A , Diachenko G W. Comparison of headspace-SPME-GC-MS and LC-MS for the detection and quantification of coumarin, vanillin, and ethyl vanillin in vanilla extract products[J]. Food Chemistry, 2008, 107( 4):1701-1709.
[28] XU Xing , PENG Feijin , SHU Ping , et al. Determination of vanillin and ethyl vanillin in milk tea by headspace solid-phase microextraction coupled with gas chromatography[J]. Food Industry Science and Technology , 2016, 37(16):80-83.
[29] Gao Haiyan. Determination of vanillin in cow's milk by GC-QQQ headspace injection[J]. Agricultural Product Processing , 2016(9):43-45.
[30] PENG Feijin , XU Xing , SHU Ping , et al. Determination of vanillin and ethyl vanillin in beverages by GC and GC-MS[J]. Food Industry Science and Technology , 2015, 36(15):303-306.
[31] ZHANG Jianhui,LI Sha,HUANG Hui,XIA Lixin. Determination of vanillin in milk powder by liquid-liquid extraction-acceptance phase solidification-reverse extraction-gas chromatography/mass spectrometry[J]. Food and Machinery , 2015(2):98-101.
[32] DU Guitao , FAN Yunchang , DONG Xing , et al. Progress of electrochemical sensors in food analysis[J]. Materials Herald , 2015, 029(019):40-45.
[33] WANG Shumin , PENG Ji , TAN Haonan , et al. Determination of ethyl vanillin in chocolate by dissolution voltammetry with Nafion-graphene modified electrode[J]. Chemical Analytical Metrology , 2017(4):21-23.
[34] SI Xiaojing , HAN Jingting , ZHU Wenjing ,et al. Establishment of graphene/tricobalt tetraoxide electrochemical sensor and analysis of vanillin in cookies[J]. Food Industry Technology , 2021, 42(8):221-226.
[35] Lv Yu , Du Navy , Ji Shaofan , et al. Electrochemical behavior and voltammetric determination of vanillin at an electrically activated glassy carbon electrode[J]. Journal of Analytical Science , 2013, 29(4):543-546.
[36] Mu GF. Application of high efficiency capillary electrophoresis in food and pharmaceutical analysis [D]. Yantai: Yantai University , 2013.
[37] ZHAO Jian-Fen , WEI Shou-Lian , CHEN Jin-Ding. Separation and Determination of Vanillin, Vanillinol, Vanillic Acid and Ferulic Acid by Capillary Electrophoresis[J]. Food Science , 2012, 33(24):289-292.
[38] Xing Xiaoping , Cui Gang. Rapid determination of vanillin in chocolate by capillary electrophoresis with amperometric detection[J]. Food Industry Science and Technology , 2006, 27(10):186-188.
[39] YANG Guijun , GAO Wenhui. Determination of eight additives in food by capillary electrophoresis[J]. Food Science , 20107(10): 377-380.
[40] Elbashir A A , Elgorashe R , Alnajjar A O , et al. Capillary electrophoresis method for simultaneous analysis of caffeine, vanillin and ethyl vanillin in beverages[J]. Separation Science Plus, 2021, 4(6-7):1-7.
[41] ZHANG Yong , LIN Guowei , XIA Jinhua , et al. Determination of vanillin in cereals by visible spectrophotometry[J]. Food Science , 2010(4):233-235.
[42] WANG Shi , CHENG Jie , SU Xiaoou. Rapid detection of vanillin in larger infant formulae by surface-enhanced Raman spectroscopy[J]. Chinese Agricultural Science , 2014, 000(011):2224-2232.
[43] CHEN Da , ZOU Jian , TAN Brown , et al. A new method to detect vanillin in milk powder based on Fourier transform infrared spectroscopy[J]. Nanotechnology and Precision Engineering , 2017, 15(6):438-443.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






