What Is the Use of Beta Glucan in Aquaculture?
With the development of aquaculture, the quantity and quality of aquaculture products have also been continuously improved, and people's needs have gradually shifted from quantity to quality. To address the problems of antibiotic residues and drug resistance, research on alternatives to antibiotics has received widespread attention. The use of immunostimulants to improve the immune function of aquatic animals and enhance their body's defenses has become one of the important solutions to this problem. Among them, β-glucan is currently one of the most intensively studied immunostimulants.
After entering the bloodstream, β-glucan can bind to specific immune receptors, activate the innate immune response, enhance the body's immune and antioxidant functions, and thus improve growth performance [1]. In addition, the structural characteristics of β-glucan may be the key to its diverse biological activities [2]. β-Glucan has various biological functions such as growth regulation, immune regulation, anti-oxidation, improving the intestinal environment, lowering blood lipids and anti-stress. It has shown good application results in improving the production performance of aquatic animals and promoting their health. It has broad development and application prospects in the aquaculture industry. The author reviews the structure and main biological functions of β-glucan and its application in aquaculture production, with a view to providing a reference basis for the use of β-glucan as an additive in aquaculture.
1 Structural characteristics of β-glucan
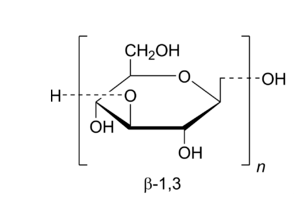
β-glucan is widely found in nature and is composed of D-glucose monomers linked by β-bonds. Its molecular formula is C18 H 32 O16 . Dextran can be divided into α and β types. α-Dextran is not biologically active, while β-dextran has a variety of biological activities. Beta-glucans are complex polysaccharides found in many natural products, such as oats, barley, yeast and algae [3-4]. Due to the different sources of beta-glucans, the structural characteristics and biological functions they exhibit also differ. Beta-glucans from different sources have different primary structures and conformations. The type of primary structure is determined by glycosidic bonds, branching and degree of polymerization. The conformation of beta-glucans usually appears as irregular curls, single helices or triple helices, and is affected by primary structure, intermolecular forces, temperature and solvents [5]. Cereal β-glucans are mainly linked by β-1,3 and β-1,4 glycosidic bonds[6]; microbial or algal β-glucans are mainly linked by β-1,3 and β-1,6 glycosidic bonds. The biological activity of β-glucans from different sources varies due to the different types and numbers of side chain residues[ 4] .
2 Biological functions of β-glucan
2.1 Immunomodulatory effects
Fish defend themselves against pathogen invasion via nonspecific immune pathways. β-Glucan enhances the body's immune response by increasing lysozyme activity, the number of phagocytes and activation of the complement pathway [7-8] . Yeast β-glucan can reduce the inflammatory response by down-regulating the expression of cytokines. A reduction in leukocyte infiltration in the lamina propria and submucosa can be observed under the light microscope [9]. β-Glucan can bind to several receptors on leukocytes and activate the innate immune system, thereby enhancing the immune response [10]. β-Glucan can also regulate macrophages and dendritic cells by activating macrophages and dendritic cells and natural killer cells. It can also bind to complement receptor 3 (CR3), C-type lectin receptor 1 ( Dectin-1) or Toll-like receptor (TLR), which induces B cells to produce immunoglobulin G (IgG), ultimately stimulating the body to produce an immune response [8].
Studies have found that the addition of β-glucan to the feed significantly increases the survival rate of Vibrio parahaemolyticus-infected Litopenaeus vannamei [11]; significantly improves the survival rate of Oncorhynchus mykiss infected with Aeromonas salmonicida survival rate and relieve stress response, and the best protective effect was observed when the addition amount was 0. 2% was the optimal protective effect [12]. Regla et al. [13] used intraperitoneal injection of carp virus (Spring viraemia of carp virus, SVCV) to infect zebrafish (Brachydanio rerio var), and found that feeding yeast β-glucan significantly up-regulated the expression of zebrafish non-specific immune-related genes IL-1 b, IL-6, IL-8, IL-10 and TNF-α, indicating that yeast β-glucan has a positive effect on zebrafish resistance to SVCV. Alvarez-Rodríguez et al. [14] also reached a similar conclusion in a study in which zebrafish were injected intraperitoneally with SVCV.
Krishnan et al. [15] showed that β-glucan can alleviate the pro-inflammatory response of macrophages in Epinephelus septemfasciatus, enhance the immune response of fish against nervous necrosis virus (NNV), and significantly improve the survival rate of infected fish. The addition of β-glucan to the feed significantly increased the expression levels of antibacterial factors such as Alf-1, lysozyme and crustin-1 in the swimming crab Portunus trituberculatus, improving its ability to resist ciliate infections [16]; the addition of yeast β-glucan can significantly increase the serum lysozyme activity and IgM, CR3 and CR4 levels in turbot (Scophthalmus maximus), and significantly reduce the gene expression levels of inflammatory cytokines in the lamina propria and submucosa [17]. lysozyme activity and IgM, CR3 and CR4 levels, and significantly reduced the gene expression levels of inflammatory cytokines in the lamina propria and submucosa[17] . Deltamethrin can cause down-regulation of the immune-related genes IL-1β and IL-8 in Nile tilapia, and up-regulation of the pro-inflammatory factors interferon γ (IFN-γ), heat shock protein 70 (HSP70), and caspase 3 (CASP3). expression levels were elevated, but supplementation with β-glucan could alleviate the stress response caused by deltamethrin, restoring the expression of antioxidant and immune genes to normal levels [17]. Adding yeast β-glucan to the feed can significantly increase the activity of acid phosphatase and alkaline phosphatase in the intestines of Pengze crucian carp, significantly enhancing the intestinal immune response [18]; increase the mRNA expression of TLR and HSP70 in the hepatopancreas and intestines of Vanamei shrimp, and reduce the expression of TNF-α and Dectin-3 [19].
It was found that feeding Caspian brown trout (Salmo trutta caspius) with a feed supplemented with brewer's yeast beta-glucan can significantly increase plasma lysozyme activity and IgM levels and reduce the expression of inflammation-related genes[10]; adding yeast beta-glucan to the feed of juvenile yellow sand turtles can significantly increase acid phosphatase and alkaline phosphatase activity, thereby enhancing nonspecific immune function [20]; adding 1. 0% β-glucan can significantly increase the expression levels of TNF-α and INF-γ in yellowfin sea bream (Tor putitora), but has no significant effect on the expression level of IL-10 [21]. IL-6 and Toll-like receptor 1 (Toll interleukin 1 receptor domain adaptor protein (TIRAP) mediates the JAK-STAT and TLR signaling pathways, which can regulate the body's immune response. Adding β-glucan to the feed can significantly increase the IL-6 and TIRAP gene expression levels in the pearl grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀), thereby enhancing the body's immune response [22]. expression levels, thereby enhancing the body's immune response [22].
Copper ions can cause a significant upregulation of pro-inflammatory factors such as TNF-α, IL-1β and IL-6 in IgM+ B cells. Beta-glucan can inhibit the pro-inflammatory response caused by copper ions. In beta-glucan-cultured IgM+ B cells, the expression levels of B-cell antigen receptor (BCR) complex-related proteins alpha chain and β chain (CD79a and CD79b) were significantly increased, indicating that β-glucan can also regulate immunity through the BCR protein in IgM+ B cells [23]. Studies have found that moderate amounts of β-1,3-glucan in the feed can increase the phagocytic activity of the blood cells of the red swamp crayfish (Cherax quadricarinatus). The timing and dosage of the addition are key factors affecting the immune response of the body [24]; the immune response induced by intraperitoneal injection of β-glucan in zebrafish is very short, generally 2-14 days [13]. K Koch et al. [25] added β-glucan to the feed of tilapia for 15 to 45 days, which increased the non-specific immune response and disease resistance of tilapia, and did not cause immunosuppression. The specific reasons for this need to be further explored.
2.2 Regulation of lipid metabolism
Beta-glucan can reduce the concentration of glucose, total cholesterol and triglycerides in the blood[26]. There are two possible mechanisms by which beta-glucan affects lipid metabolism in the body: (1) the intestinal flora ferments the polysaccharide to produce short-chain fatty acids, which are captured by the portal vein and transported to the liver, thereby reducing lipid production; (2) beta-glucan binds to bile acids in the intestine, causing an increase in intestinal viscosity and thereby interfering with lipid absorption [27]. Lopes et al. [28] added brewer's yeast beta-glucan to the feed of juvenile fat-belly tilapia (Piaractus mesopotamicus), which reduced plasma triglycerides and lipid levels in the liver, muscles and viscera.
Zhao et al. [29] added different amounts of β-1,3-glucan to the feed of Vanamei shrimp and found that an addition of 250 mg/kg significantly increased serum cholesterol levels. Yang et al. [30] found that yeast β-glucan can significantly reduce the concentrations of serum triglycerides, total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol in grass carp (Ctenopharyngodon idella) when added to the basic feed of grass carp. . Cao et al. [18] conducted a 70-day experiment by adding yeast β-glucan to the feed of Pengze crucian carp (Carassius auratus var. Pengze). The results showed that the addition of yeast β-glucan can reduce total cholesterol and high- and low-density lipoprotein levels. However, other studies have found that the addition of β-glucan has no significant effect on cholesterol. For example, feeding β-1,3-glucan to the basal diet of Japanese sea bass (Lateolabrax japonicus) for 60 days had no significant effect on serum cholesterol in Japanese sea bass [31]; and the addition of β-glucan to the water had no significant effect on the serum cholesterol concentration of Nile tilapia (Oreochromis niloticus) [32]. non-fish (Oreochromis niloticus) had no significant effect on serum cholesterol concentration [32]. Given the differences in the effects of β-glucan from different sources on lipid metabolism in aquatic animals, and the relatively few studies on the metabolic mechanisms of β-glucan in aquatic animals, it is necessary to continue to conduct relevant research to reveal the mechanism by which β-glucan affects lipid metabolism in aquatic animals.
2.3 Antioxidant effect
Under normal circumstances, free radicals are a by-product of metabolism, and a dynamic balance between production and clearance exists. This dynamic balance mainly depends on the antioxidant system. If the level of a certain type of free radical exceeds the limit, the body's homeostasis will be disrupted, and excessive inflammation will deplete antioxidants and lead to oxidative damage [23]. Studies have shown that β-glucan can enhance the body's antioxidant capacity by increasing the activity of antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD), as well as the scavenging activity of free radicals and superoxide anions. In addition, β-glucan can enhance the body's antioxidant capacity by activating the Nrf2 signaling pathway and upregulating the expression of antioxidant genes [33-34]. Yang et al. [30] found that the addition of yeast β-glucan to low-fishmeal feed and feeding grass carp resulted in high levels of antioxidant parameters in serum and testicular tissue, confirming that yeast β-glucan can produce antioxidant properties. Nr f2-Keap1 signaling pathway plays a critical role in regulating antioxidant responses, and yeast β-glucan can activate the Nrf2 signaling pathway and upregulate the expression of antioxidant genes to enhance antioxidant responses.
With the increasing amount of β-glucan added to the feed, the malondialdehyde (MDA) content in the liver of young Atlantic salmon gradually decreased, while the activity levels of GSH, GSH-Px and SOD showed an upward trend before decreasing, indicating that adding an appropriate amount of β-glucan to the feed can increase the liver antioxidant capacity of young Atlantic salmon, but excessive the antioxidant capacity showed a downward trend [35]. Wang Yonghong et al. [36] injected 0.1 mL of β-glucan at a concentration of 0 or 5 mg/kg body weight into the abdominal cavity of juvenile yellow croaker (Larimichthys crocea). 1mL, under normal oxygenation conditions, β-glucan had no effect on the MDA content of the liver of juvenile big yellow croaker, but under hypoxic stress, β-glucan could significantly increase the expression levels of Cu/Zn-SOD, Mon-SOD, CAT, GRx1b and Nrf2 and the activity of antioxidant enzymes, and reduce the MDA content, thereby reducing the oxidative damage caused by hypoxic stress on juvenile yellow croaker.
Zhu et al. [37] found that the addition of β-1,3-glucan to the feed significantly enhanced the activities of phenoloxidase (PO), SOD and GSH-Px, and reduced the MDA content in mudskippers, indicating that the addition of β-1,3-glucan significantly improved the antioxidant capacity of mudskippers. β-Glucan can enhance the antioxidant capacity of Nile tilapia by reducing the subacute deltamethrin stress. Nile tilapia's antioxidant capacity can be enhanced by β-glucan, which can reduce the expression levels of HSP70 and CASP3 genes [17]. In summary, β-glucan can enhance the antioxidant capacity of animals, but excessive addition may weaken it. Therefore, the amount of β-glucan added should be adjusted in production practice according to the different growth stages of different animal bodies or different breeding environments, so as to avoid negative effects on animal growth and economic losses.
2.4 Anti-stress effect
Environmental stress can often be fatal for aquatic animals. The immunostimulant β-glucan can improve the ability of fish to resist harsh environments [38]. Blood levels of cortisol and glucose can reflect the health status of fish in response to environmental stress, and the reduction in blood cortisol and glucose levels in Nile tilapia is related to the ability of β-glucan to enhance their stress resistance [39]. Using subacute deltamethrin stress Nile tilapia for 15 and 30 days, it was found that Nile tilapia fed β-glucan had significantly lower blood cortisol and glucose levels, while the control group had significantly higher blood cortisol and glucose levels [17]. Souza et al. [32] found that the administration of 0.1 mg/L β-glucan in water could enhance the tolerance of tilapia to hypoxia and increase survival rates, while also regulating blood glucose levels.
HSP70 and HSP90α are important indicators for detecting heat stress. When 1% β-glucan and 0.05% heat-killed lactic acid bacteria (HK-LP) were added to the feed of mud loaches, it was found that the expression levels of HSP70 and HSP90α in the liver were significantly increased [40]. Zhao Hong 0.05% heat-killed lactic acid bacteria (HK-LP) was found to significantly increase the expression levels of HSP70 and HSP90α in the liver [40]. Zhao Hongxia et al. [41] fed 0. 1% β-1,3-glucan was fed at 14-day intervals, which may improve the resistance of Vanamei shrimp to nitrite . Wu Chunyu et al. [31] used feed with different β-glucan content of brewer's yeast to feed flower bass for 60 days, and then used ammonium chloride to conduct an ammonia nitrogen stress test. The results showed that the mortality rate of the 400 and 600 mg/kg brewer's yeast β-glucan addition groups was significantly lower than that of the no addition group. A combination of parabola and broken line models was used to obtain 41 5.10 mg/kg was the appropriate amount to add, indicating that the addition of brewer's yeast β-glucan to the feed can alleviate the damage caused by ammonia nitrogen stress to the flower bass and improve its ability to resist ammonia nitrogen stress. Therefore, β-glucan, as a feed additive, can alleviate the stress response of different fish and shrimp under specific conditions.
3 Application of β-glucan in aquaculture
3.1 Effect on growth performance of aquatic animals
Studies have shown that adding β-glucan to the feed can promote the growth of aquatic animals. Changes in growth performance are closely related to changes in digestive enzymes. The activity of digestive enzymes directly reflects the degree of feed digestion and utilization. Higher activity indicates that the feed is more fully utilized and is more beneficial to animal growth [19]. Wang Wanliang et al. [35] found that feeding young Atlantic salmon (Salmo trutta) with feed containing different amounts of β-glucan in a circular glass tank at a water temperature of 13–14°C had a positive effect on growth. However, as the amount of β-glucan added gradually increased, the specific growth rate and digestive enzyme activity of the young Atlantic salmon first increased and then decreased, indicating that the effect of β-glucan on the growth performance of young Yalong salmon may be related to the amount added, and the strength of digestive enzyme activity may be the main factor affecting the growth performance of young Yalong salmon. The addition of 0.2% to 0.4% β-glucan to the feed can significantly increase the protease and amylase activities in the intestine of Vanamei shrimp, and the growth performance is significantly improved. It is inferred that the improvement of the growth performance of Vanamei shrimp may be closely related to the increase in digestive enzyme activity [19].
Zhao et al. [29] found that in a long-term culture experiment with Penaeus vannamei for 84 days, a low dose of β-glucan (250 mg/kg) may promote growth performance mainly as an energy source, while a high dose of β-glucan (500 mg/kg) can stimulate the immune response. Divya et al. [42] added Saccharomyces cerevisiae ) β-glucan significantly increased the final body weight and improved the growth performance of Mozambique tilapia (Oreochromis mossambicus) cultured under ammonia stress. The reason may be that β-glucan enhances the cellular immunity, humoral immunity and antioxidant response of Mozambique tilapia, reduces the consumption of nutrients for immune response, and thus obtains more nutrients to supply the needs of growth [43 -44] . Rainbow trout cultured in concrete pools at 11–13 ℃ and fed a diet supplemented with an appropriate amount of β-glucan (a mixture of β-1,3-glucan and β-1,6-glucan) can significantly increase their weight gain rate, specific growth rate, and increase the plumpness of the fish body [4 5-46]. β-glucan can promote the metabolism of nutrients such as fat and protein by altering the intestinal flora of loach (Misgurnus anguillicaudatus), thereby improving the absorption of nutrients and promoting animal growth [40].

However, some studies have shown that β-glucan has no promoting effect on the growth of aquatic animals. Liang Zhi-ling et al. [20] added yeast β-glucan to the feed of juvenile yellow-snouted soft-shelled turtles (Truogx sinensis), and found that it had no significant effect on the tissues, organs or growth. Gu et al. [9] fed turbot containing yeast β-glucan, and found that it had no significant effect on growth performance or feed utilization. Li Yong-juan et al. [47] fed yellow Pelteobagrus fulvidraco juveniles did not show a significant growth-promoting effect. The reason may be that feeding β-glucan causes the aquatic animal's body to be in a state of long-term immune activation, and the body redistributes nutrients, with the final result being no significant effect on the growth of aquatic animals [35]. Therefore, the application of β-glucan in aquaculture production should take into account various factors such as its source, the amount added, the type of aquatic animal and the breeding environment.
3.2 Effect on intestinal health of aquatic animals
The intestinal microecology of fish is susceptible to external environmental changes, invasion of harmful bacteria and other factors, which can lead to disruption of the intestinal homeostasis and affect the health of the fish. Adding an appropriate amount of β-glucan to the feed can improve the activity of digestive enzymes, enhance the digestive and absorptive capacity of the intestine, and improve feed utilization efficiency [48]. Studies have found that adding an appropriate amount of β-1,3-glucan to the feed can significantly increase the activity of the loach's intestinal digestive enzymes, but high doses of β-1,3-glucan do not continue to increase digestive enzyme activity [37]; feeding young Yalong salmon with β-glucan-containing feed can increase the activity of intestinal digestive enzymes and enhance the intestinal digestion capacity [35]; ; feeding Penzer crucian carp yeast β-glucan feed was found that, compared with the control group, the addition of yeast β-glucan can significantly increase the height of intestinal folds and microvilli, increase the activity of amylase and trypsin, and significantly improve intestinal digestion [18]; adding 0.02% yeast β-glucan to the feed can significantly increase the abundance of Bacillus bacillus and Bacillus subtilis in the intestine, significantly increasing the activity of intestinal amylase and protease.
This may be related to the ability of Bacillus to produce protease, amylase and lipase in the intestine. Adding 0. 0.04% β-glucan significantly increased the abundance of Bacillus and Chitinibacterium, while the abundance of Citrobacter, Microbacterium and Mycobacterium was significantly reduced [19]; feeding rainbow trout with β-1,3-glucan significantly increased the number of lactobacilli in the intestine, improving intestinal health [45]. Souza et al. [32] applied β-glucan to the culture water of Nile tilapia, and found that β-glucan could significantly increase the abundance of the phyla Firmicutes, Fusobacteria and Proteobacteria under hypoxic stress.
Substituting fish meal with soybean meal can have a negative impact on the intestines of Atlantic salmon (Salmo salar) and sockeye salmon (Oncorhynchus tshawytscha). After feeding on a diet supplemented with yeast β-glucan, the height of the intestinal mucosal folds of turbot was significantly increased, the fusion of mucosal folds was reduced, and decreased width of the lamina propria and leukocyte infiltration [9, 49]; β-glucan alleviates the negative effects of enteritis on aquatic animals by promoting the expression of neurotransmitters such as acetylcholinesterase, serotonin, and substance P, thereby reducing mechanical damage to the intestines [50]. Ji et al. [46] studied the effect of yeast β-glucan on intestinal inflammation induced by trinitrobenzenesulfonic acid (TNBS) in rainbow trout enteritis, and found that yeast β-glucan can reduce TNBS-induced enteritis, reduce the mortality rate of rainbow trout, and slow down the percentage of weight loss during rainbow trout enteritis. Therefore, in the future, experiments should be carried out on the effects of β-glucan on the intestinal health of different aquatic animals, and the dosage and addition cycle of β-glucan should be adjusted according to the intestinal absorption characteristics and the structure and abundance of the flora of different aquatic animals.

3.3 Effect of combined β-glucan supplementation on aquatic animals
Studies have shown that the combined use of multiple immune stimulants has a better immune effect than the addition of a single type of immune stimulant [51]. The combined addition of multiple substances is mainly based on the stability, adaptability, safety and synergistic effect of various ingredients to determine the type and ratio of added substances [52].
Miao et al. [53] studied the effect of β-glucan (Saccharomyces cerevisiae β-glucan and Torulopsis ferment lysate) in combination with a probiotic strain of Pediococcus acidilactici PA-GY2 isolated from the intestine of Macrobrachium rosenbergii (rosenberg shrimp). The combined use of PA-GY2 and brewer's yeast β-glucan can significantly improve the growth performance and lipase activity of Macrobrachium rosenbergii. The combined addition of PA-GY2, brewer's yeast β-glucan and yeast β-glucan significantly reduces the feed coefficient and improves growth performance. The combined use of PA-GY2 and brewer's yeast β-glucan or yeast β-glucan can increase immune expression and reduced mortality.
Jami et al. [10] added brewer's yeast IgM, lysozyme, complement and serum-substituted complement (ACH50) activity to the basal diet of Caspian brown trout to enhance non-specific immunity. The individual and combined addition of immune stimulants can reduce or increase the transcription of inflammation-related genes (TNF-α, IL-1β and IL-8). Brewer's yeast β-glucan glucan alone or in combination with Lactobacillus plantarum (Lp) significantly increased IL-8 mRNA expression, but the combination of β-glucan, mannan-oligosaccharide (MOS) and Lp significantly reduced IL-8 mRNA expression. Studies have found that MOS can improve growth performance by increasing body weight, feed conversion efficiency, improving intestinal structure and positively regulating intestinal flora[54]; Lp can promote the absorption of β-glucan, improve the activity of digestive tract enzymes, growth performance and feed utilization efficiency of aquatic animals, inhibit the adhesion and growth of pathogenic bacteria, improve the level of digestive tract enzymes, immunity, and enhance the host's disease resistance and survival rate[ 55-56].

The combined addition of β-glucan powder and MOS to the feed of carp (Cyprinus carpio) can significantly improve the growth rate, intestinal health and immunity of carp [57]; the combined addition of β-glucan and heat-inactivated lactobacillus (HK-LP) to the feed significantly reduces the feed coefficient and improves the growth performance of young loach, among which the feed The most significant effect was achieved by adding 1% β-glucan and 1% HK-LP to the feed[40] ; synbiotics (β-1,3-glucan combined with protein and Bacillus licheniformis) can improve growth performance by stimulating the secretion of extracellular enzymes by the intestinal flora of Mozambique fish and increasing intestinal absorption. It can also enhance antioxidant capacity by enhancing the activity of active oxygen and stimulating the release of antioxidant enzymes to enhance antioxidant capacity [58]; synbiotics (Saccharomyces cerevisiae, mannan-oligosaccharides and β-glucan) increased the survival rate and antioxidant capacity of Nile tilapia infected with Pseudomonas aeruginosa, possibly because the mannan-oligosaccharides in the synbiotics can attach to certain Gram bacteria, preventing infection and thus improving the fish's immunity and defence against infectious diseases [59].
3.4 The effects of different feeding methods of β-glucan on aquatic animals
Studies have shown that short-term ( <30d) feeding of β-glucan can enhance non-specific immunity, and long-term feeding of β-glucan may have immunosuppressive effects on aquatic animals [14, 60]. Li Fudong et al. [61] reported that in a temperature-controlled aquarium at (25±5) °C, continuous long-term (60d) feeding of feed supplemented with 0.15% and 0.50% β-glucan significantly reduced the lysozyme and acid phosphatase activities and survival rate of carp, with no significant effect on growth performance.
Koch et al. [25] found that feeding tilapia with feed supplemented with 1% β-glucan for 60 days in a temperature-controlled aquarium at 26 °C significantly promoted growth and enhanced immunity. It is recommended that long-term feeding with β-glucan be considered. Zhao Hongxia et al. [41] added 0. 1% β-1,3-glucan continuously or intermittently fed to Vanamei shrimp, the results showed that whether the interval or continuous feeding of β-1,3-glucan had no significant effect on the growth performance of Vanamei shrimp, but intermittent feeding significantly increased the activity of lipase in Vanamei shrimp, promoted the metabolism of energy and protein in shrimp, and 14-day intermittent feeding of β-1,3-glucan significantly reduced the nitrite nitrogen stress mortality of Vanamei shrimp. 3-glucan significantly reduced the mortality rate of Vannamei shrimp under nitrite nitrogen stress.

Li Yongjuan et al. [47] designed a basal feed group, added 200, 600 and 1000 mg/kg yeast β-glucan to three continuous feeding groups and three intermittent feeding groups to study the effect of yeast β-glucan on the physiological indicators of juvenile yellow croakers. The results showed that the The continuous feeding group had a significantly higher weight gain rate than the corresponding intermittent feeding group. In addition, neither intermittent nor continuous feeding had a significant effect on the serum biochemical indicators, anti-infection ability and growth performance of juvenile yellow croakers. In summary, whether intermittent feeding can have a positive effect on aquatic animals may be related to the source of β-glucan, the type of cultured animal, the culture cycle, the culture environment, the formula and the amount added, etc.
4 Summary
As the aquaculture industry develops, various problems in the breeding process have gradually become more prominent. Environmental pollution, the indiscriminate use of antibiotics, and fish infections have begun to threaten the health of aquatic animals and humans.
The immunostimulant β-glucan powder has been used in a variety of fields, and a large number of studies have proven its functions of promoting growth, enhancing immunity, resisting oxidation, and resisting stress. As a green additive in aquaculture, β-glucan is one of the aquafeed additives with the greatest development potential in the future. However, there are still many problems that need to be solved in its application, and research is still needed in the following areas: ① There are many species of aquaculture in China, and further research is needed to evaluate the appropriate dosage and timeliness of β-glucan in different aquatic animals. ② China is a vast country, and the appropriate requirements of aquatic animals in different breeding environments, breeding modes, breeding seasons, and breeding stages the appropriate requirements for β-glucan still need to be studied in detail; ③ the appropriate requirements for β-glucan should be assessed according to actual production conditions based on different evaluation criteria such as optimal growth performance, intestinal function, product quality, disease resistance and stress resistance; ④ when multiple additives are used in combination in feed, whether there is synergy or antagonism between β-glucan and other additives, as well as the impact of nutrient metabolism on the aquatic environment, still need to be studied in depth; ⑤ the signal pathways by which β-glucan affects the physiological functions and metabolic functions of aquatic animals still need to be further explored. reaction, as well as the impact of nutritional metabolism on the aquatic environment, etc., still require in-depth research; (5) the signal pathways by which β-glucan affects the physiological functions and metabolic functions of aquatic animals have yet to be further explored.
References
[ 1 ] XU B Y , SHAO Q J . Research progress in immune regulation effect of β-g lucans on fish[J] . Chinese Journal of Animal Nutrition , 2019 , 31(7) : 2971-2980. (in Chinese)
[ 2 ] ZHAO L Y , FANG L Y , JIANG L S , et al. Biological functions of β-g lucan and its application in ruminant feeding[J] . Chinese Journal of Animal Nutrition , 2020 , 32(5) :2003-2009. (in Chinese)
[ 3 ] MURPHY E J , REZOAGLI E , MAJOR I , et al. β-g lucan metabolic and immunomodulatory properties and potential for clinical application[J] . Journal of Fungi , 2020 , 6(4) :356.
[ 4 ] CHEN C F , CHEN X , CHEN C R , et al. Characteristics of β-g lucan and its regulation on animal immune function[J] . Journal of Huazhong Agricultural University , 2003 , 22(1) : 95-100. ( in Chinese)
[ 5 ] WANG Q , SHENG X , SHI A , et al. β-g lucans : Relationships between modification , conformation and functional activities[J] . Molecules , 2017 , 22(2) :257.
[ 6 ] JIN S. Research status of main functional components in oat[J] . China Science and Technology Information , 2010 , 9 :114-115.
[ 7 ] YI J H , YANG F , NIE Q , et al. Research progress of β-g lucan in yeast cell wall in aquaculture[J] . China Feed , 2016 , 14:41-44.
[ 8 ] LIU Y , WU Q , WU X , et al. Structure , preparation , modification , and bioactivities of β-g lucan and mannan from yeast cell wall : A review[J] . International Journal of Biological Macromolecules , 2021 , 173 :
445-456.
[ 9 ] GU M , PAN S , LI Q , et al. Evaluation and compare of yeast β-g lucan and carboxymethylg lucan to improve the immunity and gut health of turbot fed diet containing 400 g/kg of soybean meal[J] . Aquaculture Reports , 2021 , 21 :100882.
[10] JAMI M J , ABEDIAN K A , PAKNEJAD H , et al.Effects of dietary b-g lucan , mannan oligosaccharide , lactobacillus p lantarum and their combinations on g rowth p erformance , immunity and immune related gene expression of Caspian trout , Salmo trutta caspius ( Kessler , 1877 )[J] . Fish & Shellfish Immunology , 2019 , 91 :202-208.
[11] FELIX D M , JACINTO E C , CRDOVA AI C , et al. Physiolo gical and antioxidant response of Litopenaeus vannamei against Vibrio parahaemolyticus infection after feeding supplemented diets containing Dunaliella sp. flour and β-g lucans [J] . Journal of Invertebrate Pathology , 2022 , 187:107702.
[12] JI L Q , SUN G X , WANG Y , et al. Regulation of β- g lucan on stress process of rainbow trout infected withAeromonassalmonicense[J] . Journalof Fishery SciencesofChina , 2018 , 25(1) :178-188. (in Chinese)
[13] REGLA M M , MARA D M O , LUIS M , et al. Beta g lucan enhances the response to SVCV infection in zebrafish[J] . Developmental and Comparative Immunology , 2018 , 84:307-314.
[14] ALVAREZ-RODRGUEZ M , PEREIRO P , REYESLPEZ F E , et al. Analysis of the long-lived responses induced by immunostimulants and their effects on a viral infection in zebrafish (Danio rerio) [J] . Frontiers in Immunology , 2018 , 9 :1575.
[15] KRISHNAN R , JANG Y , OH M. Beta g lucan induced immune p riming protects against nervous necrosis virus infection in sevenband grouper[J] . Fish & Shellfish Immunology , 2022 , 121 :163-171.
[16] PERVEEN S , YANG L , ZHOU S , et al. β-1 , 3-g lucan from Euglena gracilis as an immunostimulant mediates the antip arasitic effect against Mesanophrys sp. on hemoc ytes in marine swimming crab (Portunus trituberculatus) [J] . Fish & Shellfish Immunology , 2021 , 114:28-35.
[17] DAWOOD M A O , ABDO S E , GEWAILY M S ,et al. The influence of dietary β-g lucan on immune , transcrip tomic , inflammatory and histo p atholo gy disorders caused b y deltamethrin toxicity in Nile tilap ia (Oreochromisniloticus) [J] . Fish & Shellfish Immunology , 2020 , 98:301-311.
[18] CAO H , YU R , ZHANG Y , et al. Effects of dietary supplementation with β-g lucan and bacillus subtilis on g rowth , fillet quality , immune capacity , and antioxidant status of Pengze crucian carp (Carassius
auratus var. Pengze ) [J] . Aquaculture , 2019 , 508 : 106-112.
[19] LI H , XU C , ZHOU L , et al. Beneficial effects of dietary β-g lucan on g rowth and health status of Pacific white shrimp Litopenaeus vannamei at low salinity[J] . Fish& Shellfish Immunology , 2019 , 91 : 315-324.
[20] LIANG Z L , MA Y P , FENG G Q , et al. Effects of β-g lucan on g rowth p erformance , immune and antioxidant indices of Trionyx sinensis [J] .Guangdong Agricultural Sciences , 2019 , 46(4) : 95-100. (in Chinese)
[21] AKHTAR M S , TRIPATHI P H , PANDEY A , et al. β-g lucan modulates non-specific immune gene expression , thermal tolerance and elicits disease resistance in endang ered Tor putitora fry challeng ed with Aeromonas salmonicida [J] . Fish & Shellfish Immunology , 2021 , 119 :154-162.
[22] CHEN J , DAI B T , WANG H M , et al. Effects of adding β-g lucan to feed on the g rowth p erformance , immune indexes , transcriptome and intestinal flora of Epinephelus fuscoguttatus × Epinephelus lanceolatus ♂ [J] . Journalof SouthernAgriculture , 2022 , 53(5) :1434-1447.
[23] CHEN J , LEI Y , DONG Z , et al. Toxicological damages on copper exposure to IgM+ B cells of nile tilap ia (Oreochromis niloticus) and mitigation of its adverse effects b y β-g lucan administration[J] . Toxicology inVitro , 2022 , 81 :105334.
[24] WANG L , ZHANG X X , WANG D M , et al. Immune stimulationeffectofβ-1 , 3-g lucan on blood cells of red crayfish[J] . Journalof Southern Agriculture , 2019 , 50(4) :883-890. (in Chinese)
[25] KOCH J F A , DE OLIVEIRA C A F , ZANUZZO F S. Dietary β-g lucan ( MacroGard® ) improves innate immune responses and disease resistance in Nile tilapia regardless of the administration p eriod[J] . Fish& Shellfish Immunology , 2021 , 112 :56-63.
[26] SHITULENI A S , FANG G , SONIA A N , et al.Effects of yeast pol ysaccharide on biochemical indices , antioxidant status , histo p atholo gical lesions and g enetic expressions related with lip id metabolism in mice fed with high fat diet[J] . Bioactive Carbohydrates and Dietary Fibre , 2016 , 8(2) :51-57.
[27] BELL S , GOLDMAN V M , BISTRIAN B R , et al. Effect of beta-g lucan from oats and yeast on serum lip ids[J] . Critical Reviews in Food Science and Nutrition , 1999 , 39(2) :189-202.
[28] LOPES L M F , MELLO M M M D , URBINATI E C. β-g lucan reduces cortisol p lasma levels , enhances innate immune system after A. hydrophila inoculation , and has lipol ytic effects on the pacu(Piaractus mesopotamicus) [J] . Aquaculture , 2022 , 546 :737411.
[29] ZHAO H X , CAO J M , WANG A , et al. Effect of long- term administration of dietary β-1 , 3-g lucan on g rowth , p hysiolo gical , and immune responses in Litopenaeus vannamei ( Boone , 1931 ) [ J ] . Aquaculture International , 2012 , 20(1) :145-158.
[30] YANG G , QIU H , YU R , et al. Dietary supplementation of β-g lucan , inulin and emodin modulates antioxidant response and suppresses intestinal inflammation of grass carp (Ctenopharyngodon idellus ) [ J ] . Animal Feed ScienceandTechnology , 2021 , 272:114789.
[31] WU C Y , CAO J M , HUANG Y H , et al. Effects ofβ- g lucan supplementation on g rowth p erformance , body composition , serum biochemical indices and resistance to ammonia nitro gen stress of seabass (Lateolabrax japonicus ) [ J ] . Chinese Journal of Animal Nutrition , 2013 , 25(12) :3033-3040.
[32] SOUZA F P D , LIMA E C S D , PANDOLFI V C F ,et al. Effect of β-g lucan in water on g rowth p erformance , blood status and intestinal microbiota in tilap ia under hypoxia[J] . AquacultureReports , 2020 , 17:100369.
[33] CAO J M , ZHAO H X , HUANG Y H , et al. β-g lucan and its application in aquatic animals[J] . Feed Industry , 2013 , 34(18) :1-6.
[34] LIU Y , HUANG G. The derivatization and antioxidant activities of yeast mannan[J] .InternationalJournalofBiologicalMacromolecules , 2018 , 107(PtA) :755-761.
[35] WANG W L , ZHOU J S , CHEN M Q , et al. Effects of β-g lucan on g rowth , intestinal dig estive enzyme activities and liver antioxidant capacity of j uvenile Asian salmon[J] . Southwest China Journal of Agricultural Sciences , 2021 , 34(3) : 673-678.
[36] WANG Y H , ZHANG J S , ZENG L. Protective effect and mechanism of β-g lucan on j uvenile Large Yellow cropfish under hypoxic stress [J] . Journal of Fisheries of China , 2018 , 42(6) : 828-837. ( in Chinese)
[37] ZHU M , WU S. The g rowth p erformance and nonspecific immunity of loach Paramisgurnus dabryanus as affected b y dietary β-1 , 3-g lucan[J] . Fish& Shellfish Immunology , 2018 , 83 :368-372.
[38] BAGNI M , ROMANO N , FINOIA M G , et al. Short- and long- term effects of a dietary yeast β-g lucan
( macrogard) and alginic acid ( ergosan) preparation on immune response in sea bass ( Dicentrarchus labrax) [J] . Fish & Shellfish Immunology , 2005 , 18(4) :311-325.
[39] DAWOOD M A O , KOSHIO S , ISHIKAWA M , et al. Physiolo gical response , blood chemistry p rofile and mucus secretion of red sea bream ( Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress [J] . Fish Physiology and Biochemistry , 2017 , 43(1) :179-192.
[40] CHEN J W , GUO D Y , ZHAO B , et al. Effects of dietary β-g lucan and inactivated lactic acid bacteria on g rowth p erformance , intestinal fatty acid composition and immune p erformance of j uvenile loach[J] . Acta Hydrobiologica Sinica , 2019 , 43(1) : 52-59.
[41] ZHAO H X , CHEN B , MO W Y , et al. Effects of dietary β-1 , 3-g lucan on g rowth p erformance , serum metabolism , immune-related gene expression and resistance to nitrite nitro gen stress in Litopenaeus vannamei [J] . Acta Hydrobiologica Sinica , 2021 , 45(3) :593-600. (in Chinese)
[42] DIVYA M , GOPI N , ISWARYA A , et al. β-g lucan extracted from eukaryotic sing le-celled microorganism Saccharomyces cerevisiae : Dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus[J] . Microbial Pathogenesis , 2020 , 139 :103917.
[43] WANG Y , JI L Q , SUN G X , et al. Effects of dietary β-g lucan on p artial g rowth indices and blood p hysiolo gical indices of Rainbowtrout [J] . Advances inFishery Science , 2018 , 39(3) :65-71. (in Chinese)
[44] SUN J W , QI Z L. Effects of β-g lucan on g rowth p erformance and serum biochemical indices of yellow catfish ( Pelteobagrus fulvidraco ) [ J ] . Hebei Fisheries , 2017 , 3 :1-4.
[45] KHANJANI M H , GHAEDI G , SHARIFINIA M. Effects of diets containing β-g lucan on survival , g rowth p erformance , haematolo gical , immunity and biochemical parameters of rainbow trout (Oncorhynchus mykiss) fing erlings[J] . Aquaculture Research , 2022 , 53(5) :1842-1850.
[46] JI L , FU S , JI R , et al. β-g lucan mitigated trinitrobenzene sulfonic acid-induced enteritis in the rainbow trout ( Oncorhynchus mykiss ) [ J ] . Aquaculture , 2019 , 513 :734393.
[47] LI Y J , WANG W M , HUANG Y H , et al. Effects of β-g lucan supplementation on g rowth p erformance , serum biochemical indices and resistance to aeromonas hydro p hila infection of j uvenile Yellow catfish (Pelteobagrus fulvidraco) [J] . Chinese Journal of Animal Nutrition , 2015 , 27(12) : 3754-3762.
[48] PILARSKI F , FERREIRA D O C , DARPOSSOLO D S F ,et al. Different beta-glucans improve the growth performance and bacterial resistance in Niletilapia[J] . Fish& Shellfish Immunology , 2017 , 70:25-29.
[49] BOOMAN M , FORSTER I , VEDERAS J C , et al. Soybean meal-induced enteritis in atlantic salmon (Salmo salar ) and chinook salmon ( Oncorhynchus tshawytscha) but not in pink salmon (O.gorbuscha)[J] . Aquaculture , 2018 , 483 :238-243.
[50] CHEN Z , LIN S , JIANG Y , et al. Effects of bread yeast cell wall beta-g lucans on mice with lo p eramide- induced constip ation[J] . Journalof Medicinal Food , 2019 , 22(10) :1009-1021.
[51] BERNAL M G ,MARRERO R M ,CAMPA-CRDOVA A I , et al. Probiotic effect of streptomyces strains alone or in combination with Bacillus and Lactobacillus in j uveniles of the white shrimp Litopenaeus vannamei [ J ] . Aquaculture International , 2017 , 25(2) :927- 939.
[52] CAO H , WANG A G , LIU G D. Compound additives and their application[A] . The 8th China International Food Additives and Ingredients Exhibition and the 11th National Food Additives Production and
Application Technology Exhibition[C] . 2004.
[53] MIAO S , HAN B , ZHAO C , et al. Effects of dietary Pediococcusacidilactici GY2single or combined with saccharomyces cerevisiae or/and β-g lucan on the g rowth , innate immunity response and disease resistance of Macrobrachiumrosenbergii[J] . Fish & Shellfish Immunology , 2020 , 98:68-76.
[54] DIMITROGLOU A , MERRIFIELD D L , SPRING P , et al. Effects of mannan oligosaccharide ( MOS) supplementation on g rowth p erformance , feed utilisation , intestinal histolo gy and gut microbiota of gilthead sea bream (Sparus aurata)[J] . Aquaculture , 2010 , 300(1-4) :182-188.
[55] CHIU C , GUU Y , LIU C , et al. Immune responses and gene expression in white shrimp , lito penaeus vannamei , induced b y Lactobacillus plantarum[J] . Fish & Shellfish Immunology , 2007 , 23(2) :364-377.
[56] DASH G , RAMAN R P , PANI PRASAD K , et al.Evaluation of paraprobiotic applicability of Lactobacillus plantarum in imp roving the immune response and disease protection in giant freshwater prawn , acrobrachiumrosenbergii (de Man , 1879) [J] .Fish & Shellfish Immunology , 2015 , 43(1) :167-174.
[57] MAGOUZ F I , SALEM M F I , EMARA A E I , et al. A mixture of β-g lucan and mannanoligosaccharide ameliorated the g rowth rate , dig estive enzyme activity , intestinal morphometry , and immunity of common carp (Cyprinus carpio) [J] . Annals of Animal Science , 2021 , 21(3) :1027-1041.
[58] ANJUGAM M , ISWARYA A , SIBIYA A , et al.Molecular interaction anal ysis of β-1 , 3 g lucan binding p rotein with Bacilluslicheniformis and evaluation of its immunostimulant property in Oreochromis
mossambicus [J] . Fish & Shellfish Immunology , 2022 , 121 :183-196.
[59] EL-NOBI G , HASSANIN M , KHALIL A A , et al.Synbiotic effects of Saccharomycescerevisiae , mannan oligosaccharides , and β-g lucan on innate immunity , antioxidant status , and disease resistance of Nile tilap ia , Oreochromis niloticus [J] . Antibiotics , 2021 , 10(5) :567.
[60] YAMAMOTO F Y , YIN F , ROSSI W J , et al. Beta- 1 ,3 g lucan derived from euglena g racilis and Algamune enhances innate immune responses of red drum (Sciaenops ocellatus L. ) [J] . Fish & Shellfish Immunology , 2018 , 77:273-279.
[61] LI F D , YE J D , WANG K , et al. Effects of long- term continuous feeding ofβ-g lucan on g rowth p erformance and non-specific immune function of cyprinus carpio[J] . Chinese Journal of Animal Nutrition , 2009 , 21(4) :499-505.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese