What Is the Use of Beta Carotene in Animal Feeding?
Beta-carotene is the most abundant provitamin A carotenoid in the human diet and tissues. Beta-carotene and its metabolites are critical regulatory signaling factors in tissue metabolism and exert numerous beneficial functions in mammals, including humans. Although beta-carotene is considered a safe form of vitamin A, its harmful effects are at least in some cases due to inappropriate intake, due to its highly regulated intestinal absorption mechanism. This article provides an overview of the metabolism of β-carotene, clearly differentiating between its possible beneficial or harmful effects on animal health, and thus providing a theoretical basis for the appropriate dosage of β-carotene that can be ingested by different animals.
1 Structure and properties of β-carotene
There are many different types of carotenoids in nature, which can be divided into carotenes and xanthophylls according to their chemical structure [1]. Carotenoids (such as β-carotene, α-carotene and β-cryptoxanthin) are non-oxidised carotenoids with linear or cyclic hydrocarbons at one or both ends of the molecule, while xanthophylls (such as zeaxanthin, meso-zeaxanthin, astaxanthin and canthaxanthin) are the oxidised derivatives of carotenoids [2]. Beta-carotene is found in carrots, along with its three isomers (alpha, beta, and gamma). Beta-carotene is the most active and is widespread in nature [3].
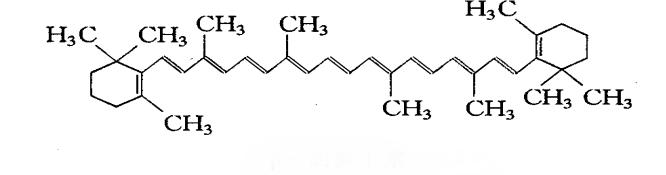
The connection between β-carotene and vitamin A was established by Von Euler et al. [4], who further demonstrated that crystalline carotene has vitamin A activity. Moore [5] further showed that β-carotene can be converted to vitamin A in rats. According to whether carotenoids themselves have the function of vitamin A precursors, they are divided into provitamin A and non-provitamin A. Provitamin A and its metabolites after enzymatic and non-enzymatic cleavage (provitamin A) can produce vitamin A. β-carotene is the most abundant vitamin A precursor in nature [6]. Pro-vitamin A and its metabolites (provitamin A) after enzymatic and non-enzymatic cleavage can produce vitamin A. Beta-carotene is the most abundant pro-vitamin A in nature [6]. Beta-carotene is a short, straight-chain molecule with 40 carbon atoms, 15 conjugated double bonds at each end of the molecule, and two β-ionone rings. This structural feature makes beta-carotene inherently highly hydrophobic and non-polar. In animals, polar carotenoids appear to be more readily absorbed than non-polar carotenoids [7]. In general, carotenoids are highly hydrophobic.
2 Absorption, transport, metabolism and deposition of β-carotene in animals
2.1 Absorption of β-carotene in the animal intestine
The kinetic parameters of β-carotene in the digestive tract are mostly derived from the results of studies on non-ruminants. Since β-carotene is transported together with lipids in animals, its transport and absorption in the small intestine are greatly affected by the type and content of fat in the feed. The non-polar properties of β-carotene determine that it is located in the particle core of the digestive tract during transport, and the efficiency of its transfer from the emulsion to the chylomicrons is 12% to 18% [8]. When the intake of β-carotene is high or the intake of fat is low, the transport of β-carotene from the emulsion to the chylomicrons in the small intestine is the rate-limiting step. The small intestine is mainly responsible for absorbing lipids, fat-soluble vitamins and β-carotene, and subsequently delivering them to peripheral tissues.
Studies have shown that even though the human intestine expresses large amounts of β-carotene 15, 15 'oxygenase (CM O Ⅰ or BCMO1 or BCO1), it does not completely convert ingested β-carotene into vitamin A in the intestine. 17% to 45% of the actually ingested β-carotene is released into the peripheral circulation in its intact, uncut form [9]. Studies have shown that multiple polymorphisms in the CMO Ⅰ gene associated with the CM O Ⅰ variable enzyme may be the cause of the lower efficiency of some individuals in breaking down β-carotene [10]. Studies have also shown that the intestines of mice and other rodents can more efficiently absorb β-carotene, and that only when it is ingested in superphysiological amounts can this provitamin A carotenoid be detected in their circulation [11]. Other animal models such as Mongolian gerbils, silkworms and ruminant calves can also absorb β-carotene in its intact form, and the distribution of vitamin A precursors in serum and tissues is similar to that in humans. At present, the mechanism of β-carotene absorption and transport in ruminants is not well understood, but ruminants can be used as a good model for studying carotenoid transport. Due to the modification and resynthesis of fat by rumen microorganisms in ruminants before entering the duodenum, the process is more complicated than in non-ruminants. Lutein appears earlier than β-carotene in the serum of calves whose rumen has not yet developed [12].
2.2 Transport of β-carotene in animal serum
It has been established that due to the high lipophilicity and non-polarity of β-carotene (including other carotenoids), its transport is closely related to various lipid proteins in the circulation. It may enter various lipoprotein particles such as chylomicrons and their remnants [very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL) and low-density lipoprotein (LDL)] into the hydrophobic core, as well as other lipids such as cholesterol esters and retinoids [13]. These lipoproteins promote the transfer of β-carotene from the intestinal barrier to various tissues of the body and its intertissue transport. Different animal species transport β-carotene in different types of lipoprotein. Studies have shown that high-density lipoprotein (HDL) is the main carrier of β-carotene in the blood circulation of cattle [14].
Overall, although the order in which different lipoproteins transport different provitamin A carotenoids varies significantly among species, β-carotene can be transported bound to various lipoproteins in the circulation. Studies have found that β-carotene can enter all types of lipoproteins to varying degrees, with HDL accounting for about 82%, LDL 12%, and VLDL 0.3% [15]. A study in rats found that most of the β-carotene in the serum is bound to the larger VLDL and LDL [16]. Pei Lingpeng [17] found that most carotenoids in animals, such as β-cryptoxanthin, are mainly distributed in LDL and HDL; about 53% of lutein and zeaxanthin are bound to HDL, 31% are found in LDL, and about 16% are bound to VLDL. The fate and final metabolism of β-carotene depends to a large extent on the affinity of β-carotene and lipoproteins. Gugger et al. [18] studied the transport mechanism of β-carotene between intracellular organelles in vitro and found that the transport of β-carotene in cells is not regulated by transport proteins in the cytoplasm, but may be regulated by vesicle transport or membrane-bound proteins.
2.3 Metabolism of β-carotene in animals
Retinal aldehyde formed after the decomposition of vitamin A precursors can be oxidized by retinal dehydrogenase to form all-trans-retinoic acid, which is the biologically active form of vitamin A. Retinoic acid is not only a transcriptional regulator, but also acts as a ligand for specific nuclear receptors, retinoic acid receptors (RARs) or retinoid X receptors (RXRs), forming homo- or heterodimers to regulate the transcription of hundreds of target genes [19]. When the production of retinoic acid in tissues exceeds a certain limit, it can be oxidatively degraded by transcriptional repressors belonging to the cytochrome P450 family to produce more polar compounds such as 4-hydroxy or 4-oxo retinoic acid [20] (Figure 1).
As shown in Figure 1, CMO I symmetrically oxidatively cleaves the 15,15' double bond of β-carotene to produce two molecules of retinaldehyde; retinaldehyde can be oxidized to retinal by aldehyde dehydrogenase (ALDH1) or retinal dehydrogenase (RALDH); Further oxidation of retinol to more polar compounds, including 4-hydroxytamoxifen, which is thought to be responsible for transcriptional inactivation, is carried out by cytochrome P450 26 family enzymes (Cyp26). Alternatively, different forms of alcohol dehydrogenase (ADH) from the medium-chain dehydrogenase/reductase (MDR) family and various retinol dehydrogenases (RDH) from the short-chain dehydrogenase/reductase (SDR) family can reduce retinal to retinol, and can be further esterified to retinal by retinal acyltransferase (LRAT). In addition, apocarotenoids can be produced from β-carotene; the cleavage of the 9',10'-double bond is catalyzed by β-carotene 9',10'-oxygenase 2 (BCMO2 or BCO2) and produces β-apo-10'-carotenal and β-ionone. and asymmetric cleavage at other double bonds can occur either nonenzymatically or be catalyzed by enzymes. Figure 1 lists some of the potential apocarotenoids produced by asymmetric cleavage of β-carotene. The dashed arrow indicates that apocarotenoids can be ultimately converted to a retinal molecule. The mechanism of this conversion has not yet been fully elucidated.
In humans and mice, β-carotene cleavage enzymes such as CM O Ⅰ and CM O Ⅱ are expressed in various adult tissues, including liver and fat, as well as developing tissues such as the placenta, yolk sac, and embryo [21]. These enzymes can biologically convert β-carotene into vitamin A in situ, which indicates that β-carotene can be used as a local source of retinoids in various parts of the body. CM O Ⅰ is a cytoplasmic enzyme with strong substrate specificity that only interacts with carotenoids (with at least one unsaturated β-ionone ring). is the major enzyme responsible for the cleavage of β-carotene to vitamin A in adult tissues [22]. In vitro studies have found that retinal and retinoic acid formed by cleavage of β-carotene by CMOⅠ may affect lipid metabolism in adipocytes by regulating the peroxisome proliferator-activated receptor (PPARγ) and retinoic acid receptor (RAR) signaling pathways [23]. However, it is still unclear whether CM O Ⅰ affects lipid metabolism in various tissues in a similar way, and whether this effect is independent of its ability to break down β-carotene. β-Carotene can also be asymmetrically cleaved by CM O Ⅱ to produce a β-ionone ring and apocarotenoid, which is ultimately converted into a molecule of retinaldehyde [2], but the mechanism of conversion has not yet been fully elucidated.
2.4 Deposition of β-carotene in animals
In animals, β-carotene is mostly stored in the liver, with a small amount deposited in adipose tissue, adrenal glands and skin [16]. The distribution and storage location of β-carotene in various animal tissues varies greatly. Shapiro et al. [24] found that β-carotene was detected in the liver but not in the adipose tissue of rats after β-carotene supplementation. It is inferred that β-carotene is not simply stored in fat, and that there may be a β-carotene-binding protein that reduces the deposition of β-carotene in fat due to the lipophobic nature of this protein.
Studies have also found that β-carotene is mainly deposited in the small intestine and liver of three-yellow chickens [25], but the carotenoid content detected in the abdominal fat of laying hens is higher than that in the liver. This may be due to the different distribution of carotenoids in animals at different growth stages. During growth and development, carotenoids are mainly distributed in the liver, adipose tissue, blood, skin and feathers, and gradually transferred to the reproductive organs after sexual maturity [26]. The distribution of different carotenoids in different species of animals also varies significantly. β-Carotene is higher in the livers of sheep and goats, while lutein is higher in adipose tissue and serum. In bovine serum and adipose tissue, β-carotene predominates, and lutein is also higher in adipose tissue, but β-carotene is lower in the liver [27].
3. Biological functions of β-carotene
3.1. Effect of β-carotene on animal production performance
Studies have found that adding β-carotene to the diet of dairy cows can improve milk quality and production. In heat-stressed cows, milk production increased by 11% when supplemented with 400 mg of β-carotene per day, and milk production increased by 6.4% when supplemented with 300 mg of β-carotene. Supplementation with β-carotene has a positive effect on milk yield and milk quality in dairy cows [28]. He Wenjuan [29] added β-carotene to the feed of Chinese Holstein cows and found that when vitamin A was sufficient, the addition of β-carotene had no significant effect on milk yield, milk composition, and somatic cell count in the early lactation period, but milk yield increased to varying degrees 3 months after calving. Xia Yun et al. [30] added 90 mg/d β-carotene to the feed of Holstein cows in Australia. The results showed that the milk yield on the 20th day after addition was significantly higher than that of the control group by 11.03%, and the milk yield on the 40th day was significantly higher than that of the control group by 13.83%. The milk fat content of the β-carotene addition group was significantly higher.
Sun Shengxiang [31] added 900 mg of β-carotene to the diet of each cow every day, and found that the milk yield of the cows increased the most, and they were able to maintain a high lactation level for a certain period of time. The contents of milk fat, milk protein, and dry matter in milk were all significantly improved. Wu Hongjiu et al. [32] found that after adding different concentrations of β-carotene to the feed of Chinese Holstein cows, milk production, milk fat rate, and milk protein rate were significantly higher than those of the control group. Oliveira et al. [33] added 1.2 g of β-carotene to dairy cows every day, and found that the milk protein content increased from 2.90% to 2.96%, and the ratio of milk fat to milk protein greater than 1.5 decreased from 22.6% to 6.5%. Ma Jifeng et al. [34] added 100, 200 and 300 mg/d β-carotene to the basal diet of dairy cows, and their milk production was increased by 3.53%, 9.06% and 13.39%, respectively, compared with the control group.

The coloring effect of β-carotene has an impact on the meat quality of beef cattle. Beta-carotene deposited in adipose tissue tends to turn body fat yellow and lower the grade of beef, so the amount of beta-carotene in the diet should be reduced during the later stages of fattening of beef cattle [35]. Studies have also shown that feeding low doses of vitamin A and carotene is beneficial for the formation of beef marble [12].
3.2 Effect of β-carotene on animal immune function
Beta-carotene can improve the humoral, cellular and non-specific immune functions of animals, enhance disease resistance, and increase serum lysozyme activity when added to the feed. Chew et al. [36] found that beta-carotene can stimulate the proliferation of lymphocytes in animals and enhance the cell-mediated humoral immune response, which has a positive effect on the immune response. He Wenjuan [29] added β-carotene to the feed of Chinese Holstein cows and found that it reduced the incidence of retained afterbirth, metritis and mastitis in the first three months after calving.
Cucco et al. [37] found that β-carotene supplementation can promote growth and improve immunity in poultry. Ma Sihui et al. [38] found that adding β-carotene to the mouse diet can alleviate the immunosuppressive effect caused by cyclophosphamide and enhance the immune function of mice by increasing the content of cytokines and immunoglobulins in immunosuppressed mice. Ma Jifeng et al. [34] showed that the somatic cell count in the milk of the β-carotene-supplemented group was lower than that of the control group, with a decrease of 18.54%, 35.27% and 46.10%, respectively. Nishijima et al. [39] found that feeding dried carrots to Japanese black cattle could increase the concentration of IgA and IgG in the colostrum of β-carotene-deficient cattle. The incidence of mastitis in early lactation was reduced by 60% when serum retinol concentration increased to 100 ng/mL in the last week before calving [40]. However, serum β-carotene concentration was not associated with retained afterbirth or mastitis. The results of a large number of experiments have all demonstrated that the addition of β-carotene has a positive effect on the immune function of animals.
3.3 Effect of β-carotene on animal reproductive performance
Dietary β-carotene may be related to reproductive performance. Ruminant animals have higher β-carotene concentrations in the ovaries, especially in the corpus luteum. Studies have shown that a lack of β-carotene may lead to delayed ovulation in dairy cows, corpus luteum dysfunction, and an increased incidence of ovarian cysts [41]. At present, domestic and foreign scholars have conducted a large number of studies on adjusting the nutrition of dairy cow feed to improve fertility. The benefits of β-carotene supplementation in dairy cow feed on reproductive performance may be related to the conversion of β-carotene to vitamin A, especially in the uterus and ovaries [42]. Serum β-carotene concentrations are associated with progesterone secretion by luteal cells. The mean serum β-carotene concentration 3 weeks before parturition was higher in cows that ovulated in the first postpartum wave than in non-ovulating cows. Periparturient β-carotene supplementation (500 or 2,000 mg/d) significantly increased the number of cows that ovulated in the first postpartum wave [43].
In postpartum cows exposed to heat stress for 120 days, a supplementation of 400 mg beta-carotene for more than 90 days increased the pregnancy rate [28]. Researchers in the United States and Germany found that beta-carotene supplementation can shorten the age of first estrus, increase the pregnancy rate, promote uterine repair and ovulation, and reduce the incidence of ovarian cysts and early embryonic death.
The important role of beta-carotene in reproduction has been widely studied in Japan. It was found that the serum carotene concentration of cows with ovarian cysts [(11 ± 2) μg/dL] was significantly lower than that of healthy cows [(33 ± 4) μg/dL]; serum beta-carotene concentration was related to the quality of embryos from superovulated Japanese Black cattle. When the serum β-carotene concentration is higher than 200 μg/dL, there is a tendency for an increase in the number of corpora lutea and total recoverable embryos, and a significant increase in the number of normally implantable embryos [44]. Adding β-carotene to other animals can also improve fertility. Studies have found that supplementation with vitamin A (4,000 IU/kg) and β-carotene (100 mg/kg) increased the β-carotene content of the adrenal glands and ovaries of young sows [45]. β-carotene also enhances rumen function. In vitro, the growth of rumen bacteria and cellulose digestion capacity were significantly improved by the addition of β-carotene [46].
4 Summary
Studies have shown that β-carotene not only functions as a precursor of vitamin A, but also functions in the body as an antioxidant and anticancer agent, and has varying degrees of effect on improving animal production performance, enhancing reproductive performance, and improving immune function. At the same time, research on the amount of β-carotene supplementation in animals is also increasing year by year, but it is not yet sufficient to conclude the optimal supplementation strategy. More research is still needed to determine the β-carotene requirements of animals at different production stages. There has been relatively in-depth research on the conversion, transport, deposition and metabolism of carotenoids and vitamin A, but the biological effects of β-carotene, such as its antioxidant and anticancer properties, have yet to be fully explored.
References:
[1] Wang Shaoxia, Tang Xiaohua, Zhou Yonghong, et al. Research on the extraction process of paprika red [J]. Food Industry Science and Technology, 2008, 29(8): 196-199.
[2] VON LINTIG J,SIES H.Carotenoids[J].Archives of Biochemistry and Biophysics,2013,539(2) :99-101.
[3] Yan Hongxiang. Research on the digestion and absorption of β-carotene in the gastrointestinal tract of goats and its antioxidant effects [D]. Master's thesis. Yangzhou: Yangzhou University, 2007.
[4] VON EULER H,KARRER P,KRAUSS E V,et al. Zur biochemie der tomatenfarbstoffe [J].Helvetica Chimica Acta,1931,14( 1) :154-162.
[5 ] MOORE T.Vitamin A and carotene:the absence of the liver oil vitamin A from carotene. Ⅵ. The conversion of carotene to vitamin A in vivo [J].Biochemical Journal,1930,24(3) :692.
[6 ] KRINSKY N I,JOHNSON E J.Carotenoid actions and their relation to health and disease[J].Molecular As- pects of Medicine,2005,26(6) :459-516.
[7 ] VAN HET HOF K H,BROUWER I A,WEST C E,et al.Bioavailability of lutein from vegetables is 5 times higher than that of β-carotene[J].The American Journal of Clinical Nutrition,1999,70(2) :261-268.
[8 ] GARRETT D A,FAILLA M L,SARAMA R J.Devel-opment of an in vitro digestion method to assess carotenoid bioavailability from meals[J].Journal of Agricultural and Food Chemistry,1999,47 ( 10 ) :4301 - 4309.
[9 ] VON LINTIG J.Colors with functions:elucidating the biochemical and molecular basis of carotenoid metabo- lism[J].Annual Review of Nutrition,2010,30 ( 1) : 35-56.
[10] LIETZ G,OXLEY A,LEUNG W,et al.Single nucleotide polymorphisms upstream from the β-carotene 15, 15' -monoxygenase gene influence provitamin A con- version efficiency in female volunteers[J].The Jour- nal of Nutrition,2012,142( 1) :161S-165S .
[11] Dong Hongwei, Wu Min, Zhao Yanli, et al. Protective effect of β-carotene on tight junction proteins in intestinal mucosal epithelium of mice [J]. China Animal Husbandry Journal, 2016, 52(23): 52-55.
[12] Bi Yulin. Study on the deposition law of β-carotene at different levels in beef cattle and its effect on beef cattle production performance, blood biochemistry and metabolic key enzymes [D]. Master's thesis. Tai'an: Shandong Agricultural University, 2014.
[13] Wei Qiaoli. Study on the effect of β-carotene on fat synthesis in beef cattle [D]. Master's thesis. Tai'an: Shandong Agricultural University, 2014.
[14] Zhou Limei and Zhou Guanghong. Research progress of carotenoids in animal nutrition [J]. Grain and Feed Industry, 2001, 1 (2): 39-41.
[15] Song Jianting. Research on the effects of some factors in the diet and intestinal environment on the activity of β-carotene 15,15' plus dioxygenase [D]. Nanjing: Nanjing Agricultural University, 2003.
[16] Zhu Xudong. Research on the distribution of β-carotene in rats using isotope tracing technology [D]. Master's thesis. Nanjing: Nanjing Agricultural University, 2004.
[17] Pei Lingpeng. Research on the regulation of carotenoid metabolism in osteoblasts [D]. Doctoral dissertation. Beijing: China Academy of Traditional Chinese Medicine, 2008.
[18] GUGGER E T,BIERER T L,HENZE T M ,et al. β - carotene uptake and tissue distribution in ferrets (Mus- tela putorius furo) [J].The Journal of Nutrition, 1992,122( 1) :115-119.
[19] TANOURY Z A,PISKUNOV A,ROCHETTE-EGLY C.Vitamin A and retinoid signaling:genomic and non- genomic effects:thematic review series:fat-soluble vi- tamins : vitamin A [J].Journal of Lipid Research, 2013,54(7) :1761-1775.
[20] ABU -ABED S,DOLLE P,METZGER D,et al.The retinoic acidmetabolizing enzyme,CYP26A1,is essential for normal hindbrain patterning,vertebral identity,and development of posterior structures[J].Genes & Development,2001,15(2) :226-240.
[21] KIM Y K,WASSEF L,CHUNG S,et al. β-Carotene and its cleavage enzyme β-carotene-15,15' -oxygenase( CM O Ⅰ ) affect retinoid metabolism in developing tissues[J].FASEB Journal,2011,25(5) :1641-1652.
[22] TOURNIA IRE F,GOURANTON E,VON LINTIG J, et al. β-carotene conversion products and their effects on adipose tissue[J].Genes & Nutrition,2009,4(3) : 179-187.
[23] AMENGUAL J,GOURANTON E,VAN HELDEN Y G,et al.Beta-carotene reduces body adiposity of mice via BCMO1[J].PLoS One,2011,6(6) :e20644.
[24] SHAPIRO S S,MOTT D J,MACHLIN L T.Kinetic characteristics of β-carotene uptake and depletion in rat tissue [J].The Journal of Nutrition,1984,114 ( 10) :1924-1933.
[25] Luo Guilan. Distribution of 14C-labelled β-carotene and lutein in the body of three-yellow chickens [D]. Master's thesis. Nanjing: Nanjing Agricultural University, 2006.
[26] Wang Fang, Li Mingzhen, Tian Qiyou, et al. Carotenoids and coloring of livestock and poultry products [J]. Shandong Animal Husbandry and Veterinary Medicine, 2002, 30(4): 37-38.
[27] Jin Qing, Bi Yulin, Cheng Haijian, et al. Effect of β-carotene on slaughter performance and carcass quality of beef cattle [J]. Journal of Animal Science and Technology, 2016, 37(5): 26-31.
[28] ARECHIGA C F,STAPLES C R , MCDOWELL L R , et al.Effects of timed insemination and supplemental β-carotene on reproduction and milk yield
of dairy cows under heat stress[J].Journal of Dairy Science,1998,81 (2) :390-402.
[29] He Wenjuan. The effect of β-carotene on the lactation and immune performance of dairy cows and the relationship between the in vitro degradation by rumen microorganisms and the degree of fat saturation of the feed [D]. Master's thesis. Beijing: China Agricultural University, 2006.
[30] Xia Yun, Han Xiangmin, Gan Bozhong. The effect of different β-carotene levels on milk yield and milk quality of dairy cows [J]. China Dairy Science, 2007, 33(4): 29-32.
[31] Sun Shengxiang. The effect of different β-carotene levels on the milk production performance of dairy cows [J]. Qinghai Animal Husbandry and Veterinary Medicine Journal, 2010, 40(6): 9-11.
[32] Wu Hongjiu, Li Hongqiu. The effect of different β-carotene levels on milk yield and milk quality of dairy cows [J]. Modern Animal Husbandry, 2013, 33 (14): 18.
[33] OLIVEIRA R C,GUERREIRO B M ,JUNIOR N N M ,et al.Supplementation of prepartum dairy cows with β-carotene[J].Journal of Dairy Science,2015, 98(9) :6304-6314.
[34] Ma Jifeng, Chang Guoxin, Wang Jiandong, et al. Research on the effect of β-carotene on the lactation and reproductive performance of dairy cows [J]. Heilongjiang Animal Husbandry and Veterinary Medicine, 2015(1): 86-88.
[35] Zhang Xingkai. Effect of vitamin A on intramuscular fat deposition and ACC/HSL and PPARγ gene expression in beef cattle [D]. Master's thesis. Yangling: Northwest A&F University, 2005.[36] CHEW B P,PARK J S.Carotenoid action on the im - mune response[J].The Journal of Nutrition,2004,134 ( 1) :257S-261S .
[37] CUCCO M ,GUASCO B,MALACARNE G,et al. Effects of β-carotene supplementation on chick growth,immune status and behaviour in the grey partridge,Perdix perdix[J].Behavioural Processes,2006, 73(3) :325-332.
[38] Ma Sihui, Yang Hong, Wu Tiancheng, et al. Effect of β-carotene on immune indicators in immunosuppressed mice [J]. Chinese Journal of Veterinary Medicine, 2014, 48 (7): 10-14.
[39] NISHIJIMA Y,TANIGUCHI S,IKEDA S,et al. Effects of β-carotene-enriched dry carrots on β-carotene status and colostral immunoglobulin in β-carotene-deficient Japanese black cows[J].Animal Science Journal,2017,88(4) :653-658.
[40] LEBLANC S J,HERDT T H,SEYMOUR W M ,et al. Peripartum serum vitamin E,retinol,and beta-carotene in dairy cattle and their associations with disease.[J]. Journal of Dairy Science,2004,87(3) :609-619.
[41]Wang B. The role of β-carotene in dairy cow feeding [J]. Modern Animal Husbandry Science and Technology, 2015(1): 34.
[42] SCHWEIGERT F J. Research note:changes in the concentration of β-carotene,α-tocopherol and retinol in the bovine Corpus luteum during the ovarian cycle [J].Archives of Animal Nutrition,2003,57(4) :307- 310.
[43] KAWASHIMA C ,NAGASHIM A S ,SAWADA K, et al.Effect of β-carotene supply during close-up dry period on the onset of first postpartum luteal activity in dairy cows[J]. Reproduction in Domestic Animals, 2010,45(6) :e282.
[44] INABA T,MEZAN M ,SHIMIZU R , et al.Plasma concentrations of β-carotene and vitamin A in cows with ovarian cyst[J].The Japanese Journal of Veteri- nary Science,1986,48(6) :1275-1278.
[45] SCHWEIGERT F J,BUCHHOLZ I,SCHUHMACH- ER A,et al.Effect of dietary β-carotene on the accu- mulation of β-carotene and vitamin A in plasma and tissues of gilts[J]. Reproduction Nutrition Development,2001,41 ( 1) :47-55.
[46] Yan Hongxiang, Kuang Wei, Wu Min, et al. Study on the effect of β-carotene on the in vitro fermentation of goat rumen microorganisms [J]. Feed Industry, 2006, 27 (22): 36-38.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese