What Is the Source of Natural Beta Carotene?
1 Introduction
Beta-carotene is widely found in green vegetables, fruits, algae and bacteria, and is formed by cyclization of the branched linear pathway from octahydro-lycopene to lycopene [1]. Beta-carotene is a good source of vitamin A and plays an important role in antioxidant, anti-inflammatory, immune enhancement, anti-tumor and other aspects. It has been recognized by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) as a non-toxic, safe and nutritious food additive. With the deepening of research, the market demand for beta-carotene is increasing. This paper reviews the sources, structural characteristics, toxicological studies and biological functions of beta-carotene. JECFA) has been recognized as a non-toxic, safe, and nutritious food additive. With the deepening of research, the market demand for β-carotene is increasing day by day. This paper reviews the sources, structural characteristics, toxicological studies, and biological functions of β-carotene to provide a reference for the development and utilization of β-carotene.
2 Structure and sources of β-carotene
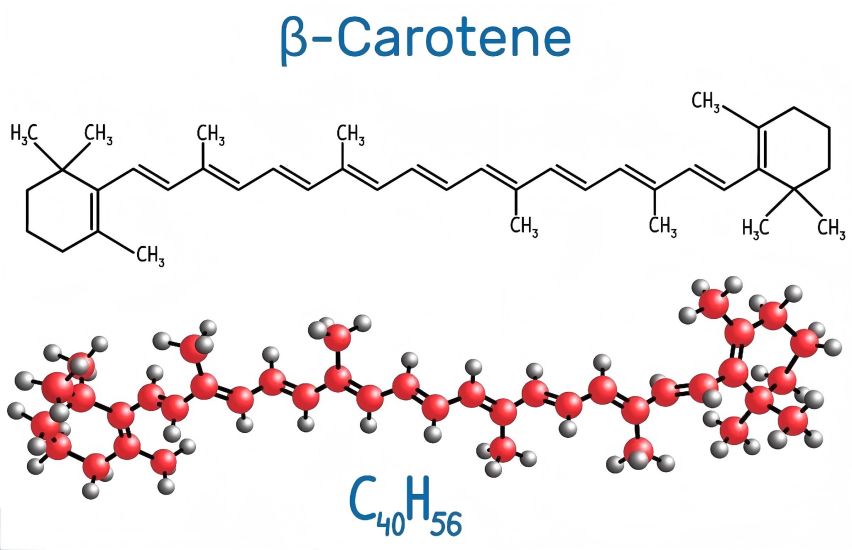
Beta-carotene cannot be synthesized in the human body and can only be obtained through food. The molecular formula of beta-carotene is C40H56, and it has a reverse-symmetrical chemical structure with eight isoprene structures on the main chain and two β-ionone ring structures at the ends (Figure 1). The molecular weight is 536.88, melting point 184°C. In nature, exists in the form of all-trans and 4 isomers: 9-cis, 13-cis, and 15-cis [3]. β-Carotene is a purplish red to dark red shiny crystalline powder. Its dilute solution is orange to yellow, and as the concentration increases it becomes orange. The polarity of the solution can be slightly reddish. β-Carotene is easily oxidized and degraded in the presence of oxygen, light, heat, and strong acids, but is stable in weak alkalis; insoluble in water, propylene glycol, and glycerin, almost insoluble in methanol and ethanol, soluble in organic solvents such as tetrahydrofuran, dinitrosulfoxide, carbon disulfide, benzene, chloroform, ethane, and vegetable oil[4].
Depending on the source, β-carotene is divided into natural β-carotene and chemically synthesized β-carotene. Natural β-carotene can be obtained by extracting from plants, algae and microbial fermentation. Beta-carotene is extracted from vegetables, fruits, palm oil, flowers and other plants using organic solvent extraction, supercritical fluid extraction or in-situ extraction. Algae, especially Dunaliella salina, can produce large amounts of beta-carotene during cultivation and breeding, and are highly adaptable [5]. However, the preparation process is affected by factors such as location and climate, resulting in high extraction costs and making it difficult to achieve large-scale production of β-carotene [6]. Microbial fermentation is a popular technology in recent years. The bacteria and fungi used includeOwenella, Blastomyces triposporus, Cladosporium cladosporioides, and Rhodotorula glutinis[7]. The use of these wild strains to produce β-carotene has a short cycle, relatively safe, and more efficient than plants and algae. However, the wild strains have poor living performance, low final yield, and are difficult to industrialize [4].
The difference between the structure of natural β-carotene and chemically synthesized β-carotene is reflected in the ratio of all-trans and cis isomers. The configuration of chemically synthesized β-carotene is almost all-trans, while natural β-carotene contains a certain proportion of cis isomers in addition to all-trans isomers [8].
The all-trans structure of β-carotene is more easily absorbed than the cis isomer. When all-trans β-carotene is administered by gavage, the accumulation of β-carotene in the serum and liver tissue of gerbils is higher than that of 9-cis and 13-cis. All-trans has obvious preferential uptake, transport, tissue accumulation and bioavailability [9]. After oral administration of a mixture of all-trans and 80% 9-cis β-carotene to healthy adult men, the all-trans isomer increased more in serum than the 9-cis isomer [10]. As a precursor of vitamin A, the all-trans isomer of beta-carotene is more biologically active than the cis isomer. When gerbils were given doses of 141, 275 and 418 nmol/d by gavage, 9-cis and 13-cis isomers fed gerbils had relative vitamin A values of 38% and 62% of the all-trans value [11]; in a rat experiment, the liver vitamin A stores of 9-cis and 13-cis groups of rats were 61% and 74% of the all-trans group [12].
Although the source activity of the cis isomers is lower than that of all-trans, studies have found other biological effects of the cis isomers. In vitro and in vivo experiments have shown that 9-cis is more prominent than all-trans isomers in terms of antioxidant capacity [13,14]. In the study by Harari et al. [15], mice fed a high-fat diet containing 50% and 25% 9-cis isomers of duscroside had a 40% to 63% reduction in plasma cholesterol concentrations and a 60% to 83% reduction in the cholesterol concentration of atherosclerotic lesions in the 50% 9-cis group. Using an in vitro digestion method combined with a Caco-2 cell model, the differences in the digestion stability, micelle formation and absorption of β-carotene isomers were compared, and it was found that the micelle formation efficiency of the cis isomer was higher than that of the all-trans isomer [16]. Currently, the main β-carotene structure on the market is all-trans. If the proportion of cis-isomers is increased, their synergistic effect can be brought into play, enhancing the health effects of β-carotene. The preparation of high cis-isomer β-carotene mainly uses isomerization techniques, such as thermo-induced isomerization, photo-induced isomerization [17], and nano-catalytic technology [18].
Recombinant DNA technology has made it possible to biosynthesize β-carotene. Exogenous genes are introduced into rice to form a carotenoid conversion enzyme system in the rice endosperm, which accumulates β-carotene. In 2000, Ye et al. [19] transferred the daffodil-derived octahydro-tomato red pigment synthase gene into rice seeds to cultivate the first generation of “golden rice”. However, the β-carotene content of the first generation of “golden rice” was only 1.6 μg/g. On this basis, Al-Babili et al. [20] replaced the original daffodil-derived octahydro-tomato red pigment synthase with a maize-derived octahydro-tomato red pigment synthase, and successfully developed the second generation of “golden rice”, which increased the β-carotene content by 23 times. In 2012, Chinese researchers developed the third generation of “golden rice” in 2012. The genes involved in carotenoid biosynthesis originated from grapes, and the accumulation of β-carotene in rice was 37 μg/g[21].
3 Toxicological studies of carotenoids
3.1 Toxicological studies of natural carotenoids
The safety of natural β-carotene has been confirmed in many studies. Mutagenicity evaluations including the Ames test and the mouse bone marrow cell micronucleus test showed that β-carotene is not mutagenic and no evidence of embryotoxicity was found. A multigeneration study in rats showed that oral administration of 1000 mg/kg/d of β-carotene did not interfere with rat reproductive function [22]. In the study by Nabae et al. [23], rats were fed a diet containing 5.0% β-carotene from Trichophora brasiliensis in irradiated feed, and no adverse health effects were observed (3127 mg/kg/d for male rats and 3362 mg/kg/d for female rats).

3.2 Toxicological studies on chemically synthesized beta-carotene
No adverse effects were observed in the mutagenicity, teratogenicity and carcinogenicity toxicological studies on chemically synthesized beta-carotene. No reproductive or developmental toxic effects were observed in the multigeneration and teratology studies, respectively. In subacute and subchronic feeding studies in rats and mice, no treatment-related adverse effects were observed at doses of 1000 mg/kg/day (equivalent to 2% of the dietary level). No organ toxicity was found in chronic feeding trials in rats, mice and dogs [24].
3.3 Toxicological studies of genetically engineered β-carotene
With the development of biotechnology, the application of genetic engineering to β-carotene has received increasing attention. Grenfell-Lee et al. [25] studied the genetic toxicity and subchronic toxicity of β-carotene crystals produced by fermentation of transgenic lipase yeast. The results of the Ames test, chromosomal aberration test and micronucleus test were all negative. with 0, 125, 250, and 500 mg/kg doses given to rats by continuous gavage for 13 weeks, no toxic abnormalities were found in clinical symptoms, ophthalmology, hematology, autopsy, or histopathology. The no-observed-adverse-effect level of β-carotene from Rhodotorula yeasts is 500 mg/kg. Wu et al. [21] studied the safety of β-carotene-rich transgenic rice and found that β-carotene-rich transgenic rice has similar nutritional value to the non-transgenic rice Zhonghua 11 and no harmful effects caused by transgenic rice were found.
4. Biological effects of β-carotene
4.1 Antioxidant effects
Due to its special structure of 11 conjugated double bonds and 2 violaxanthone rings, β-carotene has the ability to scavenge free radicals[26], quench singlet oxygen, and promote the expression of antioxidant enzymes. This structure can also protect the body by terminating lipid peroxidation reactions[27]. In Sun Xiejun's experiment [28], the reducing power, hydroxyl radical scavenging capacity and lipid peroxidation resistance of a β-carotene standard solution and a saltweed extract both increased with the concentration of β-carotene. β-Carotene was extracted by cultivating and fermenting a Trichophora strain. It was found that at the same temperature, its ability to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals gradually increased, and there was a dose-response relationship within a certain range [29]. In an in vivo antioxidant experiment with zebrafish, three experimental groups were set up with 25, 50, and 100 μg/mL, and the corresponding antioxidant capacities were 30.43%, 53.6%, and 73.91%, respectively [29].
When 0.6, 1.2, and 1.8 g of β-carotene were added to the diet of dairy cows, the total antioxidant capacity (T-AOC), antioxidant enzyme system, and glutathione (GSH) content in the blood of dairy cows in the 1.2 or 1.8 g group increased, and the malondialdehyde (MDA) concentration decreased [30]. Liu Yu [31] found that selenium, β-carotene and the combined experimental group of rats liver tissue total superoxide dismutase (total superoxide dismutase, T-SOD), glutathione peroxidase (glutathione peroxidase, GSH-Px) activity of water was higher than the control group, suggesting that the preventive and therapeutic administration of rats with the right amount of selenium and β-carotene can improve the body's antioxidant capacity.
4.2 Anti-inflammatory effect
Oxidative stress activates inflammatory signaling pathways. Beta-carotene can inhibit inflammatory signaling and tissue damage, reducing the risk of inflammatory diseases. Beta-carotene can inhibit reactive oxygen species-mediated inflammatory signaling, including mitogen-activated protein kinases and redox-sensitive transcription factors, and reduce the expression of inflammatory mediators such as interleukin-8 (IL-8), inducible nitric oxide synthase and cyclooxygenase-2 in infected tissues. Adding antioxidants such as beta-carotene to the diet can help prevent Helicobacter pylori-induced gastritis [32].
Beta-carotene can prevent atherosclerosis and inflammatory diseases such as rheumatoid arthritis by scavenging free radicals and disrupting the sequence of reactions that lead to damage [33]. Zhang Xiaoyin et al. [34] established an inflammation model by inducing RAW264.7 macrophages to release large amounts of lipopolysaccharide (LPS), and the results showed that β-carotene has a promoting effect on the cell activity of LPS-stimulated macrophages, and can inhibit the secretion of inflammatory factors interleukin-1β (IL-1β) and interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) by inhibiting the expression of NF-κB p65 protein in the NF-κB pathway. Yan Changmeng et al. [35] studied the effect of β-carotene on the expression of NF-KB, transforming growth factor βl (transforming growth factor-β1, TGF-βl) and IL-6 in rats with acute pancreatitis. The pathological scores, serum amylase and IL-6 levels, and NF-KB and mRNA expression of rats in the β-carotene treatment group were lower than those of the acute pancreatitis model group, TGF-β1 expression was higher, suggesting that in the early stages of acute pancreatitis, β-carotene may play a role in inflammation and repair and regeneration.
4.3 Modulatory effect on the immune system
Beta-carotene can enhance the body's humoral immunity, cellular immunity and non-specific immune response, and improve the body's immunity. Feeding dairy cows with 0 g/d/head, 0.6 g/d/head, 1.2 g/d/head, and 1.8 g/d/head of beta-carotene additive had no significant effect on the content of serum immunoglobulins IgA, IgG, and IgM in cows. However, the 1.2 g group was able to increase the ratio of the immune T cell subset CD4 (cluster of differentiation 4)/CD8 (cluster of differentiation 8) and reduce the content of the immune cytokines interferon Y (Interferon-Y, IFN-Y), TNF-a, IL-1, IL-2, and IL-4 [36].
Adding β-carotene to the diet of Hy-Line Brown chicks increased their daily weight gain and the organ coefficient of the bursa of Fabricius [37]. Ma Shihui et al. [38] established a cyclophosphamide mouse immunosuppression model and found that β-carotene can increase the serum levels of the cytokines IL-2 and IL-4, as well as the levels of IgA, IgG and IgM in mice. Zhang Xiaoyin et al. [39] showed that adding β-carotene to the basal feed of pregnant sows in the first half and one month before the expected date of birth increased the serum and milk IgA concentrations compared to the control group. Yoshitaka et al. [40] showed that after β-carotene supplementation during pregnancy and lactation, the number of IgA antibody-secreting cells in the mammary glands and ileums of the rats increased; the pups born to β-carotene-supplemented rats showed significantly higher levels of IgA in stomach contents on the 7th and 14th days after birth, indicating that ingestion of β-carotene during pregnancy and lactation can promote the transfer of IgA from the mother to the pups in breast milk.
4.4 Antitumor effect
The anti-tumor effect of β-carotene is manifested in the inhibition of tumorigenesis, the inhibition of tumor cell proliferation and the promotion of apoptosis. Zhao et al. [41] found that β-carotene combined with rosiglitazone can inhibit the proliferation of human leukemia K562 cells through a peroxisome proliferators-activated receptor-γ (PPAR-γ)-dependent signaling pathway. Beta-carotene isolated from spinach has been shown to reduce the viability of breast cancer cells MCF-7, and the two have a dose-dependent relationship [42].
Studies have shown that in the liver tissue of rats in the selenium and β-carotene group, the expression of the Bcl-2 protein, an important gene that controls tumor cell apoptosis, is lower than that in the control group, and the expression of the Bax protein, a gene that promotes tumor cell apoptosis, is higher [31]. However, intervention studies in smokers have reported that in people who smoke at a high frequency and for a long time or who are exposed to asbestos, the intake of β-carotene increases the risk of lung cancer and death [43]. Some studies have hypothesized that the interaction of carcinogens in cigarette smoke components with β-carotene is a potential mechanism for the increased risk of lung cancer. The results showed that the increased risk of lung cancer in smokers taking β-carotene supplements did not depend on the level of smoking tar or nicotine, and it was recommended that all smokers should continue to avoid β-carotene supplements [44]. Regina [45] believes that changes in retinoid metabolism and signaling pathways, as well as interactions with cytochrome P450 (cytochrome P450 proteins, CYP) and pro-oxidation/DNA oxidation, are potential mechanisms, and heavy smoking can affect β-carotene intake. Mathilde et al. [46] showed that for non-smokers, β-carotene intake is negatively correlated with cancer; and for smokers, β-carotene is positively correlated with cancer.
4.5 Effect on other diseases
β-Carotene is a precursor of vitamin A and has the biological activity of vitamin A, such as protecting eyesight, preventing night blindness, dry eyes, etc., and ingestion of β-carotene does not lead to excessive accumulation of vitamin A in the body. Recent epidemiological studies have shown that α-carotene and β-carotene intake may be negatively correlated with depressive symptoms in middle-aged and elderly women [47], and is associated with lower overall cardiovascular disease, heart disease, stroke and other causes of death [48]. The mechanism remains to be further explored.

5 Summary
The safety and biological effects of β-carotene in terms of antioxidant, anti-inflammatory and immunomodulatory effects have been confirmed in many studies. However, the mechanism of action in some diseases is still not fully understood, and more in-depth research is needed in the future to provide a scientific basis for the industrial production and clinical application of β-carotene.
Reference:
[1] Alagoz Y, Nayak P, Dhami N, et al. cis-carotene biosynthesis, evolution and regulation in plants: The emergence of novel signaling metabolites [J]. Arch Biochem Biophys, 2018, 654: 172-184.
[2] β-carotene Industry Research Report in China 2020-2024 [R]. China Market Research Online, 2020
[3]Shao B, Xu XD. The carotenes source and its functions [J]. China Food Addit, 2008, (5): 41-44.
[4]Wang YY, Xing JM, Chen HG. Progress in metabolic engineering of β-carotene synthesis [J]. Chin J Biotech, 2017, 33(4): 578-590.
[5] Ahmed F, Fanning K, Netzel M, et al. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters [J]. Food Chem, 2014, 165: 300-306.
[6] Zhang L. Breeding of high-yielding β-carotene strain blakeslea trispora and optimization of its fementation technique [D]. Wuhan: Huazhong University of Science and Technology, 2017.
[7] Mata-Gómez LC, Montañez JC, Méndez-Zavala A, et al. Biotechnological production of carotenoids by yeasts: An overview [J]. Microb Cell Factor, 2014, 13: 12.
[8] Flachowsky G, Aulrich K, Böhme H, et al. Studies on feeds from genetically modified plants (GMP)–Contributions to nutritional and safety assessment [J]. Anim Feed Sci Technol, 2007, 133(1): 2-30.
[9] Deming DM, Teixeira SR, Jr EJ. All-trans beta-carotene appears to be more bioavailable than 9-cis or 13-cis beta-carotene in gerbils given single oral doses of each isomer [J]. J Nutr, 2002, 132(9): 2700-2708.
[10] Johnson EJ, Krinsky NI, Russell RM. Serum response of all-trans and 9-cis isomers of beta-carotene in humans [J]. J Am Coll Nutr, 1996, 15(6): 620-624.
[11] Deming DM, Baker DH, Erdman JW. The relative vitamin A value of 9-cis beta-carotene is less and that of 13-cis beta-carotene may be greater than the accepted 50% that of all-trans beta-carotene in gerbils [J]. J Nutr, 2002, 132(9): 2709-2712.
[12] Sweeney J, Marsh A. Liver storage of vitamin A in rats fed with carotene steromers [J]. JNutr, 1973, 103: 20-25.
[13] A review on factors influencing bioaccessibility and bioefficacy of carotenoids [Z].
[14] Levin G, Yeshurun M, Mokady S. In vivo antiperoxidative effect of 9-cis-β-carotene compared with that of the all-tras isomer [J]. Nutr Cancer, 1997, 27: 293-297.
[15] Harari A, Harats D, Marko D, et al. A 9-cis β-carotene–enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice [J]. JNutr, 2008, 138(10): 1923.
[16] Ferruzzi M, Lumpkin J, Schwartz S, et al. Digestive stability, micellarization, and uptake of beta-carotene isomers by Caco-2 human intestinal cells [J]. J Agric Food Chem, 2006, 54: 2780-2785.
[17]Qiu D, Wu YC, Jin XJ, et al. Advance in isomerization of carotenoids [J]. Food Sci Technol, 2010, (11): 257-261.
[18]Yan HX, Sun QR, Li D, et al. Isomerization of β--carotene and preparation of 9-cis β-carotene [J]. J Food Sci Biotechnol, 2016, 35(7): 739-746.
[19] Ye X, Al-Babili S, Klã TA, et al. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm [J]. Science, 2000, 287(5451): 303-305.
[20] Al-Babili S, Hoa TT, Schaub P. Exploring the potential of the bacterial carotene desaturase CrtI to increase the beta-carotene content in golden rice [J]. J Exp Botany, 2006, 57(4): 1007-1014.
[21] Wu Y, Xu Y, Du Y, et al. Dietary safety assessment of genetically modified rice EH rich in β-carotene [J]. Regul Toxicol Pharmacol, 2017, 88: 66-71.
[22] Heywood R, Palmer AK, Gregson RL, et al. The toxicity of beta-carotene [J]. Toxicology, 1985, 36(2-3): 91-100.
[23] Nabae K, Ichihara T, Hagiwara A, et al. A 90-day oral toxicity study of beta -carotene derived from Blakeslea trispora, a natural food colorant, in F344 rats [J]. Food Chem Toxicol, 2005, 43(7): 1127-1133.
[24] Woutersen R, Wolterbeek AM, Van DBH, et al. Safety evaluation of synthetic beta-carotene [J]. Crit Rev Toxicol, 1999, 29(6): 515-542.
[25] Grenfell-Lee D, Zeller S, Cardoso R, et al. The safety of β-carotene from Yarrowia lipolytica [J]. Food Chem Toxicol Int J Publish British Ind Biol Res Associat, 2014, 65: 1-11.
[26] Elvira-Torales L, García-Alonso J, Periago MJ. Nutritional importance of carotenoids and their effect on liver health: A review [J]. Antioxidants, 2019, 8: 229.
[27] Burton GW, Ingold KU. Beta-carotene: An unusual type of lipid antioxidant [J]. Science, 1984, 224(4649): 569-573.
[28]Sun XJ, Pan LF, Li XX, et al. Extraction technique and antioxidant activity of β-carotenoid from Dunaliella salina [J]. Sci Technol Food Ind, 2015, 36(22): 246-251.
[29]Wang Y, Qu HM, Zhao B, et al. Study on the kinetics of antioxidant activity of beta carotene from Blakeslea trispora [J]. Food Res Dev, 2018, 39(10): 7-11.
[30]Chen LQ. Effects of β-carotene supplementation in diet on production performance and antioxidant index of dairy cows [D]. Hohhot: Inner Mongolia Agricultural University, 2018.
[31] Liu Y. Study on the lmpact of selenium and β-carotene on physiological function in rats and the prevention and treatment of liver cancer [D]. Lanzhou: Lanzhou University, 2017.
[32] Sjunnesson H, Sturegard E, Willén R. High intake of selenium, β-carotene, and vitamins A, C, and E reduces growth of helicobacter pylori in the guinea pig [J]. Comp Med, 2001, 51: 418.
[33] Kang H, Kim H. Astaxanthin and β-carotene in Helicobacter pylori-induced gastric inflammation: A mini-review on action mechanisms [J]. J Cancer Prev, 2017, 22(2): 57-61.
[34]Zhang XY, Zhang SS, Wu M, et al. Effects and mechanism of β-carotene on inflammatory factors in LPS-induced RaW264. 7 cells [J]. Chin J Immunol, 2017, 33(6): 838-843.
[35]Yan CM, Tian H, Huo MX, et al. Effects of β-carotene on expressions of NF-KB, TGF-β1 and IL-6 in rats with acute pancreatitis [J]. Jiangsu Med J, 2018, 44(4): 357-360.
[36]Li C. Effects of Different Levels of β-carotene on serum biochemical, immune function and reproductive performance of dairy cows [D]. Hohhot: Inner Mongolia Agricultural University, 2018.
[37]Liu HY, Ji YB, Wang YL, et al. Effects of dietary β-carotene on growth performance and immune organs of Hailan Brown chickens [J]. Heilongjiang Animal Sci Veter Med, 2016, (24): 75-77.
[38]Ma SH, Yang H, Wu TC, et al. Effect of β-carotene on Immune Index of the Immunosuppression mice [J]. Chin J Vet Drug, 2014, 48(7): 10-14.
[39]Zhang XY, Ji YB, Li YQ, et al. Effects of β-carotene on immunoglobulin A concentration in feces, serum and milk of pregnant sows [J]. Chin J Anim Nutr, 2016, 28(2): 572-578.
[40] Yoshitaka N, Miki S, Shuntaro I, et al. Supplemental β-carotene increases IgA-secreting cells in mammary gland and IgA transfer from milk to neonatal mice [J]. British J Nutr, 2011, 105(1): 24-30.
[41] Zhao H, Gu H, Zhang H, et al. PPARγ-dependent pathway in the growth-inhibitory effects of K562 cells by carotenoids in combination with rosiglitazone [J]. Biochim Biophys Acta (BBA)-General Subject, 2014, 1840(1): 545-555.
[42] Sowmya SG, Yogendra PK,Arpitha HS, et al. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells [J]. Mol Cell Biochem, 2017, 436(1): 1-12.
[43] Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease [J]. N Engl J Med, 1996, 2(18): 1150-1155.
[44] Middha P, Weinstein SJ, Männistö S, et al. β-carotene supplementation and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: The role of tar and nicotine [J]. Nicot Tobacco Res: off J Soc Res Nicot Tobacco, 2019, 21(8): 1045-1050.
[45] Regina G. Beta-carotene and lung cancer in smokers: Review of hypotheses and status of research [J]. Nutr Cancer, 2009, 61(6): 767-774.
[46] Mathilde T, Emmanuelle K, Fran OCC, et al. Dual Association of beta-carotene with risk of tobacco-related cancers in a cohort of French women [J]. J Nat Cancer Instit, 2005, 97(18): 1338-1344.
[47] Li D, Li Y. Associations of α-carotenoid and β-carotenoid with depressive symptoms in late midlife women [J]. J Affective Disord, 2019, 256: 424-430.
[48] Huang J, Weinstein JS, Yu K, et al. Serum beta carotene and overall and cause-specific mortality: A prospective cohort study [J]. Circ Res, 2018, 123: 1339-1349.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese