What Is the Extraction Method of Ginsenoside?
Ginseng (Panax ginseng C. A. Meyer) is a perennial herb in the family Araliaceae. It is a traditional precious Chinese medicine with the effects of tonifying qi and producing blood, strengthening the positive and dispelling the negative. Ginsenosides are one of the main active ingredients in ginseng, accounting for about 4% of the total mass of ginseng. They have the effects of enhancing the human immune system, anti-aging, anti-fatigue, and treating cardiovascular diseases, and have now become the main ingredient in some special effect medicines. Extraction and separation technology is crucial to the efficient extraction and concentration of ginsenosides from ginseng and the purification of preparations by removing as many impurities as possible. This paper reviews the reported extraction and separation methods of ginsenosides, with the aim of providing a reference for the extraction and separation of ginsenosides.

1 Extraction methods for ginsenosides
1.1 Traditional extraction methods
1.1.1 Boiling method
The decoction method mainly uses water as the extraction solvent. The medicinal material is heated and boiled for a certain period of time to obtain a decoction. This needs to be repeated many times and is mainly used to extract the better water-soluble components of Chinese herbal medicines. It is suitable for medicinal materials whose active ingredients are soluble in water and not sensitive to heating. It is one of the earliest and most commonly used extraction methods for extracting Chinese herbal medicine components. Chen Ali et al. used the extraction rates of ginsenosides Rb1, Re, and Rg1 as the evaluation indicators, and used an orthogonal test method to optimize the boiling extraction conditions of ginseng. The results showed that the highest extraction rate of ginsenosides was obtained by boiling 8 times the mass of ginseng in water for 2 times, each time for 1 hour [1].

1.1.2 Maceration method
The maceration method is to extract the active ingredients from medicinal materials by immersing them in a solvent at room temperature or under heated conditions according to the principle of like dissolves like. Zhang Chunhong et al. used a maceration method with an extraction temperature of 60 °C, a maceration time of 2 h, and a solvent volume of 10 times the amount of the macerate to extract ginsenosides, with a maximum total saponin yield of 8.33% [2]. Sun Guangzhi et al. determined the optimal extraction process by investigating the effects of solvent multiple, extraction time, number of extractions and volume fraction of solvent on the extraction rate of propanoyl ginsenosides [3].
1.1.3 Reflux method
In the reflux method, an organic solvent is used as the extraction solvent. The volatile solvent is distilled off by heating the medicinal material, and then condensed and returned to the extractor to continue the extraction cycle until the active ingredients are completely extracted. At present, the traditional reflux operation for extracting ginsenosides in the laboratory is to reflux with 80% methanol at (75 ± 1) °C for 3 h and repeat 4 times. Yan Guangjun et al. used the total content of ginsenoside Rg1 and ginsenoside Re as the index, and through comparison and comprehensive analysis of several processes, it was shown that the reflux extraction process was the most effective [4]. Zhang Ling et al. studied the effect of different extraction processes on the content of effective components in ginseng and determined the optimal extraction process conditions for reflux extraction [5]. Hao Shaojun et al. used the content of ginsenosides as the evaluation index and used the orthogonal test method to optimize the optimal extraction process [6]. Kim et al. used the extraction of diol-type and triol-type saponins as the index to optimize the optimal process for the ethanol reflux method [7].
1.1.4 Soxhlet extraction method
The medicinal material is packed in gauze or filter paper and placed in the Soxhlet extraction extraction vessel. A certain amount of extraction solvent is added to the flask, heated and kept boiling. The solvent vapor condenses and refluxes into the extraction vessel to come into contact with the medicine. After that, the active ingredients dissolve in the solvent. After the solvent reaches a certain volume, the solvent that has dissolved the active ingredients is refluxed into the flask. the solvent is reheated and evaporated, and after cooling, it is re-exposed to the medicine to extract it in a cycle. Zhang Jing et al. took 2 g of ginseng powder and 60 mL of methanol were added. After extraction in a Soxhlet extractor for 8 h, the total ginseng saponin content was measured by spectrophotometry and found to be 3.27% [8]. Wood et al. used the Soxhlet extraction method at 80 to 90 °C to effectively extract ginsenosides [9]. Qu et al. placed 500 mg of American ginseng sample in a Soxhlet extractor and and extracted ginsenosides with 70% ethanol [10].
1.2 Modern extraction methods
1.2.1 Supercritical fluid extraction
A single-phase state formed when the temperature and pressure exceed the critical point of a substance is called a supercritical fluid. Supercritical fluids have a density similar to that of liquids, low viscosity and strong diffusivity, which gives them relatively strong solubility and enables efficient extraction with rapid mass transfer. Zhang Le et al. used supercritical fluid extraction to extract ginsenosides Rh1 and Rh2 [11]. Luo et al. used ultrasonic-assisted supercritical fluid extraction to obtain ginsenosides with a high yield [12]. Wood et al. used methanol and DMSO as modifiers for supercritical fluid extraction of ginsenosides from American ginseng, extracted 90% of the total saponins [9]. Wang et al. found that the yield of ginsenosides extracted by supercritical fluid increased with increasing temperature [13].

1.2.2 Foam separation method
The foam separation method is a technique that uses the differences in the adsorption properties of substances on the surface of bubbles to separate them. Because ginseng saponins have the properties of a surfactant, they can produce stable foam when stirred or gas is passed in, so they can be separated and enriched using flotation separation technology. Xiu et al. used the foam separation method to separate and concentrate five types of saponins, including Rb1 and Rb2 [14]. Zhang Dajia et al. used foam separation to isolate ginsenosides Rb1, Rb2, Rd, Rc, and Rf [15]. Wang Yutang et al. used dynamic foam flotation to isolate and enrich diol-type ginsenosides in ginseng extract [16]. Zhang et al. used foam flotation-solid phase extraction to isolate trace saponins from American ginseng root [17].
1.2.3 Ultrasonic-assisted extraction
Ultrasonic-assisted extraction is a process that applies the combined effects of cavitation, vibration, crushing, and agitation generated by ultrasound to traditional Chinese medicine extraction to achieve efficient and rapid extraction. Zhang Chongxi et al. compared the traditional methods of water decoction, warm soaking, ethanol reflux, microwave-assisted extraction, and ultrasonic-assisted extraction, and the results showed that the ultrasonic method was the best [18]. Zhang Xianchen et al. used orthogonal design to determine the content of ginsenosides under different ultrasonic treatment conditions by colorimetry, and optimized the ultrasonic extraction process of ginsenosides [19]. Wu et al. found that ultrasonic-assisted extraction with water, methanol, and n-butanol as solvents at 38.5 kHz is three times faster than traditional extraction [20].
1.2.4 Microwave-assisted extraction technology
Microwave-assisted extraction uses microwaves to heat the solvent in the extraction system, so that the active ingredients in the plant sample being extracted are separated and enter the solvent in contact with it. This technology mainly uses the microwave heating effect to complete the extraction and separation process. The microwave energy absorbed by the extracted substance causes the internal temperature of the cell to rise rapidly, resulting in cell rupture and the active ingredients dissolving in the solvent.
Kwon et al. optimized the conditions for microwave-assisted extraction of ginseng saponins using response surface methodology [21]. Shu et al. investigated the effects of microwave intensity, extraction time and other factors on microwave-assisted extraction [22]. Shi et al. used microwave-assisted extraction to isolate seven types of ginsenosides, including Rg1, Re and Rb1 and seven other ginsenosides from ginseng roots using microwave-assisted extraction [23]. Wang et al. used pressurized microwave-assisted extraction to extract ginseng roots and American ginseng samples, and investigated the effects of extraction time, pressure, and solvent on the extraction yield [24]. Shi Wei et al. used microwave-assisted extraction technology to quickly and effectively extract and separate six ginsenosides, Rg1, Re, Rb1, Rc, Rb2, and Rd, from ginseng root [25].
1.2.5 High-pressure and ultra-high-pressure extraction
High-pressure and ultra-high-pressure (above 100 MPa) extraction applies hydrostatic pressure to a mixture of extraction solvent and traditional Chinese medicine. After the pressure inside and outside the plant cells reaches equilibrium, the pressure is quickly released, causing the cells to permeabilize. The active ingredients in the cells pass through the various membranes of the cells and are transferred to the extracellular extraction solution, thereby achieving the purpose of extracting the active ingredients. Supercritical extraction can achieve the highest extraction efficiency in the shortest time. If the operation is carried out properly, a pure extract can be obtained, and the extraction can be carried out at room temperature, which is conducive to the separation of thermally unstable substances.
High-pressure and supercritical extraction have been applied in the extraction of ginsenosides. Chen Ruizhan et al. used supercritical extraction to extract ginsenosides under the conditions of a solvent of 50% ethanol, a pressure of 500 MPa, extraction time of 2 min using the ultra-high pressure method [26]. Chen et al. used ultra-high pressure to extract ginsenosides at room temperature and optimized the extraction process conditions using the uniform design method [27]. Lee et al. compared the yields of total ginsenosides and ginsenoside metabolites under high-pressure extraction and thermal extraction conditions, and showed that the yield of high-pressure extraction was higher [28].
1.3 New methods
1.3.1 Biomimetic extraction method
The biomimetic extraction method is based on the basic principles of drug metabolism and uses an in vitro simulation of the gastrointestinal system to extract ginsenosides. Chen Xin et al. used ginseng ultrafine powder as the raw material and extracted ginsenosides with biomimetic solvents and water as the extraction solvent [29]. The results showed that the extraction efficiency of total ginsenosides, ginsenoside Rg1 and ginsenoside Re by the biomimetic extraction method was higher than that by the water extraction method, and the chromatogram of the biomimetic extract showed the production of new components.
1.3.2 Pulsed electric field extraction method
The pulsed electric field extraction method is a new extraction method that has been applied in food engineering to extract active ingredients from biological materials. Hou et al. used pulsed electric field extraction to extract ginsenosides Rg1, Re, Rb1, Rc, Rb2, and Rd from ginseng, and compared the method with hot reflux extraction and microwave-assisted extraction. The results showed that pulsed electric field extraction had the highest yield and the shortest time [30].
1.3.3 Matrix solid-phase dispersion extraction
The process of solid-phase dispersion extraction is to first mix the sample with an abrasive dispersant, then load the mixture into a glass column, and finally elute and extract with an appropriate solvent. Shi et al. used solid-phase dispersion extraction for the extraction of ginseng leaves, extracting 8 ginsenosides such as Rb2, Rc, and Rd, and comparing it with the hot reflux method. The results showed that the solid-phase dispersion extraction method had a higher yield, took less time, and consumed less solvent [31].
2 Separation methods
2.1 Solid-liquid separation
Ginsenosides are usually separated using solid-liquid chromatography. The sample is extracted once or several times with methanol or ethanol, and then the extract is collected and combined and extracted by vacuum drying. The residue suspended in water is separated into fractions by different organic solvents, such as the n-hexane layer, ethyl acetate layer, n-butanol layer, and water layer. The n-hexane layer contains high molecular weight and oil-soluble impurities, while the other fractions are further separated into smaller parts by chromatography on a macroporous resin column and a silica gel column using a gradient solvent system. The fractions are then subjected to further separation by normal-phase silica gel column chromatography, reversed-phase silica gel column chromatography, gel column chromatography, and gradient elution with different solvent systems. The separated substances can be purified by preparative liquid chromatography, and their structures can be determined by chemical and spectroscopic methods.
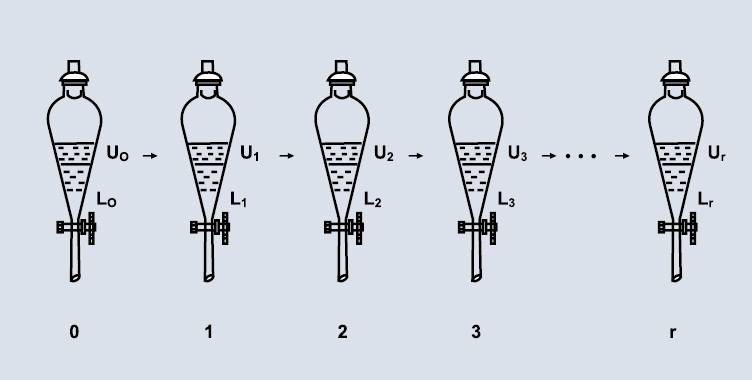
2.2 Liquid-liquid separation
Liquid-liquid partitioning technology relies on the different partitioning ratios of samples in immiscible solvents to separate them. Since there is no solid support, the problem of irreversible adsorption of the stationary phase to the sample from conventional column chromatography is avoided. Liquid-liquid partitioning mainly includes high-speed counter-current chromatography and centrifugal partition chromatography.
2.2.1 High-speed counter-current chromatography (HSCCC)
High-speed counter-current chromatography (HSCCC) is widely used in the preparation and separation of ginsenosides. Before HSCCC separation, the ginseng sample is extracted with organic reagents, and the saponin fraction is concentrated and enriched by passing through a macroporous resin column, a reverse-phase C-18 column, and a medium-pressure liquid column chromatography. Effective selection of HSCCC conditions includes the selection of a two-phase solvent system and the method of eluting the sample. The choice of mobile phase is particularly important. Recent applications of HSCCC to the separation of ginsenosides in ginseng products have resulted in the isolation of ginsenosides Rb1 [32–34], Rg1 [32, 34, 37], Re [32, 34, 37], Rf [33], Rd [33–34], Rg3 [35], Rg5 [35], Rk1 [35], F4 [35] and Ro [36].
2.2.2 Centrifugal partition chromatography (CPC)
Centrifugal partition chromatography (CPC) is a liquid-liquid separation chromatography without adsorption that operates in a continuous gravitational field. At present, the solvent system of chloroform-methanol-water has been successfully used in CPC to separate saponins. Wang et al. used CPC to separate ginsenosides Rc, Rb1, and Re from American ginseng using an ethyl acetate-n-butanol-water (1:1:2) solvent system [38].
2.3 New methods
2.3.1 Activated carbon selective adsorption
Kuang et al. used activated carbon selective adsorption to separate and purify ginsenoside Re from ginseng flower buds [39].
2.3.2 Demodulation technology
The composition and function of ginseng are usually studied using one of two methods: “separation-bioassay” or “bioassay-guided separation”. In order to prove whether the extracted component is biologically active, an extract without the component needs to be prepared as a demodified extract. In the process of comparing biological activities, if the biological activity of the demodified extract is lower than that of the original extract, it means that the component is a biologically active substance. Therefore, the method of obtaining the demodified extract is one of the focuses of research, including chemical chromatography and immunoaffinity chromatography.

2.3.2.1 Chemical chromatography
Some demulcent extracts can be prepared by column chromatography. For example, in order to prepare the Rb1 demulcent extract, the ginseng flower bud extract is first separated through a macroporous resin column using water and aqueous ethanol as the eluent. The aqueous ethanol stream is then separated by reverse-phase high-performance liquid chromatography. The separation can be divided into three parts: the water part, the Rb1 part, and the other saponin part. The Rb1 part is removed, and the remaining water part and other saponin parts are combined to form the Rb1 demoulding extract. In order to improve efficiency, Liu et al. invented an online control chromatography technique to prepare the demoulding extract [40].
2.3.2.2 Immunoadsorbent chromatography
Immunoadsorbent chromatography is a chromatographic method in which the stationary phase is a monoclonal antibody against the target compound. It is an effective method for separating and enriching trace components from complex mixtures. The high selectivity of immunoaffinity chromatography for target compounds comes from the proteins cross-linked to the stationary phase. Tanaka et al. have prepared monoclonal antibodies against ginsenosides Rb1 [41-43], Rg1 [44], Rd [45] and Re [46].
Compared with the chemical chromatographic method for preparing the extract, the immunoaffinity chromatographic method increases the selectivity of the analysis, reduces the steps of sample preparation, and increases the volume of the sample carrier. On the other hand, it greatly shortens the time required for chromatographic separation and the time required to select the optimal experimental conditions. However, the immunoaffinity chromatographic method also has some disadvantages, namely the complexity of the monoclonal antibody preparation process and the instability of the immunoaffinity column.
3 Prospects
Although traditional extraction and separation methods (decoction, reflux, etc.) each have their own advantages, they have limitations such as long extraction times, low efficiency, high solvent consumption, and are not conducive to the extraction of thermally stable or volatile components. Therefore, people have been looking for more efficient and convenient methods. With the continuous development of traditional Chinese medicine extraction technology, new methods suitable for the extraction and separation of ginsenosides are constantly emerging. They have the advantages of short extraction time, low organic solvent usage, stronger selectivity of the extract, and less environmental pollution. This provides a basis for the further development and efficient use of ginsenosides, and it is believed that the extraction, separation, and further development and utilization of ginsenosides will have a broader future.
References:
[1] Chen A-li, Cui Y-xia, Zhou L-yan, et al. Optimization of the process for extracting ginseng water by orthogonal test method [J]. China Modern Drug Application, 2009, 3 (9): 21-22.
[2] Zhang C-hong, Zhang C-xi, Zheng Y-lan, et al. Study on the optimal process for extracting ginsenosides by maceration [J]. Journal of Jilin Agricultural University, 2003, 25 (1): 73-74, 78.
[3] Sun Guangzhi, Wang Jiyan, Liu Zhi, et al. Research on the optimal extraction process of propanoyl ginsenosides from ginseng using orthogonal experiments [J]. Chinese Herbal Medicine, 2006, 37 (8): 1194-1195.
[4] Yan Guangjun, Zhang Baojiang, Xu Daojuan. Comparative study of several commonly used ginseng extraction processes [J]. Shandong Pharmaceutical Industry, 2002, 21 (4): 8-8.
[5] Zhang Ling, Shan Weihua, Liang Ruixue, et al. Research on ginseng extraction process [J]. Shizhen Traditional Chinese Medicine, 2000, 11 (9): 777-778.
[6] Hao Shaojun, Zhang Zhengchen. Orthogonal design method for studying the optimal extraction process of ginsenosides [J]. Chinese Journal of Practical Medicine, 2007, 6(1): 48-50.
[7]Kim S J,Murthy H N,Hahn E J,et al.Parameters affecting the ex- traction of ginsenosides from the adventitious roots of ginseng(Panax ginseng C.A.Meyer) [J].Separation and Purification Technology, 2007,56(3) :401-406 .
[8] Zhang Jing, Chen Quancheng, Gong Xiaojie, et al. Effect of different extraction methods on the extraction rate of ginsenosides [J]. Journal of Jilin Agricultural University, 2003, 25 (1): 71-72.
[9]Wood J A,Bernards M A,Wan W K,et al.Extraction of ginsenosides from north American ginseng using modified supercritical carbon diox- ide[J].The Journal of Supercritical Fluids,2006,39( 1) :40-47 .
[10]Qu C L,Bai Y P,Jin X Q,et al.Study on ginsenosides in different parts and ages of Panax quinquefolius L.[J]. Food Chemistry, 2009,115 ( 1) : 340-346 .
[11] Zhang Le, Song Fengrui, Wang Qi, et al. Supercritical carbon dioxide extraction of rare ginsenosides from ginseng [J]. Applied Chemistry, 2010, 27 (12): 1483-1485.
[12]Luo D L,Qiu T Q,Lu Q.Ultrasound-assisted extraction of ginsen- osides in supercritical CO2 reverse microemulsions[J]. Journal of the Science of Food and Agriculture,2007,87 (3) :431-436 .
[13]Wang H C,Chen C R , Chang C J.Carbon dioxide extraction of gin- seng root hair oil and ginsenosides[J]. Food Chemistry,2001,72 (4) : 505-509 .
[14]Xiu Z L,Zhang D J,Jia L Y,et al.Foam separation of ginsenosides of Panax ginseng[J].The Chinese Journal of Process Engineering, 2001,1 (3) : 289-293 .
[15] Zhang Daijia, Xiu Zhilong, Lin Xinhua, et al. The effect of drying methods on the extraction and separation of ginsenosides from fresh ginseng [J]. Journal of Integrated Traditional Chinese and Western Medicine, 2004, 2 (4): 292-294.
[16] Wang Yutang, Liu Xuebo, Yue Tianli, et al. Separation and enrichment of diol-type ginsenosides in ginseng extract by dynamic foam flotation [J]. Journal of Chemical Sciences in Colleges and Universities, 2009, 30 (9): 1713-1716.
[17]Zhang R, Zhang H Q,Wu L W,et al. Foam Floatation -SPE for separation and concentration of trace ginsenosides[J]. Chroma- tographia,2010,72( 1 /2) : 39-46 .
[18] Zhang Chongxi, Zheng Youlan, Zhang Chunhong, et al. Optimization of the process for extracting ginseng total saponins by different methods [J]. Ginseng Research, 2003, 15 (4): 5-8.
[19] Zhang Xianchen, Wang Shumin, Chen Guang, et al. Study on the ultrasonic extraction process of ginseng total saponins [J]. Research and Practice of Modern Traditional Chinese Medicine, 2005, 19(6): 55-57.
[20]Wu J,Lin L,Chau F T.Ultrasound -assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells[J].Ultrason- ics Sonochemistry,2001,8 (4) : 347-352 .
[21]Kwon J H,Bélanger J M,Paré J R. Optimization of microwave -as- sisted extraction( MAP) for ginseng components by response surface methodology[J].Journal of Agricultural and Food Chemistry,2003, 51 (7) : 1807 -1810 .
[22]Shu Y Y,Ko M Y,Chang Y S. Microwave -assisted extraction of ginsenosides from ginseng root[J].Microchemical Journal,2003,74 (2) : 131 -139 .
[23]Shi W,Wang Y T,Li J,et al.Investigation of ginsenosides in differ- ent parts and ages of Panax ginseng[J].Food Chemistry,2007,102 (3) : 664-668 .
[24]Wang Y T,You J Y,Yu Y,et al.Analysis of ginsenosides in Panax ginseng in high pressure microwave -assisted extraction[J].Food Chemistry,2008,110( 1) : 161 -167 .
[25] Shi Wei, Wang Yutang, Quan Xinjun, et al. Determination of ginsenosides in ginseng root by high performance liquid chromatography-evaporative light scattering detection [J]. Analytical Chemistry, 2006, 34 (2): 243-246.
[26] Chen Ruizhan, Zhang Shouqin, Wang Changzheng. Research on the optimization of the process of extracting ginsenosides from ginseng by ultra-high pressure using orthogonal experiments [J]. Chinese Herbal Medicine, 2005, 36(3): 365-368.
[27]Chen R Z,Meng F L,Zhang S Q,et al.Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng[J].Separation and Purification Technology,2009,66 (2) : 340-346 .
[28]Lee H S,Lee H J,Yu H J,et al.A comparison between high hydrostatic pressure extraction and heat extraction of ginsenosides from ginseng(Panax ginseng C.A.Meyer) [J].Journal of the Science of Food and Agriculture,2011,91 (8) : 1466 -1473 .
[29] Chen Xin, Hu Chaoqi, Zhang Hongchang, et al. Preliminary study on the extraction of ginsenosides by biomimetic chemistry [J]. Chinese Pharmacy, 2012, 23 (19): 1752-1754.
[30]Hou J G,He S Y,Ling M S,et al.A method of extracting ginsen- osides from Panax ginseng by pulsed electric field[J].Journal of Separation Science,2010,33 ( 17 /18) : 2707-2713 .
[31]Shi X L,Jin Y R , Liu J B,et al.Matrix solid phase dispersion ex- traction of ginsenosides in the leaves of Panax ginseng C.M.Mey [J].Food Chemistry,2011,129(3) : 1253 -1257 .
[32]Du Q Z,Jerz G,Waibel R , et al.Isolation of dammarane saponins from Panax notoginseng by high -speed counter -current chroma- tography[J].Journal of Chromatography a,2003,1008 (2) : 173 - 180.
[33]Qi X C,Ignatova S,Luo G,et al.Preparative isolation and purifica- tion of ginsenosides Rf,Re,Rd and Rb1 from the Roots of Panax ginseng with a salt / containing solvent system and flow step -gradi- ent by high performance counter -current chromatography coupled with an evaporative light scattering detector[J].Journal of Chroma- tography a,2010,1217 ( 13) : 1995-2001 .
[34]Cao X L,Tian Y,Zhang T Y,et al.Separation of dammarane-sapo- nins from notoginseng,root of Panax notoginseng ( Burk.) F. H. Chen,by HSCCC coupled with evaporative light scattering detector [J]. Journal of Liquid Chromatography & Related Technologies, 2003,26(9 /10) : 1579 -1591 .
[35]Ha Y W,Lim S S,Ha I J,et al.Preparative isolation of four ginsen- osides from Korean red ginseng ( steam -treated Panax ginseng C. A.Meyer) ,by high -speed counter -current chromatography cou- pled with evaporative light scattering detection[J].Journal of Chro- matography a,2007,1151 ( 1 /2) : 37-44 .
[36]Cheng Y J,Liang Q L,Hu P,et al.Combination of normal -phase medium -pressure liquid chromatography and high -performance counter-current chromatography for preparation of ginsenoside - Ro from Panax ginseng with high recovery and efficiency[J].Separa- tion and Purification Technology,2010,73 (3) : 397-402 .
[37]Chen F Q,Luo J G,Kong L Y.Fast isolation of ginsenosides Re and Rg1 from the roots of Panax ginseng by HSCCC -ELSD combined with MCI gel CC guided by HPLC -MS[J].Journal of Liquid Chro-
matography & Related Technologies,2012,35 (7) : 912-923 .
[38]Wang J,Bai H L,Liu C M,et al.Isolation and purification of gin- senosides from plant extract of panax quinquefolium L.by high per- formance centrifugal partition chromatography coupled with ELSD [J].Chromatographia,2010,71 (3 /4) : 267-271 .
[39]Kuang P Q,Wang G,Yuan Q P,et al.Separation and purification of ginsenoside Re from ginseng bud by selective adsorption of active Carbon and preparative high-performance liquid chromatography [J].Natural Product Research,2012,26(3) : 286-290 .
[40]Liu Y,Zhou J L,Liu P,et al.Chemical markers' fishing and knock- out for holistic activity and interaction evaluation of the components in herbal medicines[J].Journal of Chromatography a,2010,1217 (32) : 5239-5245 .
[41]Tanaka H,Fukuda N,Yahara S,et al.Isolation of ginsenoside Rb1 from Kalopanax pictus by eastern blotting using anti -ginsenoside Rb1 monoclonal antibody[J]. Phytotherapy Research,2005,19 (3) : 255-258 .
[42]Fukuda N,Tanaka H,Shoyama Y.Isolation of the pharmacologically active saponin ginsenoside Rb1 from ginseng by immunoaffinity col- umn chromatography[J]. Journal of Natural Products,2000,63 (2) : 283-285 .
[43]Putalun W,Fukuda N,Tanaka H,et al.Immunoaffinity column for i- solation of bioactive compounds using monoclonal antibodies[J]. Journal of Liquid Chromatography & Related Technologies,2002,25 ( 13 /15) : 2387-2398 .
[44]Tanaka H,Fukuda N,Shoyama Y.Formation of monoclonal antibody against a major ginseng component,ginsenoside Rb1 and its charac- terization[J].Cytotechnology,1999,29(2) : 115 -120 .
[45]Morinaga O,Fukuda N,Tanaka H,et al.Chromatographic resolution of glucosidic compounds,ginsenosides on polyethersulphone mem- brane,and its application to the quantitative immunoassay for ginseng saponins[J].Glycobiology,2005,15 ( 10) : 1061 -1066 .
[46]Morinaga O,Tanaka H,Shoyama Y.Detection and quantification of ginsenoside Re in ginseng samples by a chromatographic immunos- taining method using monoclonal antibody against ginsenoside Re [J].Journal of Chromatography.B,Analytical Technologies in the Biomedical and Life Sciences,2006,830( 1) : 100 -104 .


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese