What Is the Benefit of Mogroside Extract?
Siraitia grosvenorii (Swingle) C. Jeffery is a perennial herbaceous vine in the family Cucurbitaceae. The ripe fruit of Siraitia grosvenorii is a medicinal and edible plant native to southern China, with Guangxi as its main production area. It is also found in Guizhou, Jiangxi, Hunan and other regions [1]. Luohanguo has been used medicinally in Guangxi for more than 300 years. It tastes sweet, is cool in nature, and has the effects of clearing away heat and relieving summerheat, nourishing the lungs and quenching thirst. Clinically, it can be used to treat high blood pressure, tuberculosis, asthma, gastritis, whooping cough, acute and chronic bronchitis, and acute and chronic tonsillitis [2].
Since the 1960s, research on Luo Han Guo has received increasing attention. Research shows that the main active ingredient in Luo Han Guo is the triterpene glycoside mogroside, which accounts for 3.7% to 3.9% of the total content in the dried fruit. It is an ideal natural sweetener with low calories, 300 times sweeter than sucrose but only 1/50 the calories, and is safe to eat, has no peculiar smell and and has good thermal stability. It is an excellent sweetener for people with obesity, hypertension and diabetes[3-4]. In recent years, there have been continuous reports on the biological activity of Mogroside, mainly focusing on its antioxidant, hypoglycemic, anti-diabetic, liver-protecting, anti-fatigue, hypolipidemic, and anti-cancer activities. This article provides a review of the main biological activities of Mogroside to provide a reference for follow-up research.
1 Chemical composition and structure of mogroside
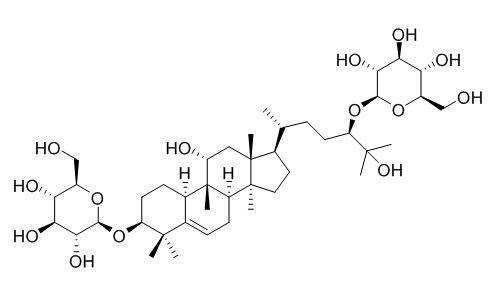
Mogroside, also known as mogroside, is a triterpene glucoside, and its aglycone is a triterpene alcohol. Two glucoside side chains consisting of four or fewer glucose units are linked to the aglycone by β-glycosidic bonds. The side chains are linked to the aglycone mainly by β-1,6 and β-1,2 glycosidic bonds. Mogrosides have the common aglycone mogrol (Fig. 1a), the main difference being the different attached sugar groups and the oxidation of the 11-OH (Fig. 1b) or 7-H (Fig. 1c) of individual glycosides to =O.
Twenty-six types of cucurbit-type triterpene saponins, three types of triterpene benzoates and two types of pentacyclic triterpene alcohols have been isolated and identified (Fig. 1). In addition, two types of cucurbit-type triterpenoids, siraitic acid F (Fig. 1-m) and siraitic acid C (Fig. 1-n), have been isolated from the root of Siraitia grosvenorii [5-6]. Recently, 12 types of cucurbitane-type saponins were isolated and identified from the root and immature fruit of Siraitia grosvenori. Among these compounds, 20-hydroxy-11-oxomogroside IA1, β, 19-epoxy-29-nor-3,11-dioxo-cucurbit-24-ene-27-oic acid 27-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside and 19,29-nor-3,11-dioxo-cucurbit-4,24-diene -27-oicacid 27 - O - β - D - glucopyranosyl - (1 → 6) - β - D - glucopyranoside, with the typical structure of cucurbitane triterpenoid glycosides, which is slightly chemically modified [7-9], see Figure 2.
2 Biological activity of Mogroside
2.1 Antioxidant
Existing literature mainly studies the antioxidant properties of mogroside in vitro. Zhang Liqin found that different mogroside extracts have scavenging effects on both hydroxyl radicals and superoxide anion radicals, can inhibit lipid peroxidation in rat liver tissue, have a protective effect on Fe2+ and H2O2-induced liver tissue peroxidation damage, can reduce mitochondrial swelling and reduce the occurrence of red blood cell hemolysis. Among them, Mogroside V has a very strong scavenging activity for hydroxyl radicals and superoxide anion radicals, indicating that Mogroside V, the main saponin component of Luo Han Guo, is its main antioxidant active ingredient. However, the antioxidant activity of Mogroside extract is closely related to the antioxidant system [10]. Chen et al. used a chemiluminescence method to measure in vitro the antioxidant activity of two saponins isolated from Luo Han Guo, namely Mogroside V and 11-O-Mogroside V. The results showed that these two types of cucurbitane triterpene saponins have a significant inhibitory effect on active oxygen (O2-, H2O2 and ·OH) and the oxidative damage to DNA that they induce.
In addition, 11-O-Mogroside V has a stronger ability to scavenge O2- and H2O2 than Mogroside V, while Mogroside V is stronger than 11-O-Mogroside V in scavenging ·OH [11]. Xu et al. used the mouse insulinoma NIT-1 cell line as an experimental subject to study the inhibitory effect of Mogroside on intracellular oxidative stress induced by palmitic acid, and mainly investigated the effects of 1 mmol/L Mogroside on intracellular reactive oxygen species (ROS) and apoptosis. The results showed that compared with the palmitic acid-induced group, the Mogroside co-culture group significantly reduced the intracellular ROS concentration and restored the mRNA expression levels of GLUT2 and pyruvate kinase, but Mogroside was unable to reduce palmitic acid-induced apoptosis. These results suggest that mogroside may exert its antioxidant effect by reducing intracellular ROS and regulating the expression of glucose metabolism-related genes [12]. At present, there is no literature on the in vivo antioxidant activity of mogroside. Whether mogroside, which has strong antioxidant properties in vitro, also has strong antioxidant properties in vivo requires further research.
2.2 Hypoglycemic and anti-diabetic
It has been reported that after a single oral dose of 30% Mogroside at a dose of 200 mg/kg in healthy adults, the results showed that 30% Mogroside had no significant effect on blood glucose levels and liver enzyme activity in healthy people [1, 13-14]. Some scholars have also studied the effect of Mogroside on blood glucose through animal experiments. Qi Xiangyang et al. [15] found that 0.5, 1.0, and 3.0 g/kg Mogroside-containing Luo Han Guo extract had no effect on blood glucose in normal mice. The medium (1.0 g/kg) and low (0.5 g/kg) dose groups had a hypoglycemic effect on diabetic mice, while the blood glucose of mice in the high dose group (3.0 g/kg) increased instead. This indicates that when the dosage of the Mogroside extract is 0.5–1.0 g/kg, it has the effect of lowering the fasting blood glucose of diabetic mice, and there is a dose-effect relationship between the blood glucose lowering effects. In addition, Mogroside has a significant blood glucose lowering effect on mice with streptozotocin-induced diabetes, and it has a preventive effect on the abnormal increase in serum triglycerides and serum cholesterol in diabetic mice can increase the content of high-density lipoprotein cholesterol in the blood, normalize the body's blood lipid levels, and prevent lipid metabolic disorders caused by diabetes [16]. Its mechanism of lowering blood sugar may be related to improving the antioxidant and free radical scavenging capacity of diabetic mice, improving blood lipid levels, thereby reducing the damage of allopurinol to pancreatic β cells or improving the function of damaged cells [15-16].
Monk fruit extract has an anti-diabetic effect on spontaneously diabetic Goto-Kakizaki rats. It can improve insulin response in an oral glucose tolerance test, the accumulation of pancreatic insulin in the fasting state, improve renal function, and enhance the antioxidant properties of the liver and plasma [17]. Insulin-dependent diabetic (IDDM) mice showed significant damage to islet cells. In addition, treatment with 4-hydroxy-2-oxopyrimidine caused a significant increase in the expression of CD8+ lymphocytes, resulting in a sharp decrease in the CD4+/CD8+ ratio (but the CD4+ level remained unchanged).
Mice in the normal group and the experimental diabetes group that were given mogroside for 4 weeks significantly alleviated the early clinical symptoms, biochemical abnormalities, and pathological damage to islet cells of diabetes, and the low-dose mogroside treatment regulated the immune imbalance that occurred in mice with IDDM induced by tetrazine by upregulating the subpopulation and proportion of CD4+ lymphocytes and changing the intracellular cytokine profile. The expression of proinflammatory Th1 cytokines (such as IFN-γ and TNF-α) in splenic lymphocytes shifts towards a favorable Th2 type. Mogroside has no effect on normal mice, except for the low-dose Mogroside, which upregulates IL-4 expression levels. These results suggest that Mogroside has an anti-diabetic effect, and HPLC analysis showed that the main anti-diabetic active component is Mogroside V, the main component of Luo Han Guo saponin [18].

Mogroside also inhibits the increase in blood glucose levels after a single meal of maltose in rats. Suzuki et al. found that Mogroside extract and the isolated saponin also inhibit maltase activity in vitro, which may contribute to the observed anti-glycemic activity in vivo. Mogroside therefore appears to be a useful, non-caloric sucrose substitute and may alleviate postprandial blood glucose via the inhibition of maltase activity [19]. After treatment with 100 mg/kg Mogroside, the mice had significantly lower blood glucose and blood lipids and higher antioxidant activity than the other diabetic groups, and were similar to the commercial positive control group. In vitro analysis of antioxidant capacity showed that Mogroside and MogrosideV both exhibited strong oxygen radical scavenging activity. These results confirm that the extract may have an inhibitory effect on diabetes-induced hyperglycemia. In addition, this study suggests that the administration of MogrosideV may have an inhibitory effect on oxidative stress and hyperglycemia-related diabetic complications [20].
At present, there is not much literature on the anti-diabetic effects of Mogroside. The main research subjects are experimental animals, and there are few reports on its in vitro protective effects on pancreatic islet cells, its in vivo and in vitro anti-diabetic effects, and its mechanism of action in relation to complications.
2.3 Liver protection
Wang Qin et al. studied the protective effect of Mogroside on experimental chronic liver injury. The results showed that Mogroside can reduce serum ALT and AST activity, enhance liver tissue SOD and GSH-Px activity, reduce liver tissue MDA levels, and significantly reduce the degree of pathological changes in liver tissue. There was a significant difference compared to the model group (p<0.05 or p<0.01), suggesting that Mogroside has a significant hepatoprotective effect on chronic liver damage caused by CCl4 in rats, and its mechanism may be related to the inhibition of lipid peroxidation [21]. In addition, Mogroside has a protective effect on carbon tetrachloride-induced acute liver injury in mice and immune liver injury induced by Bacillus Calmette-Guérin (BCG) plus lipopolysaccharide (LPS).
It can significantly reduce the serum ALT and AST activities of mice with acute and immune liver injury, enhance the SOD activity of liver tissue homogenates and reduce the MDA content of mice with immune liver injury, and significantly reduce the degree of pathological changes in liver tissue. These results suggest that Mogroside's protective effect on acute liver injury and immune liver injury in mice may be related to its anti-lipid peroxidation effect [22]. Liao Changxiu found that 0.5 g/kg·d Mogroside administered by continuous gavage for 7 days can significantly reduce the increase in serum glutamic pyruvic transaminase caused by acute alcoholic liver injury in mice caused by ethanol, increase the activity of liver catalase and superoxide dismutase, reduce pathological changes in liver tissue, suggesting that Mogroside's protective effect on ethanol-induced liver damage may be related to enhancing the liver's antioxidant function [23].
Further studies have shown that Mogroside at different doses can significantly reduce the levels of ALT, AST, MDA, CⅣ, and PⅢNP in rats with CCl4-induced liver fibrosis, enhance SOD activity, reduce the degree of pathological damage to liver tissue, inhibit the expression of TGF-β1 in liver tissue, and have a significant protective effect on rats with CCl4-induced liver fibrosis [24]. Wang Qin et al. found that Mogroside had no specific cytotoxicity on rat hepatic stellate cells HSC-T6. Mogroside at 20% and 10% volume fractions significantly inhibited the proliferation of HSC-T6 (p<0.05 or p<0.01) and significantly inhibited the expression of Col-I, TGF-β1 and TIMP-1 mRNA expression (p<0.05, p<0.01), suggesting that Mogroside has no specific toxicity to HSC-T6 cells, but can inhibit HSC-T6 cell proliferation and Col-I expression and promote extracellular matrix (ECM) degradation, which may be part of its mechanism of action against liver fibrosis [25].
Xiao Gang and Wang Qin studied the protective effect of Mogroside on CCl4-induced acute liver injury in mice. The results showed that Mogroside can reduce the serum ALT and AST activities and significantly reduce the degree of pathological changes in liver tissue. For chronic liver injury caused by CCl4 in rats, Mogroside can reduce the serum ALT and AST activities; reduce the content of hyaluronic acid (HA), type III procollagen amino-terminal peptide (P III NP), and hydroxyproline (Hyp) content; enhances SOD and GSH-Px activity in liver tissue; reduces MDA levels in liver tissue; inhibits TGF-β1 expression and significantly reduces the degree of pathological changes in liver tissue. These results suggest that Luo Han Guo saponins have a preventive and therapeutic effect on acute liver injury caused by CCl4 in mice and chronic liver injury caused by CCl4 in rats, and also have a certain anti-hepatic fibrosis effect [26].
2.4 Anti-fatigue
Yao Jiwei et al. explored the effect of taking different doses of Luo Han Guo extract on the physiological functions of mice undergoing incremental load training by measuring the time of exhaustion swimming and exhaustion climbing, as well as the time of tolerance to hypoxia and high temperatures. The results showed that within a certain range, the degree of improvement in mouse physiological function was significantly dependent on the dose of Luo Han Guo taken. The effect of different doses of Luo Han Guo on mouse physiological function showed a typical two-way effect, with the effect gradually increasing with increasing dose, but when the dose continued to increase, inhibition occurred instead. The 15.0 g/k·w·d dose of Luo Han Guo had the most significant effect on improving mouse physiological function [27]. In addition, Luo Han Guo extract can increase the time to exhaustion during exercise in mice and promote Hb synthesis in the body [28].
Further studies have shown that the exercise capacity of mice in the medication group was significantly enhanced, and the decrease in Hb and the increase in serum GOT and CK, whole blood LD, and myocardial MDA in the immediate exhaustion group were significantly reduced. The Hb in the 24-hour recovery group was significantly higher, while serum GOT and CK and myocardial MDA were significantly lower than those in the recovery control group. The GSH-Px activity in the quiet medication group was significantly higher than that in the quiet control group.

The SOD and GSH-Px activities of the heart muscle in the drug-taking group immediately after exhaustion and after 24 hours of recovery were significantly higher than those of the corresponding control group, indicating that Luo Han Guo extract can significantly improve the exercise capacity of trained mice and the function of the heart muscle in resisting free radical oxidation, and has a significant protective effect against free radical damage caused by high-intensity exercise [29]. Xia Xing et al. showed that mogroside significantly prolonged the exhaustion swimming time of mice (p<0.05), and the hypoxia tolerance and survival time of mice in the 75 and 150 mg/kg groups were significantly prolonged (p<0.05). After taking mogroside, the liver glycogen and muscle glycogen content of mice increased significantly (p<0.001), significantly reduced blood urea nitrogen and lactic acid production after exercise (p<0.001), and significantly increased lactate dehydrogenase activity (p<0.05), suggesting that Mogroside can significantly enhance the mouse's resistance to fatigue and hypoxia. Its mechanism of action may be related to increasing the body's glycogen reserves and accelerating lactic acid metabolism [30], but its mechanism of action is still unclear.
2.5 Anti-cancer
In their search for natural cancer chemopreventive agents, Takasaki et al. screened for phytochemicals and food additives and identified two natural sweeteners, mogroside V and 11-O-mogroside V, showed strong inhibitory activity in a primary screening test for Epstein-Barr virus early antigen (EBV-EA) induced by the cancer promoter 12-O-tetradecanoylphorbol-13-acetate (TPA). These two sweeteners with cucurbitane triterpenoid aglycones have significant inhibitory effects on a two-stage carcinogenesis experiment in which mouse skin cancer was induced using peroxynitrite anion (ONOO-) as the initiator and TPA as the promoter. In addition, 11-O-MogrosideV also showed significant inhibitory effects in a two-stage carcinogenicity experiment in mice induced by 7,12-dimethylbenz [a]anthracene (DMBA) as a initiator and TPA as a promoter [31].
Mizushina et al. found that mogroside I E1, a steroidal glycoside, inhibits the growth of human cancer cells, while the two components that make up mogroside I E1, mogroside and D-glucose, have no inhibitory effect on cancer cells [32]. Li et al. discovered three new cucurbitane-type triterpene saponins, 11-O-Mogroside Ⅲ (11-oxomogroside Ⅲ), 11-dehydroxymogroside Ⅲ and 11-oxomogroside Ⅳ A. and tested the cytotoxicity of 12 Mogrosides and their analogues, including these three new compounds, on the colon cancer cell line HCT-116 and the liver cancer cell line SMMC-7721. Although there was no obvious cytotoxicity to these two liver cancer cells, a longer period of time-dependent relationship would indicate that they may be cytotoxic [8]. Matsumoto's research found that Luo Han Guo extract can inhibit Cyp1a1 induction, causing a block in ROS production, thereby inhibiting the occurrence of liver cancer, which is likely due to the inhibition of Ahr activity [33]. Weerawatanakorn et al. used TPA (12-O-tetradecanoyl phorbol 13-acetate) to stimulate mouse skin to establish a model. The results showed that the mechanism by which monascus extract inhibits inflammation-related tumorigenesis is related to its regulation of various signal pathways and inhibition of the upregulation of nuclear factor NF-кB [34].
2.6 Anti-inflammatory
Pan et al. studied the inhibitory effect of Luo Han Guo extract on the inducible expression of iNOS and COX-2 in mouse RAW 264.7 macrophages activated by lipopolysaccharide (LPS). The results showed that Luo Han Guo extract could interfere with the PI3K/Akt/IKK and MAPK signal pathways, inhibit the activation of nuclear factor NF-κB, and down-regulate the gene expression of inflammatory factors iNOS and COX-2 in macrophages [35]. Chen Yao et al. used the xylene and carrageenan-induced inflammation experiment to observe the anti-inflammatory effect of Mogroside. The results showed that Mogroside had no significant effect on the xylene and carrageenan-induced inflammation model [36]. However, Di et al. found that Mogroside can inhibit inflammation induced by lipopolysaccharide (LPS) in RAW 264.7 cells by downregulating key inflammatory genes iNOS, COX-2 and IL-6 and upregulating certain inflammatory protective genes such as PARP1, BCL2l1, TRP53 and MAPK9. Similarly, for the mouse ear swelling model, Mogroside down-regulates COX-2 and IL-6 and up-regulates the expression of PARP1, BCL2l1, TRP53, MAPK9 and PPARδ genes to inhibit inflammation induced by 12-O-tetradecanoylphorbol-13-acetate, suggesting that Mogroside's anti-cancer and anti-diabetic effects may be partially mediated by its anti-inflammatory activity [37]. Whether Mogroside's anti-diabetic effect is partially contributed by its anti-inflammatory effect and its related mechanism needs to be further explored and clarified.
2.7 Other biological activities
Wang Qin et al. used Mogroside to treat normal and cyclophosphamide (CTX)-suppressed mice by gavage to test the effects on macrophage phagocytic function and T cell proliferation. The results showed that Mogroside had no significant effect on the immune function of normal mice, but could significantly improve the macrophage phagocytic function and T cell proliferation of CTX immunosuppressed mice [38]. Zhao Yan et al. used a high-fat diet to feed ICR mice to establish a hyperlipidemia animal model. After feeding a certain dose of Mogroside for 60 days, the average serum TC content of the mice was 86.07 mg/dL, which was 27.5% lower than that of the high-fat model group, and the effect was significant (p<0.05). The TG content was 118.98 mg/dL, a 44.5% decrease, significantly lower than the high-fat model group (p<0.05), and an average HDL-C content of 0.01 mg/dL, a 27.3% increase, reaching a significant level (p<0.05), suggesting that Mogroside has the physiological function of positively regulating blood lipid metabolism in the body[39].
In addition, Streptococcus mutans hardly grows and multiplies in Mogroside culture medium, suggesting that Mogroside has an anti-caries effect [40]. A study found that a single dose of Mogroside extract had no significant effect on the histamine-induced nose-rubbing and scratching behavior of ICR mice, but 300 or 1000 mg/kg Mogroside extract had a significant inhibitory effect after 4 weeks of continuous feeding [41]. Chen et al. found that mogroside's main aglycone mogroside (mogrol) is a potent activator of AMPK in HepG2 cells, suggesting that mogroside's aglycone mogrol activates AMPK, which at least in part contributes to mogroside's anti-hyperglycemic and hypolipidemic effects in vivo [42].

3 Outlook
Currently, mogroside is mainly used as a new non-sugar, low-calorie sweetener. In recent years, although related studies have suggested that it has a variety of biological activities, mainly at the animal level, its mechanism of action is still unclear, and there is not much literature on in-depth research at the cellular, molecular, and genetic levels. The use of modern pharmacology and molecular biology techniques to further study the mechanism of its biological activity is expected to lead to the development of Mogroside functional foods or pharmaceutical precursors. In addition, there are few reports on the metabolic pathways of Mogroside in the body and the related metabolites. As a triterpene glycoside, it is not clear whether it will be degraded by gastrointestinal digestive enzymes and intestinal microbial flora after ingestion, and how it will be degraded. Therefore, it is necessary to use modern analytical techniques and metabolomics to further study the biotransformation, metabolic pathways, products, and metabolic mechanisms of Mogroside after it is ingested.
References
[1] Zhang Hong, Li Xiaohong. Research progress on the pharmacological effects and toxicity of Luo Han Guo [J]. Chinese Agricultural Science Bulletin, 2011, 27(5): 430-433.
[2] Zhang Liqin. Isolation, analysis and biological activity evaluation of the low-calorie sweetener Luo Han Guo saponin [D]. Wuhan: Huazhong Agricultural University, 2004.
[3] Ju Wei, Cheng Yuyan, Zhang Jian. Overview of research on Luo Han Guo [J]. Guangxi Light Industry, 2001, 4: 4-6.
[4] Li Dianpeng, Zhang Hourui. Research and application of Luo Han Guo, a specialty plant in Guangxi [J]. Guangxi Plants, 2000, 20(3): 270-276.
[5] JIN J S , LEE J H. Phytochemical and pharmacological aspects of Siraitia grosvenorii,luo han guo. Oriental Pharmacy and Experimental Medicine[J]. 2012,12(4):233-239.
[6] SI J Y,CHEN D H,TU G Z. Siraitic Acid F,a New nor- Cucurbitacin With Novel Skeleton,from the Roots of Siraitia Grosvenorii [J] . Journal of Asian Natural Products Research , 2005,7(1):37-41.
[7] LI D,IKEDA T,MATSUOKA N,et al. Cucurbitane glycosides from unripe fruits of Lo Han Kuo(Siraitia grosvenori)[J]. Chemical and Pharmaceutical Bulletin,2006,54(10):1425-1428.
[8] LI D,IKEDA T,NOHARA T,et al. Cucurbitane glycosides from unripe fruits of Siraitia grosvenori [J] . Chemical and Pharmaceutical Bulletin,2007,55(7):1082-1086.
[9] LI D P,EL-AASR M,IKEDA T,et al. Two new cucurbitane- type glycosides obtained from roots of Siraitia grosvenori SWINGLE [J]. Chemical and Pharmaceutical Bulletin,2009,57(8):870-872.
[10] Zhang Liqin, Qi Xiangyang. Research progress on Luohanguo saponins and pharmacological activities [J]. Guangxi Tropical Agriculture, 2005, 6: 22-24.
[11] CHEN W,WANG J,QI X,et al. The antioxidant activities of natural sweeteners,mogrosides,from fruits of Siraitia grosvenori [J]. International Journal of Food Sciences and Nutrition,2007, 58(7):548-556.
[12] XU Q ,CHEN S ,DENG L ,et al. Antioxidant effect of mogrosides against oxidative stress induced by palmitic acid in mouse insulinoma NIT-1 cells[J] . Brazilian Journal of Medical and Biological Research,2013,46(11):949-955.
[13] Xu Qing, Liang Ronggan, Su Xiaojian, et al. Effects of Luo Han Guo sweet saponin on blood glucose levels and liver enzyme activities in normal people [J]. Food Science, 2007, 28 (6): 315-317.
[14] Su Xiaojian, Xu Qing, Liang Ronggan, et al. Research on the toxic effects of mogroside [J]. Food Science, 2005, 26 (3): 221-224.
[15] Qi Xiangyang, Chen Weijun, Song Yunfei, et al. Hypoglycemic effect of Luo Han Guo extract on diabetic mice [J]. Chinese Journal of Public Health, 2003, 19(10): 1226-1227.
[16] Zhang Liqin, Qi Xiangyang, Chen Weijun, et al. Effects of Mogroside extract on blood glucose, blood lipids and antioxidant activity in diabetic mice [J]. Chinese Journal of Pharmacology, 2006, 22(2): 237-240.
[17] SUZUKI YA,TOMODA M,MURATA Y,et al. Antidiabetic effect of long -term supplementation with Siraitia grosvenori on the spontaneously diabetic Goto-Kakizaki rat[J]. British Journal of Nutrition,2007,97(4):770-775.
[18] QI X,CHEN W,LIU L,et al. Effect of a Siraitia grosvenori extract containing mogrosides on the cellular immune system of type 1 diabetes mellitus mice [J] . Molecular Nutrition & Food Research,2006,50(8):732-738 .
[19] SUZUKI Y A ,MURATA Y ,INUI H ,et al. Triterpene glycosides of Siraitia grosvenori inhibit rat intestinal maltase and suppress the rise in blood glucose level after a single oral administration of maltose in rats[J]. Journal of Agricultural and Food Chemistry,2005,53(8):2941-2946.
[20] QI X Y,CHEN W J,ZHANG L Q,et al. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress,serum glucose,and lipid levels in alloxan-induced diabetic mice[J]. Nutrition Research,2008,28(4):278-284.
[21] Wang Q, Xiao G. Experimental study on the protective effect of mogroside on chronic liver injury in rats [J]. Guangxi Traditional Chinese Medicine, 2007, 30(5): 54-56.
[22] Xiao G, Wang Q. Study on the protective effect of mogroside on experimental liver injury in mice [J]. Chinese Pharmacy, 2008, 19(3): 163-165.
[23] Liao Changxiu, Li Shubo, Xue Qiang, et al. Antagonistic effect of mogroside on ethanol-induced liver injury [J]. Shizhen Traditional Chinese Medicine, 2010, 21(12): 3116-3117.
[24] Xiao G, Chen Z, Li W, et al. Protective effect of mogrosides on carbon tetrachloride-induced liver fibrosis in rats [J]. Shandong Medicine, 2012, 52(16): 19-24.
[25] Wang Q, Wang W, Long Y, et al. Effects of Luo Han Guo sweet extract on proliferation of hepatic stellate cells HSC-T6 and genes related to liver fibrosis [J]. Chinese Herbal Medicine, 2013, 44(3): 331-334.
[26] Xiao G, Wang Q. Experimental study on the hepatoprotective effect of Luo Han Guo sweet extract [J]. Chinese Journal of Experimental Formulas, 2013, 19 (2): 196-200.
[27] Yao Jiwei, Tang Hui, Shen Weihua, et al. Observation of the effects of different doses of Luo Han Guo extract on the physiological functions of mice trained with incremental loads [J]. Liaoning Sports Science and Technology, 2007, 29 (3): 24-26.
[28] Yao Jiwei, Tang Hui, Zhou Liang, et al. Effects of Luo Han Guo extract on exercise endurance and antioxidant damage to liver tissue in mice [J]. Chinese Journal of Sports Medicine, 2008, 27(2): 221-223.
[29] Yao J W, Yang Y L, Tang H, et al. Effects of Luo Han Guo extract on exercise capacity and myocardial free radical metabolism in trained mice [J]. Journal of Beijing Sport University, 2009, 32(3): 67-69.
[30] Xia Xing, Zhong Zhenguo, Lin Caiyun, et al. Anti-fatigue and hypoxia tolerance effects of mogroside [J]. Chinese Journal of Experimental Traditional Medicine, 2012, 18(17): 198-201.
[31] TAKASAKI M , KONOSHIMA T , MURATA Y , et al. Anticarcinogenic activity of natural sweeteners ,cucurbitane glycosides,from Momordica grosvenori[J]. Cancer Letters,2003, 198(1):37-42.
[32] MIZUSHINA Y,AKIHISA T,HAYAKAWA Y,et al. Structural Analysis of Mogrol and its Glycosides as Inhibitors of Animal DNA Polymerase and Human Cancer Cell Growth[J]. Letters in Drug Design & Discovery,2006,3(4):253-260.
[33] MATSUMOTO S,JIN M,DEWA Y,et al. Suppressive effect of Siraitia grosvenorii extract on dicyclanil-promoted hepatocellular proliferative lesions in male mice[J]. The Journal of Toxicological Sciences,2009,34(1):109-118.
[34] WEERAWATANAKORN M,YANG J R,TSAI M L,et al. Inhibitory effects of Momordica grosvenori Swingle extracts on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mouse skin[J]. Food & Function,2014.
[35] PAN M H,YANG J R,TSAI M L,et al. Anti-inflammatory effect of Momordica grosvenori Swingle extract through suppressed LPS -induced upregulation of iNOS and COX -2 in murine macrophages[J]. Journal of Functional Foods,2009,1(2):145-152.
[36] Chen Y, Wang Y, Fan X, et al. Research on the laxative and anti-inflammatory effects of monk fruit sweeteners [J]. Chinese Journal of Pharmaceutical Sciences, 2011, 27(3): 202-204.
[37] DI R,HUANG M T,HO C T. Anti-inflammatory Activities of Mogrosides from Momordica grosvenori in Murine Macrophages Food Chemistry,2005,53(8):2941-2946.
[20] QI X Y,CHEN W J,ZHANG L Q,et al. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress,serum glucose,and lipid levels in alloxan-induced diabetic mice[J]. Nutrition Research,2008,28(4):278-284.
[21] Wang Q, Xiao G. Experimental study on the protective effect of mogroside on chronic liver injury in rats [J]. Guangxi Traditional Chinese Medicine, 2007, 30(5): 54-56.
[22] Xiao G, Wang Q. Study on the protective effect of mogroside on experimental liver injury in mice [J]. Chinese Pharmacy, 2008, 19(3): 163-165.
[23] Liao Changxiu, Li Shubo, Xue Qiang, et al. Antagonistic effect of mogroside on ethanol-induced liver injury [J]. Shizhen Traditional Chinese Medicine, 2010, 21(12): 3116-3117.
[24] Xiao G, Chen Z, Li W, et al. Protective effect of mogrosides on carbon tetrachloride-induced liver fibrosis in rats [J]. Shandong Medicine, 2012, 52(16): 19-24.
[25] Wang Q, Wang W, Long Y, et al. Effects of Luo Han Guo sweet extract on proliferation of hepatic stellate cells HSC-T6 and genes related to liver fibrosis [J]. Chinese Herbal Medicine, 2013, 44(3): 331-334.
[26] Xiao G, Wang Q. Experimental study on the hepatoprotective effect of Luo Han Guo sweet extract [J]. Chinese Journal of Experimental Formulas, 2013, 19 (2): 196-200.
[27] Yao Jiwei, Tang Hui, Shen Weihua, et al. Observation of the effects of different doses of Luo Han Guo extract on the physiological functions of mice trained with incremental loads [J]. Liaoning Sports Science and Technology, 2007, 29 (3): 24-26.
[28] Yao Jiwei, Tang Hui, Zhou Liang, et al. Effects of Luo Han Guo extract on exercise endurance and antioxidant damage to liver tissue in mice [J]. Chinese Journal of Sports Medicine, 2008, 27(2): 221-223.
[29] Yao J W, Yang Y L, Tang H, et al. Effects of Luo Han Guo extract on exercise capacity and myocardial free radical metabolism in trained mice [J]. Journal of Beijing Sport University, 2009, 32(3): 67-69.
[30] Xia Xing, Zhong Zhenguo, Lin Caiyun, et al. Anti-fatigue and hypoxia tolerance effects of mogroside [J]. Chinese Journal of Experimental Traditional Medicine, 2012, 18(17): 198-201.[31] TAKASAKI M , KONOSHIMA T , MURATA Y , et al. Anticarcinogenic activity of natural sweeteners ,cucurbitane glycosides,from Momordica grosvenori[J]. Cancer Letters,2003, 198(1):37-42.
[32] MIZUSHINA Y,AKIHISA T,HAYAKAWA Y,et al. Structural Analysis of Mogrol and its Glycosides as Inhibitors of Animal DNA Polymerase and Human Cancer Cell Growth[J]. Letters in Drug Design & Discovery,2006,3(4):253-260.
[33] MATSUMOTO S,JIN M,DEWA Y,et al. Suppressive effect of Siraitia grosvenorii extract on dicyclanil-promoted hepatocellular proliferative lesions in male mice[J]. The Journal of Toxicological Sciences,2009,34(1):109-118.
[34] WEERAWATANAKORN M,YANG J R,TSAI M L,et al. Inhibitory effects of Momordica grosvenori Swingle extracts on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mouse skin[J]. Food & Function,2014.
[35] PAN M H,YANG J R,TSAI M L,et al. Anti-inflammatory effect of Momordica grosvenori Swingle extract through suppressed LPS -induced upregulation of iNOS and COX -2 in murine macrophages[J]. Journal of Functional Foods,2009,1(2):145-152.
[36] Chen Y, Wang Y, Fan X, et al. Research on the laxative and anti-inflammatory effects of monk fruit sweeteners [J]. Chinese Journal of Pharmaceutical Sciences, 2011, 27(3): 202-204.
[37] DI R,HUANG M T,HO C T. Anti-inflammatory Activities of Mogrosides from Momordica grosvenori in Murine Macrophages [J]. Bmc Syst Biol,2010,4:141.
[42] Gasch A P,Werner Washburne M. The genomics of yeast responses to environmental stress and starvation[J]. Funct Integr Genomics,2002,2:181-192.
[43] Vanz A L ,Luensdorf H ,Adnan A ,et al. Physiological response of Pichia pastoris GS115 to methanol -induced high level production of the Hepatitis B surface antigen:catabolic adaptation,stress responses,and autophagic processes[J]. Microb Cell Fact,2012,11:103.
[44] Carnicer M,Ten Pierick A,Van Dam J,et al. Quantitative metabolomics analysis of amino acid metabolism in recombinant Pichia pastoris under different oxygen availability conditions [J]. Microb Cell Fact,2012,11:83.
[45] Dragosits M,Stadlmann J ,Graf A,et al. The response to unfolded protein is involved in osmotolerance of Pichia pastoris [J]. BMC Genomics,2010,11:207.
[46] Jorda J,Jouhten P,Camara E,et al. Metabolic flux profiling of recombinant protein secreting Pichia pastoris growing on glucose:Methanol mixtures[J]. Microb Cell Fact,2012,11:57.
[47] Sola A ,Jouhten P ,Maaheimo H ,et al. Metabolic flux profiling of Pichia pastoris grown on glycerol/methanol mixtures in chemostat cultures at low and high dilution rates [J] . Microbiology,2007,153:281-290.
[48] Jorda J ,De Jesus S S ,Peltier S ,et al. Metabolic flux analysis of recombinant Pichia pastoris growing on different glycerol/methanol mixtures by iterative fitting of NMR -derived 13C - labelling data from proteinogenic amino acids [J] . New Biotechnol,2014,31(1):120-132.
[49] Unrean P. Pathway analysis of Pichia pastoris to elucidate methanol metabolism and its regulation for production of recombinant proteins[J]. Biotechnol Prog,2014,30(1):28-37.
[50] Guan Bo, Jin Jian, Li Huazhong. Research progress on improving the ability of Pichia pastoris to secrete and express foreign proteins [J]. Acta Microbiologica Sinica, 2011, 51(7): 851-857.
[51] Zhu T,Guo M,Zhuang Y,et al. Understanding the effect of foreign gene dosage on the physiology of Pichia pastoris by transcriptional analysis of key genes[J]. Appl Microbiol Biotechnol, 2011,89(4):1127-1135.
[52] Lin X Q,Liang S L,Han S Y,et al. Quantitative ITRAQ LC-MS/MS proteomics reveals the cellular response to heterologous protein overexpression and the regulation of HAC1 in Pichia pastaris[J]. J Proteomics,2013,91:58-72.
[53] Liberek K,Lewandowska A,Zietkiewicz S. Chaperones in control of protein disaggregation[J]. EMBO J,2008,27(2):328-335.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese