Study on Luo Han Guo Sweetener Mogroside V
Siraitia grosvenorii (Swingle) C. Jeffrey, also known as Luohanguo, is the fruit of a perennial vine in the family Cucurbitaceae. It is mainly produced in Guangxi and is an economically and medicinally important plant that is endemic to China[1]. Siraitia grosvenorii is cool in nature and sweet in taste. It has been used as a traditional medicinal herb for more than 300 years in folk medicine. It has the effects of clearing away heat, moistening the lungs, relieving the pharynx and opening the voice, and lubricating the intestines to promote bowel movement[2⁃3].
At present, more than 100 compounds have been isolated from Luo Han Guo, including 46 triterpenes, 7 flavonoids, 19 amino acids, 2 polysaccharides, etc. [4]. Mogroside is the main active ingredient in Luo Han Guo. It is a type of cucurbitane triterpene glycoside, also known as Luo Han Guo extract, Mogroside, Luo Han Guo saponin, etc. [5]. Mogroside is also the main component of Luo Han Guo's sweetness.
In 1975, Lee[6] determined that the glycoside of the triterpene is the main sweetening component. In 1983, Mogroside Ⅳ, Mogroside Ⅴ and Mogroside Ⅵ were first isolated from Luohanguo, and more than 30 similar compounds have been isolated from Luohanguo since then[7⁃8]. These compounds all have mogroside as the aglycone, followed by different numbers of sugar moieties, as shown in Figure 1. The natural sweetener mogroside has a high sweetness and low calories. Compared to artificial sweeteners, it has less aftertaste and is safer. It also has a hypoglycemic effect and is an ideal substitute for sucrose. It has now been included in the list of food additives in many countries, including the United States, Japan, Australia, and New Zealand [5]. This paper reviews the sweet taste structure-activity relationship, periodic changes, biosynthesis pathway, hypoglycemic effect and other biological activities, and safety of Mogroside, with a view to providing further reference for the application of Mogroside as a natural sweetener.

1 Sweetness structure-activity relationship
Mogrosides are named Mogroside Ⅲ, Mogroside ⅢE, Mogroside Ⅳ, Mogroside Ⅴ, 11⁃O ⁃Mogroside Ⅴ, Luohanguo new glycoside, Simonoside I, etc., among which Mogroside V has the highest content [9], see Table 1. Murata et al. [10] conducted sensory tests and found that Mogroside III, Mogroside IV, Mogroside V, Simonoside I, and 11O-Mogroside V are equivalent to 195, 300, 378, 465, and 68 times the sweetness of sucrose, respectively.
Structurally, mogroside is based on mogroside alcohol as the aglycone, with glucose units linked to the C⁃3 and C⁃24 positions via glycosidic bonds. The relationship between its sweetness and structure is as follows: ① the number of sugar moieties: to maintain the sweetness, the total number of sugar groups attached to the C⁃3 and C⁃24 positions must not be less than 3; ②the oxidation state of the 11 substituents, if the 11 hydroxyl groups are in the α configuration, they are sweet; if they are in the β configuration, they are tasteless; if the 11 hydroxyl groups are oxidized, they are bitter [11⁃13]. In addition, although only four sugar moieties are attached to it, the sweetness of simenoside I is much higher than that of mogroside IV, which also contains four sugar moieties. Therefore, the effect of the position of the sugar group connection in the structure on the sweetness of Mogroside is also worthy of further research. See Table 1 for common Luohan fruit sweet glycoside structural information.
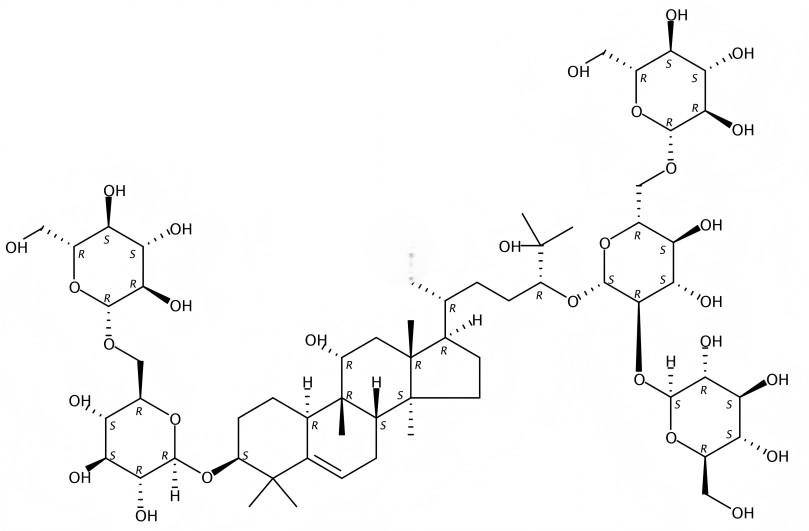
2 Periodic changes and biosynthesis
The ripe Luohanguo fruit is sweet, while its young fruit is bitter, suggesting that the content of glycosides in Luohanguo fruit changes during growth. Murata et al. [10] found that the content of sweet glycosides in ripe Luohanguo is higher than that in unripe Luohanguo. Li Dianpeng et al. [14] used thin-layer chromatography to study the changes in the composition of the glycosides in Luo Han Guo at different growth ages and found that young fruits less than 30 days old mainly contain the bitter Mogroside Ⅱ E; Mogroside Ⅲ begins to appear after 30 days, and the content reaches a peak at 55 days, and Mogroside Ⅳ is also produced, Mogroside Ⅳ content reached a maximum at 70 d; after 85 d, Luo Han Guo sweet glycoside V was the main sweet component of Luo Han Guo.
Lu Fenglai et al. [15] studied Luo Han Guo at different growth stages by RP⁃HPLC method and found that the bitter glycoside (Mogroside ⅡE, 11⁃O ⁃Mogroside ⅡE) content in Luo Han Guo was highest at 10 d; after 55 d, the sweet glycoside Mogroside Ⅴ became dominant; after 65 d, the content of Luo Han Guo sweet glycoside Ⅴ did not change much. Except for Mogroside Ⅴ, the content of all glycosides showed a trend of first increasing and then decreasing. It has also been reported in the literature that oligosaccharide chains in the biosynthesis of triterpene glycosides in plants are likely to be synthesized by sequentially adding monosaccharide molecules to the aglycone [16], suggesting a unidirectional transformation of bitter low-glycosides to sweet high-glycosides during the growth cycle of Luo Han Guo glycosides.
Itkin et al. [17] used transcriptome and genome data to fully analyze the biosynthetic pathway of monk fruit sweetener V in the fruit of the monk fruit.
The biosynthesis in the fruit can be divided into two steps: the formation of monk fruit alcohol and glycosylation. First, the production of luohangol. Acetyl coenzyme A is converted to squalene via the mevalonate pathway. Squalene is then converted to 24, 25-dihydroxy-gammahydroxysteroid, which is hydroxylated by CYP87D18 to form momordinol. In the subsequent glycosylation process, momordinol is first glycosylated by glycosyltransferase UGT720-269-1 to form bitter glycosides (Mogroside Ⅰ A1, Mogroside ⅢX, Mogroside ⅣA, and Simonin Ⅰ are formed by the next glycosylation of Luo Han Guo sweet, and Mogroside Ⅴ is finally formed. The biosynthetic pathway is shown in Figure 2.
In this biosynthetic pathway, the expression of key enzymes UGT720⁃269⁃1 and UGT94⁃289⁃3 that affect glycosylation has temporal differences. UGT720⁃269⁃1 is highly expressed in young fruits and decreases as the fruit matures. The UGT94 enzyme family, on the other hand, shows an increase in expression during the later stages of development. This is consistent with previous reports that the main component in young fruits is low-glycoside compounds such as Mogroside ⅡE, and that high-glycoside compounds continue to increase during the later stages of fruit development, with Mogroside Ⅴ eventually becoming the main component in mature fruits.
In summary, Luohanguo is generally picked after 65 days when the fruit begins to ripen. During processing, extracts from unripe and ripe fruit must not be mixed, as this will reduce the content of Mogroside and affect the quality of the extract.
3. Hypoglycemic effect
Studies have shown that Mogroside is not only effective in controlling blood sugar, but also prevents chronic complications associated with diabetes. Its hypoglycemic mechanism can be divided into repairing pancreatic damage, promoting insulin secretion, improving insulin resistance, anti-oxidative stress,and inhibiting α-glucosidase activity.
3.1 Repairing pancreatic damage and promoting insulin secretion
Pyrimidine can damage pancreatic β cells by generating free radicals and inhibiting glucokinase, suppressing proinsulin synthesis, and causing type 1 diabetes [18]. Zhang Liqin et al. [19] found that Mogroside extract can reduce blood sugar by reducing the damage of alloxan to pancreatic β cells or improving the function of damaged cells. Its main active ingredient may be Mogroside. Qi et al. [20] showed that Mogroside extract can effectively improve the morphology of islet cells in diabetic mice, increase the number of islet cells, reduce the damage to mouse islet β cells caused by tetraoxypyrimidine, and improve metabolic disorders. Zhou Ying et al. [21] used rat islet β cell tumor cells RIN⁃5F as the research object and found that Luo Han Guo extract and Mogroside Ⅴ can promote insulin secretion.

3. 2 Improving insulin resistance
Insulin resistance refers to the decreased efficiency of insulin in promoting glucose uptake and utilization, and is a major pathological feature of type 2 diabetes. Reducing insulin resistance can delay the progression of diabetic complications. Liu et al. [22] used streptozotocin (STZ) in combination with a high-fat diet to induce a type 2 diabetes model in C57BL/6 mice. The study found that Mogroside extract can improve insulin resistance in diabetic mice, as indicated by a decrease in the insulin resistance index and an increase in the insulin sensitivity index. Another study reported that activation of the c-Jun N-terminal kinase (TLR4-JNK) and oxidative stress can mediate the pathway through the TLR4 pathway [25].
3. 3 Antioxidant stress
Oxidative stress refers to a state in which the body's oxidation and antioxidant effects are out of balance, producing a large amount of reactive oxygen species (ROS) that damage body tissues, cells, proteins and other biological macromolecules. In addition to inducing insulin resistance, ROS can also directly damage pancreatic β cells during the development of diabetes, ultimately triggering or aggravating diabetes [24]. Zhu Huiling et al. [26] showed that pre-treatment with Luo Han Guo saponin can reduce the excessive accumulation of reactive oxygen species and free radicals in ethanol-damaged L⁃02 cells, reduce oxidative stress, and inhibit lipid peroxidation.
Zou Jian et al. [27] studied the antioxidant activity of crude Mogroside extract and found that it has good abilities to scavenge free radicals (DPPH, ABTS, ORAC), reduce iron ions (FRAP), and resist lipid oxidation. The antioxidant capacity increased with increasing concentration. Chen et al. [28] studied the in vitro antioxidant activity of Mogroside and found that it has an inhibitory effect on oxygen free radicals (O2-, H2O2, ·OH) and DNA oxidative damage. Among them, Mogroside V is more effective at scavenging ·OH, while 11⁃O⁃Mogroside V has a higher scavenging rate for O2- and H2O2. In addition, 11⁃O ⁃Mogroside Ⅴ has an inhibitory effect on OH-induced DNA damage. Zhang Liqin et al. [19] found that the Mogroside extract can reduce the production of lipid peroxides (MDA) in the liver of mice with diabetes induced by alloxan, and increase the activity of the antioxidant enzyme system SOD and GSH⁃Px.
The activity of glycosidase can slow down the rate of carbohydrate hydrolysis and glucose production and delay glucose absorption, thereby reducing postprandial blood glucose. This method is important for the prevention and treatment of type 2 diabetes[29]. Studies have found that Luo Han Guo extract from different growth stages has an inhibitory effect on postprandial blood glucose generation in mice. One of the mechanisms of its hypoglycemic effect is that Luo Han Guo saponin extract can directly inhibit α-glucosidase activity, and its inhibitory effect is positively correlated with the dose [30⁃31].
3. 5 Other
studies have shown that the activation of adenosine monophosphate-activated protein kinase (AMPK) by Luo Han Guo extract may help inhibit gluconeogenesis in diabetic mice, thereby lowering blood glucose levels [22]. Another study reported that Luo Han Guo saponin extract can reduce the levels of the cytokines IFN-γ and TNF-α and increase the level of the cytokine IL-4, thereby improving the cellular immune imbalance in diabetic mice and controlling diabetes [20]. In addition, mogroside extract can improve the serum high-density lipoprotein levels of diabetic mice, normalize blood lipid levels, prevent lipid metabolism disorders caused by diabetes, and prevent and treat diabetic complications [19].
In summary, mogroside not only has a good preventive and therapeutic effect on both type 1 and type 2 diabetes, but also effectively prevents diabetes-related complications.
4 Other pharmacological effects
4. 1 Cough suppressant, expectorant, and antiasthmatic
Chen Yao et al. [33] found that mogroside can reduce the number of coughs in mice and promote the production of tracheal secretions. Liu Ting et al. [34] screened the effective components of Luo Han Guo for cough suppressant and expectorant effects, and speculated that mogroside V is the main active ingredient of Luo Han Guo for cough suppression. Wang et al. [35] established a model of the spectrum of efficacy of Luo Han Guo in relieving phlegm, and the results showed that Mogroside Ⅴ and 11⁃O ⁃Mogroside Ⅴ were positively correlated with the effect of relieving phlegm and contributed significantly. Song et al. [36] found that Mogroside Ⅴ can effectively inhibit the airway hyperresponsiveness induced by ovalbumin.
4. 2 Hepatoprotective effect
Xiao Gang et al. [37] used carbon tetrachloride to induce acute liver injury and immune liver injury models in mice, respectively. The study found that Mogroside can reduce the serum ALT and AST activities of mice in the two liver injury models, increase the SOD activity of liver tissue homogenates in immune liver injury, reduce the MDA level, and improve the pathological damage of liver tissue. Hepatic stellate cells (HSCs) play an important role in the development of liver fibrosis, so inducing the apoptosis of activated HSCs is an effective means of preventing and treating fibrosis [38]. Studies have found that Mogroside-containing serum can block the cycle of rat hepatic stellate cells HSC⁃T6. Mogroside can inhibit the activation of human hepatic stellate cells LX⁃2 in vitro and promote the apoptosis of hepatic stellate cells.
4. 3 Anti-cancer
In in vitro and in vivo models of pancreatic cancer, Mogroside Ⅴ inhibited tumor growth by promoting apoptosis and cell cycle arrest in pancreatic cancer cells PANC⁃ 1 [42]. Mogroside ⅣE dose-dependently inhibited the proliferation of human colon cancer cells HT29 and human laryngeal epidermoid carcinoma cells Hep⁃2 and tumor growth in a mouse model of xenograft tumors [43]. In vivo skin carcinogenesis experiments in mice found that mogroside V and 11⁃O mogroside V can effectively inhibit the two-step carcinogenesis process using peroxynitrite as a mutagenic carcinogen and terephthalic acid as a tumor promoter. 11⁃O ⁃Mogroside Ⅴ also inhibits the two-step carcinogenesis process using 7, 12⁃dimethylbenz[a]anthracene as a mutagenic carcinogen and terephthalic acid as a tumor promoter [44].
4. 4 Anti-inflammatory and antibacterial
Di et al. [45] found that mogroside can inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 cells by downregulating the expression of key inflammatory genes iNOS, COX-2, and IL-6 and upregulating the expression of inflammatory protective genes PARP1, BCL2L1, TRP53, and MAPK9. Similarly, in a mouse ear swelling model, mogroside can inhibit the inflammation response induced by 12-oxotetradecanoylphorbol-13-acetate by down-regulating COX-2 and IL-6 expression and up-regulating PARP1, BCL2L1, TRP53, MAPK9 and PPARδ expression. He O ⁃ Mogroside Ⅴ is not only the main active ingredient of the anti-inflammatory activity of Luo Han Guo in vivo, but also one of the main active ingredients of Luo Han Guo's inhibitory effect on Staphylococcus aureus. Mogroside Ⅴ is the main active substance of Luo Han Guo's inhibitory effect on Enterococcus faecalis and Escherichia coli.
5 Safety
Su Xiaojian et al. [47] showed that the LD50 value of Luo Han Guo sweet glycoside (81.6% glycoside content) administered to mice by gavage was greater than 10,000 mg/kg, which is practically non-toxic. Mogroside 3.0 g/kg (equivalent to 360 times the human dosage) was given to dogs by gavage for 4 weeks, and there was no significant effect on the biochemical indicators of the dogs or morphological changes in the tissues and organs. Jin et al. [48] conducted a repeated dose subchronic toxicity study on Wistar rats and found that no rats in the Mogroside dose groups died within 13 weeks and that all indicators were normal. It is therefore speculated that the level of adverse reactions in humans is 2,520 mg/kg per day for men and 3,200 mg/kg per day for women or higher. Another study showed that Mogroside administered to mice by gavage at 0.25, 0.5, and 1 g/kg was not genotoxic or reproductive toxic, but when the dose reached 5 g/kg, the rate of micronuclei in the bone marrow erythrocytes and the rate of sperm deformities in male mice were higher than those in the negative control group (P<0.05) [49]. Therefore, further research is needed to determine whether mogroside is genotoxic, and the dosage of mogroside also requires attention.

6 Conclusion
With the improvement of people's living standards, metabolic diseases such as diabetes and obesity have become an important threat to human health. Therefore, high-sweetness, low-calorie artificial sweeteners are widely used in the food industry to replace sucrose. However, in recent years, with the popularization of artificial sweeteners, their safety has been questioned [50]. Some studies have reported that the intake of artificial sweeteners can lead to a craving and dependence on sugar, and that impaired caloric compensation can lead to appetite stimulation, increased intake, weight gain, and glucose intolerance [51⁃53]. Song Xiaowan's[54] research confirmed that the natural sweetener mogroside is significantly more effective than the artificial sweetener aspartame in improving systemic glucose tolerance and insulin sensitivity. In addition, artificial sweeteners can produce an unpleasant aftertaste. Mogroside extract has less bitterness than saccharin and acesulfame-K[10]. Overall, Mogroside, a natural sweetener of plant origin, is an ideal substitute for sucrose because it is highly sweet, low in calories, safe, and has little aftertaste. Mogroside also has a variety of other effects, such as lowering blood sugar, relieving coughs and phlegm, relieving asthma, and protecting the liver. Therefore, the development of Mogroside as a sweetener, health product, and medicine is of great significance for promoting public health.
Mogroside is currently used in the food industry in dairy drinks and solid drinks, but it still faces several problems. Firstly, due to unselective artificial pollination and other reasons, there are currently many varieties of Luohanguo being promoted and applied, and the overall quality of the germplasm is not satisfactory, leaving much room for genetic improvement [55]. (2) The type and content of mogroside undergo periodic changes during growth, so a method for quantitatively determining mogroside is needed to strengthen quality control. (3) At present, the relative sweetness of mogroside is still determined by sensory evaluation by professionally trained assessors, and the electronic tongue cannot replace it [56]. The evaluation process is complex, and the results are subjective; ④ Mogroside is expensive to extract and expensive, so gaining a deeper understanding of the biosynthetic mechanism of mogroside and using cell factories to produce mogroside is one of the potential ways to mass produce mogroside; ⑤ Mogroside's properties as a medicine have yet to be developed, its composition is complex, and research on its specific active ingredients needs to be further deepened to promote the development of drugs and health products.
References:
[ 1 ] Wei Xiaoqin, Lei Xiao, He Wenjing. Analysis of ecological suitability of global production areas of Luo Han Guo [J]. Guizhou Agricultural Science, 2020, 48(1): 119⁃124.
[2] National Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China: 2020 Edition I [S]. Beijing: China Medical Science and Technology Press, 2020.
[3] Li Dianpeng, Zhang Hourui. Research and application of Luo Han Guo, a specialty plant in Guangxi [J]. Guangxi Plants, 2000, 20(3): 269-275.
[ 4 ] Gong X , Chen N , Ren K , et al. The fruits of Siraitia grosvenorii : a review of a chinese food⁃medicine [ J ] . Front Pharmacol , 2019 , 10 : 1400.
[5] Wang Chen, Zhang Sicong, Wang Jian, et al. Research progress and development suggestions for the application of monk fruit sweeteners [J]. Modern Food, 2020(14): 61⁃65.
[ 6 ] Lee C. Intense sweetener from Lo Han Kuo ( Momordica grosvenori) [J] . Experientia , 1975 , 31(5) : 533 ⁃534.
[ 7 ] Takemoto T , Arihara S , Nakajima T , et al. Studies on the constituents of fructus Momordicae. I. on the sweet princple[J] . Yakugaku Zasshi , 1983 , 103(11) : 1151 ⁃ 1154.
[ 8 ] Takemoto T , Arihara S , Nakajima T , et al. Studies on the constituents of fructus Momordicae. II. structure of sapogenin [J] . Yakugaku Zasshi , 1983 , 103(11) : 1155⁃ 1166.
[ 9 ] Makapugay H C , Nanayakkara N , Soejarto D D , et al. High⁃performance liquid chromatographic analysis of the major sweet principle to Lo Han Kuo fruits[J] . J Agric Food Chem , 1985 , 33(3) : 348⁃350.
[10] Murata Y , Yoshikawa S , Suzuki Y A , et al. Sweetness characteristics of the triterpene glycosides in Siraitia grosvenori [J] . J Jpn Soc Food Sci , 2006 , 53(10) : 527⁃533.
[11] Matsumoto K , Kasai R , Ohtani K , et al. Minor cucurbitane⁃ glycosides from fruits of Siraitia grosvenori ( Cucurbitaceae ) [J] . Chem Pharm Bull (Tokyo) , 1990 , 38(7) : 2030⁃2032.
[12] Kasai R , Matsumoto K , Nie R L , et al. Glycosides from Chinese medicinal plant , Hemsleya panacis⁃scandens , and structure⁃taste relationship to cucurbitane glycosides[ J] . ChemPharm Bull (Tokyo) , 1988 , 36(1) : 234⁃243.
[13] Kasai R , Matsumoto K , Nie R L , et al. Sweet and bitter cucurbitane glycosides from Helmsleya carnosiflora [ J ] . Phytochemistry, 1987 , 26(5) : 1371 ⁃ 1376.
[14] Li Dianpeng, Chen Yueyuan, Pan Zhenghong, et al. Study on the changes of monomeric components of monk fruit at different growth ages [J]. Guangxi Botany, 2004, 24(6): 546⁃549.
[15] Lu Fenglai, Liu Jinlei, Huang Yonglin, et al. Research on the high performance liquid chromatography fingerprint of Luo Han Guo at different growth stages [J]. Food Science, 2010, 31 (18): 283-287.
[16] Haralampidis K , Trojanowska M , Osbourn A E. Biosynthesis of triterpenoid saponins in plants [ J ] . Adv Biochem Eng Biotechnol , 2002 , 75 : 31 ⁃49.
[17] Itkin M , Davidovich⁃Rikanati R , Cohen S , et al. The biosynthetic pathway of the nonsugar, high⁃intensity sweetener mogroside V from Siraitia grosvenorii [ J] . Proc Natl Acad Sci USA , 2016 , 113(47) : E7619⁃E7628.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






