What Is Lutein from Marigold Flower?
Lutein, also known as “phyto-progesterone,” is a natural carotenoid found in a wide range of vegetables, fruits, flowers, and certain algae [1]. The human body cannot synthesize it on its own and must be ingested or supplemented through the diet. Lutein is an excellent antioxidant, and as early as 1995, the U.S. Food and Drug Administration (FDA) approved its use as a food supplement in food and beverages. A large number of studies have shown that lutein plays an important role in protecting vision, preventing cataracts and cardiovascular disease, fighting cancer, acting as an antioxidant, and boosting the immune system. It is currently one of the research hotspots in the field of international functional food ingredients [2,3].
1 Structure and physical and chemical properties of lutein
Lutein is a carotenoid. Carotenoids can be divided into two categories according to their chemical structure and solubility [4]: carotenoids and xanthophylls. The former are conjugated hydrocarbons with the molecular formula C40H56, soluble in petroleum ether, and poorly or insoluble in ethanol. They include α, β, and γ-carotene, which have the function of a VA precursor, and lycopene, which does not have the function of a VA precursor. The latter are oxygen-containing derivatives of conjugated polyenes that exist as alcohols, aldehydes, ketones, and acids. They are soluble in ethanol and insoluble in ether. They include zeaxanthin, cryptoxanthin, lutein, and capsanthin.
Lutein is a derivative of α-carotene with the molecular formula C40H56O2 and a molecular weight of 568.85. The lutein molecule has three chiral carbon atoms, and in theory there are eight isomers, but in nature there is only one isomer, namely zeaxanthin. The lutein molecule has 10 conjugated double bonds and a hydroxyl group on the terminal group (Figure 1). It is these conjugated double bonds that give lutein its bright color and ability to scavenge free radicals. Lutein is present in the cell membrane in a way that the hydrophobic long carbon chain is buried in the phospholipid molecule layer, while the hydrophilic hydroxyl group remains on both sides of the membrane. This structure allows lutein to be maximally combined with the cell membrane lipids that are highly susceptible to oxidation, thereby enhancing the strength of the cell membrane. In terms of stability, studies have shown that free lutein is extremely unstable to heat, lutein monooleate (ML) is slightly more stable, and dioleate (DL) is extremely stable to heat. Both ML and DL are less sensitive to UV light than free lutein. Studies have shown that esterification of the hydroxyl group of lutein with fatty acids can enhance its stability to heat and UV light. This is one reason why lutein products are mostly supplied in the form of lutein esters [3].

2 Distribution of lutein
Lutein is widely found in nature, but its form of existence varies. It is found in the free, non-esterified form in green fruits and vegetables such as spinach, kale, cabbage, and honeydew. In contrast, in yellow or orange fruits and vegetables such as oranges, papayas, peaches and courgettes, it is esterified with fatty acids such as myristic acid, lauric acid and palmitic acid. However, after ingestion of these foods, lutein esters need to be hydrolyzed into free lutein before they can be absorbed by the animal body [5].
The lutein content in fruits and vegetables from different sources is not the same, and the results of measurements from different sources and units in different places also vary greatly.
The above data show that the lutein content in fruits and vegetables eaten in nature is low and varies greatly. If lutein is extracted directly from fruits and vegetables, the cost will be very high. Therefore, both at home and abroad, marigold (also known as marigold), which has a high lutein content, is used as raw material to extract and refine lutein for industrial production. Studies have shown that the carotenoid content of marigolds can exceed 1 mg/g fresh weight, and the specific component content is shown in Table 4.
3 Bioavailability of lutein
The bioavailability of lutein powder is closely related to its form, food processing status, cell structure, nutritional status, genetic background, etc. [6]. Lutein in plants mainly exists in the form of fatty acid esters.
After being eaten by animals, lutein esters need to be hydrolyzed by digestive juices into free lutein in order to be effectively absorbed and utilized by the intestines. Currently, marigold lutein is mainly used as a feed additive for poultry, and poultry reach marketable weight standards before their digestive tract functions mature, so they cannot make good use of lutein esters. In actual poultry farming, free lutein needs to be added for absorption and utilization. Studies have shown that the absorption rate of lutein esters in poultry is only 35% to 38%, while the absorption rate of free lutein can reach 90% [7].
The bioavailability of lutein is also closely related to food processing methods and cell structure. Lutein in food raw materials is embedded in cells, so processing methods that destroy cell structure can significantly improve the bioavailability of lutein.
The bioavailability of lutein esters is also significantly related to the amount of fat in the diet. An appropriate amount of dietary fat can induce the secretion of pancreatic esterase or lipase and promote its activity, and the hydrolysis of lutein esters requires the participation of these enzymes. Therefore, an appropriate amount of dietary fat can improve the absorption and utilization of lutein esters by the animal body, but it is not necessarily related to the bioavailability of free lutein.
4 Physiological functions of lutein
4.1 Protecting vision
Lutein is the only carotenoid found in the retina of the human eye, selectively deposited in the macula and throughout the retina. The main physiological function of lutein in the eye is as an antioxidant and photoprotectant [8]. Numerous studies have shown that lutein plays a key role in preventing and controlling common eye diseases such as presbyopia, cataracts, diabetic retinopathy, age-related macular degeneration (AMD), floaters and glaucoma. Lutein helps prevent arteriosclerosis of the eye, delays and alleviates the symptoms of presbyopia, and reduces the incidence of cataracts and AMD [9,10]. Age-related macular degeneration is the leading cause of acquired blindness, affecting 25–30 million people worldwide. Lutein is also important for the visual and even intellectual development of infants.
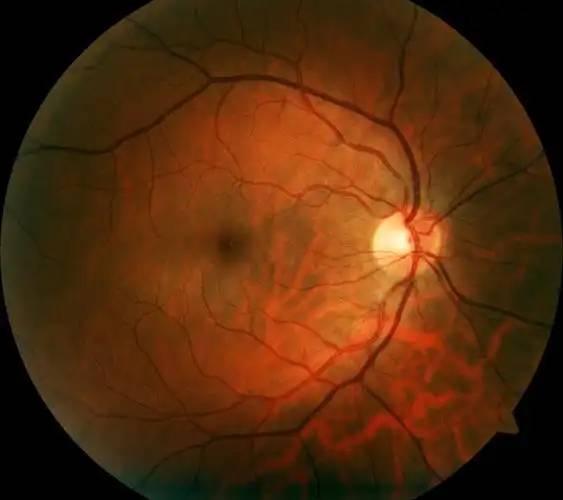
4.2 Antioxidant effect
Lutein is unique in its ability to prevent damage to biological membranes caused by free radicals, quench singlet oxygen and capture oxygen radicals[7]. Singlet oxygen and peroxide radicals mainly come from two sources: the first is produced by the body's normal metabolism; the second is produced in large quantities by factors such as smoking, air pollution, radiation and environmental toxins. Studies have found that reactive oxygen species can react with DNA, proteins and lipids, inhibiting their physiological functions and thus causing chronic diseases such as cancer, atherosclerosis and AMD. Lutein can inactivate singlet oxygen through physical or chemical quenching, protecting the body from harm. Lutein can also prevent lipid peroxidation and protect steroid-producing cells in follicles and uteri from oxidation. Therefore, adding a certain amount of lutein to food can help prevent a series of diseases caused by the aging of human organs and enhance the body's immune system.
4.3 Anti-cancer effect
Lutein has unique biological effects in inhibiting tumor growth, including antioxidant activity, inhibiting tumor angiogenesis and cell proliferation. Studies have shown that lutein is more effective than β-carotene in inhibiting lipid peroxidation of cell membranes and oxidative damage induced by oxidation [11]. As a feed additive, lutein can effectively inhibit the growth of transplantable mammary tumors in mice and promote lymphocyte growth [12]. According to the American Institute for Cancer Research (AICR), a daily intake of 400 to 600 g of fruits and vegetables per capita can reduce the relative risk of cancer by 50%. Slattery et al. [13] showed that lutein intake was significantly negatively correlated with the risk of colon cancer.
4.4 Delaying the early stages of atherosclerosis
Recent studies have shown that lutein has a delaying effect on the early stages of atherosclerosis. Dwyer et al. [14] believe that lutein can prevent the blood vessel walls of the main carotid artery from thickening. Animal experiments have found that the mice fed lutein-containing feed had lower arterial thrombosis than those that were not fed. In addition, lutein in animal wall cells can significantly reduce the oxidation of LDL cholesterol.
4.5 Coloring effect
Because lutein is bright yellow, has strong coloring power, and is resistant to light, heat, acids, alkalis, etc., it is widely used in the processing of pastries, candy, tobacco, seasonings, and feed.

5 Extraction method of lutein
Lutein was first extracted from carrot roots by Heinrich in 1831, and then from autumn yellow leaves by Berzdlius in 1837, followed by other researchers who successively extracted it from seaweed and egg yolks [15]. Currently, lutein is produced by extracting it from marigolds [16].
Lutein cannot be obtained by chemical synthesis and can only be extracted from natural plants. The following are the main methods of lutein extraction.
5.1 Membrane separation method
Traditional processes such as maceration, evaporation and concentration, and solvent purification are used to extract natural pigments. However, these processes have disadvantages such as high energy consumption, overly complex processes, and low product purity. The introduction of membrane separation [17] not only reduces costs, but also improves product quality. The extraction solution is first purified by microfiltration (MF) using a ceramic membrane, and then the filtrate is concentrated using a reverse osmosis (RO) membrane. Compared with ethanol extraction and evaporation concentration, this process, which mainly uses membrane separation technology, is simpler, and the pigment solution is basically at room temperature, which effectively ensures the quality of the pigment product.
5.2 Microwave heating method
Yang Lifei et al. [2] used tea as raw material, 6# solvent as medium, and microwave heating method to extract lutein, and obtained the optimal extraction conditions of lutein by controlling parameters such as solvent concentration, microwave power, and extraction time. The experimental results show that when the material ratio (w/v) is 1:25, the time is 30 s, and the material is extracted twice by microwave, the lutein extraction rate can reach 65.45%. The main advantage of this method is that it saves solvent and improves extraction efficiency.

5.3 Drying method
Lutein can be extracted from calendula petals by drying and tamping in a new type of rotary drum dryer [18]. When the tamping ratio is varied, the tamping efficiency fluctuates between 70% and 90%. The amount of lutein is closely related to the drying time and temperature. For the same drying time, the amount extracted at 60 °C is greater than that at 70 °C.
5.4 Extraction method
The Qingdao High-tech Industrial Park Qingdao Institute of Natural Products has been able to extract lutein from marigolds on a large scale. The process flow is: marigold petals → fermentation → drying → granulation → hexane extraction → negative pressure evaporation separation → lutein resin.
5.5 Organic solvent extraction method
This method uses ethanol as a solvent to extract lutein from marigolds under alkaline conditions. The extract is brownish yellow in color, and after being subjected to vacuum distillation, concentration, precipitation, and drying, a brown solid is obtained [19]. Song Hao et al. [16] studied the solubility of lutein in marigold flowers in four organic solvents (tetrahydrofuran, petroleum ether, hexane, and acetone) and binary mixed solvents of these solvents and ethanol. They found that the extraction effect of lutein in binary mixed solvents was better than that in pure solvents. In addition, the dissolution efficiency of lutein is also closely related to ultrasonic waves, temperature, and the size of the raw material grain.
There are also supercritical carbon dioxide fluid extraction and high-performance liquid chromatography analysis methods.
6 Outlook
China has abundant marigold resources and has already extensively carried out the extraction and processing of lutein, but the products are still crude and are mainly used as animal feed additives or for export. At present, China Agricultural University, Shanghai Jiao Tong University, Peking University, etc., have all carried out research on high-purity lutein, with purities exceeding 95% [20], but this has not yet attracted the attention of China's food industry. Domestic research institutions, including Zhejiang University, are developing innovative high-lutein rice germplasm and corresponding products, and have achieved initial results. Lutein has good protective vision, prevents atherosclerosis, anti-oxidation, anti-cancer and other effects, as a functional ingredient in food, medicine, health products and other fields will have good development prospects.
References
[1] Sun Zhen, Yao Huiyuan. The anticancer effect of lutein and the current research status [J]. Biotechnology Newsletter, 2005, 16 (1): 84-86.
[2] Yang Lifei, Deng Yu. Preliminary study on the extraction process of lutein [J]. Guangzhou Food Industry Science and Technology, 2004, 20 (1): 45-47.
[3] Meng Xianghe, Mao Zhonggui, Pan Qiuyue. Health-promoting function of lutein [J]. China Food Additives, 2003 (1): 17-20.
[4] You Xin. Lutein and its eye protection function [J]. China Food Additives, 2003 (5): 1-3, 10.
[5] Zhu Haixia, Zheng Jianxian. Structure, distribution, physicochemical properties and physiological functions of lutein [J]. China Food Additives, 2005 (5): 48-55.
[6] Jin Wenbo, Dai Ya. Tobacco Chemistry [M]. Beijing: Tsinghua University Press, 1999: 43-44.
[7] Li Haoming. Overview of research on marigold lutein and its physiological functions [J]. China Food Additives, 2001 (4): 31-33.
[8] Liu Zhen, Shen Xiaoxia, Shu Xiaoli, et al. Progress in the structure and function of lutein and its application [J]. China Science and Technology Expo, 2009 (28): 225-226.
[9] Barnes H T. Formulating beverages for healthy eyes and skin [J]. Soft Drinks Management International, 2004, 25 (6): 27.
[10] Ma Zhongjin. Foods containing lutein are good for eyesight [J]. China Food, 2000 (19): 15.
[11] Granado F, Olmedilla B, Blanco I. Nutritional and clinical relevance of lutein in human health [J]. British Journal of Nutrition, 2003, 90 (3): 487-502.
[12] Li Yongxiang, Cao Duanlin. Progress in the extraction and application of lutein [J]. Shanxi Chemical Industry, 2004, 24 (1): 16-18.
[13] Slattery M L, Benson J, Curtin K, et al. Carotenoids and colon cancer [J]. Am J Clin Nutr, 2000, 71 (2): 575.
[14] Dwyer J H, Navab M, Dwyer K M, et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: the Los Angeles atherosclerosis study [J]. Circulation, 2001, 103(24): 2922-2927.
[15] Xu Xiulan, Zhao Guohua, Kan Jianquan, et al. Research progress of lutein [J]. Cereals, Oils and Fats, 2004, (10): 3-7.
[16] Song Hao, He Zechao, Zhang Jie, et al. Extraction of lutein from marigold flowers [J]. Chemical Engineering Design, 2003, 13 (4): 10-12.
[17] Liu Mo'e. Handbook of Membrane Separation Technology [M]. Beijing: Chemical Industry Press, 2001: 12-15.
[18] Armstrong P R, Brusewitz G H, Stone M L, et al. Rotary dry- ing for threshing petals from marigold flowers[J]. Transaction of the ASAE, 2000,43(2):379-384.
[19] Ding Jiaxing. Extraction of the edible natural pigment lutein [J]. Gansu Science and Technology, 2003, 19 (7): 96-98.
[20] You Xin. Natural extracts and functional food additives [J]. Food Science, 2004, 25 (3): 216-218.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






