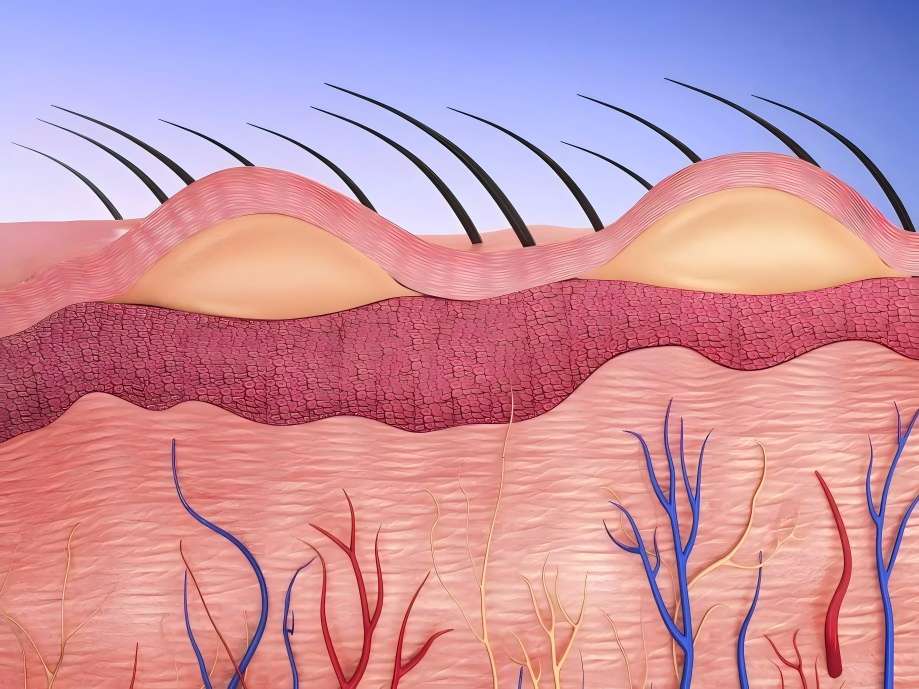
What Is High Molecular Weight Hyaluronic Acid Powder?
1 Hyaluronic acid and structural composition
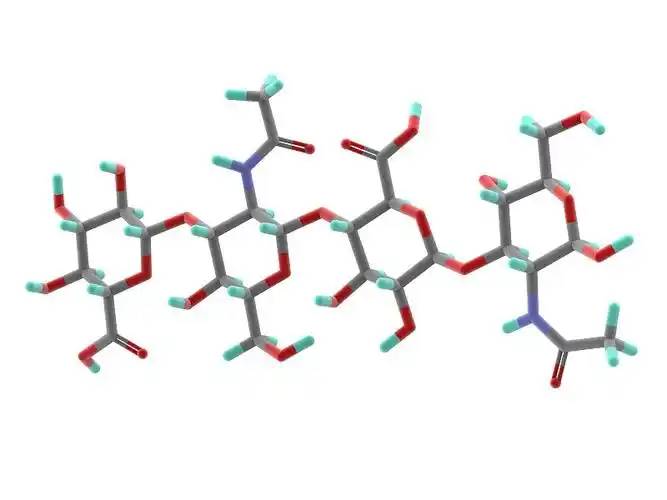
Hyaluronic acid (HA) is a non-sulfated, high molecular weight linear polysaccharide with a basic structure consisting of a disaccharide unit of D-glucuronic acid and N-acetylglucosamine, linked by a β- 1,3- ligand bond between the D-glucuronic acid and the N-acetylglucosamine, and by a β- 1,4- ligand bond between the disaccharide units (Figure 1). The molecular weight of natural hyaluronic acid contained in most tissues ranges from 1000 to 10 000 kDa, and the definition of hyaluronic acid size has not yet been fully agreed upon.According to Naro [1], high molecular weight hyaluronic acid is defined as consisting of more than 500 basic structural units; intermediate molecular weights are generally in the range of 200-500 kDa, and small molecular weight hyaluronic acid is generally defined as less than 200 kDa. According to Monslow et al [2], high molecular weight (HMW hyaluronan) is >1000 kDa, medium molecular weight (MMW hyaluronan) is 250-1000 kDa, low molecular weight (LMW hyaluronan) is >10-250 kDa, and oligo hyaluronan (oligo hyaluronan) is <10 kDa.
The role of hyaluronan in cell motility, cell adhesion and proliferation is mainly mediated by two hyaluronan receptors, CD44 and R-hyaluronan MM (the receptor for hyaluronan-mediated motility, also called CD168). Unlike other mucopolysaccharides, which are synthesised in the Golgi apparatus, hyaluronan is synthesised in the inner membrane of the plasma membrane of the cell by transmembrane synthetases (hyaluronan S1, hyaluronan S2 and hyaluronan S3). Hyaluronan S1 and S2 are responsible for the synthesis of large molecules of hyaluronan and hyaluronan S3 is responsible for the synthesis of small molecules of hyaluronan (<300 kDa), but hyaluronan S3 is more active than hyaluronan S1 and hyaluronan S2 The synthesised large molecules of hyaluronan are cleaved into small molecular weights of hyaluronan by ROS (reactive oxygen radicals) and hyaluronan hydrolytic enzyme, and further hydrolysed into low molecular weights of hyaluronan. Further hydrolysis results in oligo- or oligo-hyaluronic acid. Different molecular weights have different physiological functions, e.g. medium molecular weight hyaluronic acid stimulates cell proliferation, while small molecular weight hyaluronic acid promotes cell migration. See Table 1 [3- 19].
2 Biological Progress of Hyaluronic Acid
2.1 Hyaluronic acid and weight loss
Hyaluronic acid has been approved as a new resource food in China (Ministry of Health Announcement No. 12, 2008), and is used as a food additive and food with health benefits in Korea, as a food additive in Japan, and as a supplement in the United States, Canada, Italy, and Belgium.
Breast milk contains the highest level of hyaluronic acid in the first week of life, which decreases to a stable level over the next two months. In general, a 70 kg adult has about 15 g of hyaluronic acid in his or her body, of which nearly one-third is degraded and synthesised on a daily basis. Hyaluronic acid is taken orally to the skin and does not accumulate excessively in the body; more than 90% of hyaluronic acid is excreted in the breath or urine [19]. Hyaluronic acid is used for weight loss. When hyaluronic acid was administered to C57BL/6 mice at a dosage of 200 mg/kg for 8 weeks, it was found that hyaluronic acid had a good effect on weight loss, which not only reduced body mass, but also reduced adipose tissue and serum LDL-cholesterol, as well as total cholesterol and leptin, and also reduced adipose tissue hyperplasia, and ameliorated fatty liver. It is therefore hypothesised that the main mechanism of hyaluronic acid weight loss may be through the increase of the peroxisome proliferator-activated receptor PPAR-α and the inhibition of PPAR-γ [20].
2.2 Hyaluronic acid and aging
Hyaluronic acid and hyaluronan-binding proteins are involved in cellular senescence. Researchers have found that hyaluronic acid can heal skin damage caused by UV-B exposure through histological changes and wrinkle indicators in vivo. Moreover, high concentrations of hyaluronic acid significantly affected the expression of collagen, matrix metalloproteinase (MMP-1), interleukin IL-1β and interleukin IL-6 protein factors, but not hyaluronan S-2 and transforming growth factor (TGF-β1). Reduction of proteoglycans leads to an increase in free hyaluronan fragments interacting with CD44 protein and phosphorylation of the extracellular signal-regulated kinase ERK1/2, resulting in premature embryonic fibroblast failure [21].

Inhibition of hyaluronan synthesis by 4-methylumbelliferone or targeting of hyaluronan S-2 by miRNA induces cellular senescence, suggesting that hyaluronan plays a role in skin ageing [22]. In addition, hyaluronan concentration is greatly reduced in senescent MSCs and in the cell cycle matrix, mainly due to reduced expression of hyaluronidase [23]. Considering the ability of hyaluronan to inhibit fibroblast senescence induced by oxidative stress in vitro [24]. Thus, hyaluronic acid may be involved in ageing during normal and pathological processes. Further investigation of the role of hyaluronic acid in aging in disease states may shed light on the pathogenesis and aid in the development of therapeutic approaches for these diseases.
2.3 Hyaluronic acid and growth and development
Hyaluronan participates in and promotes cell growth through a variety of signalling pathways. In mice in which the hyaluronan gene hyaluronan s2 was knocked out, the heart and blood vessels developed abnormally, and at 9.5 weeks of development, the lack of an intact endocardial cushion resulted in embryonic lethality (TD Camenisch, 2000). Intraperitoneal injections of hyaluronic acid for 3-8 weeks increased the length of villi and depth of the glandular fossa in the small intestine, the depth of the glandular fossa in the colon, and the proliferation of epithelial cells in both. The opposite results were obtained with PEP-1, a short peptide that prevents the binding of hyaluronan to the receptor. These results suggest that endogenous hyaluronan can regulate normal small bowel and colon growth.
Hyaluronic acid and its binding proteins have been shown to play a role in fibroblast proliferation. Hyaluronic acid promotes TGF-β1-mediated fibroblast proliferation, and LMW hyaluronic acid or hyaluronan oligosaccharides stimulate fibroblast proliferation under a variety of conditions, suggesting that hyaluronic acid plays an important role in tissue fibrosis. Hyaluronic acid fragments induced myofibroblast differentiation, endothelial cell differentiation, and chondrogenic differentiation [25], whereas hyaluronic acid and mouse tumour necrosis factor α-inducible protein 6 inhibited cardiomyocyte differentiation and osteoclast differentiation of human bone marrow mesenchymal stem cells, respectively. These data suggest that hyaluronan and hyaluronan-binding proteins regulate cell differentiation in a complex manner and that this differentiation may be related to cell type and microenvironment.
2.4 Hyaluronic Acid and Immunoinflammation
Hyaluronic acid and hyaluronan-binding proteins regulate inflammation and the repair of tissue damage by modulating the infiltration of inflammatory cells, the release of inflammatory cytokines and cell migration. Therefore, hyaluronic acid may act as an immunomodulator in human diseases [26]. Synthetic hyaluronic acid has been found to promote the expression of antimicrobial peptides in intestinal epithelial cells. Oral administration of hyaluronic acid from breast milk induced an increase in mouse defensin HβD2 homologue and MuβD3 via Toll-like receptor 4 (TLR4) and CD44 protein, and the addition of hyaluronic acid cytokines to cultured cells showed that colonic mucosal epithelial cells were more resistant to the enteropathogenic bacterium Salmonella typhimurium. Hyaluronic acid regulates cell proliferation and inflammation.
Inflammatory cells activate keratinocytes to produce interleukin IL- 6, IL-1 and tumour necrosis factor (TNF- α) through the release of cytokines and the production of LMW hyaluronan fragments, which in turn increases hyaluronan synthesis partly through the action of HB-EGF, and hyaluronan feedback contributes to inflammatory responses including fibroblast migration and proliferation. hyaluronic acid has been reported by RMA et al. S. hyaluronan rma et al [25] reported that hyaluronic acid-coupled curcumin enhanced keratinocyte proliferation, reduced oxidative damage caused by hydrogen peroxide, and improved cell migration at the site of scratches.
Hyaluronic acid can be utilised by probiotics. Probiotics can use N-acetyl-D glucosamine, a precursor of hyaluronic acid, as a nutrient, and Kim et al [27] found that Lactobacillus plantarum K8 lysate significantly increased hyaluronic acid secretion from cells. Lactobacillus lysates inhibited the Th2 response induced by interleukin 4 (IL-4)-driven stimulation and increased the Th1 response, and lactobacilli controlling the Th1/Th2 balance contributed to hyaluronic acid induction and the reduction of atopic dermatitis lesions.
Hyaluronic acid and CD44 interact with each other and have the ability to modulate T-cell activation, Th1 production, B-cell activation and regulatory T-cell functions. Recent studies have shown that CD44 functions in the adaptation of regulatory T cells by interacting with galactose agglutinin 9 [28].
Hyaluronic acid and CD44 regulate neutrophil adhesion and recruitment. It is endothelial CD44, not neutrophil CD44, that mediates neutrophil migration, and hyaluronic acid is also capable of promoting the production of inflammatory vesicles in response to injury. In CD44-/- deficient mice, lymphocytes preferentially stayed in lymph nodes and delayed their entry into synovial joints with an inflammatory response compared to wild-type cells. CD44-/- deficient mice die from non-infectious pneumonia injury, a continuous inflammatory response characterised by impaired clearance of apoptotic neutrophils [26]. In addition, hyaluronan fragments can affect dendritic cell maturation, e.g. LWM hyaluronan fragments not only induce dendritic cell maturation but also switch on alloimmunity. In addition, hyaluronan oligosaccharides are potential activators of dendritic cells, allowing small molecule hyaluronan fragments to promote cell migration and subsequent allergic modifications.
In addition, TLR-4, the natural receptor for lipopolysaccharide, is one of the hyaluronan receptors. TLR-4 activates the nuclear transcription factor NF- kB protein via two pathways: one induces pro-inflammatory cytokines via the myeloid-dependent Myeloid Differentiation Factor MyD88, and the other is a non-myeloid-dependent MyD88 pathway that increases interferon-induced pro-inflammatory genes via type I interferons [26].
2.5 Hyaluronic acid and cancer
Hyaluronic acid not only acts as a cellular support and hydrophilic matrix, but also regulates cell adhesion, migration, growth and differentiation (TC Laurent, 1992). These properties have led to the involvement of hyaluronic acid in a number of pathological processes. For example, in cancer, hyaluronic acid forms a protective film on the surface of tumour cells, making them less vulnerable to attack by immune cells. At the same time, tumour cells produce more hyaluronic acid or induce the production of hyaluronic acid by releasing growth factors and cytokines. Moreover, ROS-induced hyaluronan fragments contribute to the efficient expression of hyaluronan (R Stern, 2006). Tumour cells and stromal cells are able to express hyaluronan homologues and produce hyaluronan-containing extracellular matrix, which accumulates in the tumour and pericancerous stromal tissues, thus accelerating metastasis of cancer cells [29].
In addition, hyaluronic acid also regulates fibroblast and tumour invasion (Q Yu, 2000). Transforming growth factor and tyrosine kinase, when activated, regulate hyaluronan-mediated invasive cellular motility [27]. Heterogeneity analysis of probe cells using hyaluronan resulted in the identification of subtypes of infiltrating but slow-growing breast tumours. By analysing fibroblasts isolated from transgenic mice overexpressing hyaluronan S2, we found that the cells had a greater ability to invade the stroma. If the hyaluronan S2 gene is knocked out of mesenchymal cells, the invasive fibroblast phenotype is terminated, the accumulation of myofibroblasts is discharged and the development of lung fibrosis is inhibited [30].
2.6 Correlation of hyaluronic acid with stem cells
The interaction of hyaluronic acid with stem cells has been studied in haematopoietic stem cells, MSCs and pluripotent adult progenitors, suggesting that hyaluronic acid and its binding proteins may interact with various stem cells through their ability to regulate tissue damage and repair processes. During stem cell differentiation, hyaluronan synthesis was enhanced 13-fold and 24-fold, most likely due to an increase in hyaluronan S2 expression. Hyaluronan is required for the production of haematopoietic cells during human embryonic stem cell differentiation. Knockdown of hyaluronan S2 resulted in inhibition of human embryonic stem cell differentiation. Hyaluronan oligosaccharides increase the stem cell properties of epidermal cells by regulating integrins. Hyaluronic acid promotes CD44-dependent migration of MSCs CD44 has long been used as a marker for stem cells, including embryonic, mesenchymal, haematopoietic and cancer stem cells. In addition, CD44 may play a role in regulating stem cell function. A recent study has shown that although CD44 is not a master stem cell gene, CD44 contributes to stem cell generation and the homeostasis of stem cells in their ecosystem. Hyaluronic acid is required to regulate the haematopoietic support function of bone marrow helper cells and is involved in haematopoietic site assembly.
2.7 Hyaluronan and Apoptosis
In bleomycin-induced lung injury, hyaluronic acid protects mouse lung epithelial cells from apoptosis. High molecular weight hyaluronic acid reduces UV-induced apoptosis and inflammation in human epithelial corneal cells. Dermal fibroblasts are resistant to stress-induced apoptosis in the presence of high levels of hyaluronan s2 gene expression in the presence of hyaluronan s1/3 knockout, suggesting that hyaluronan S2 protects dermal fibroblasts from environmental stress-induced apoptosis. For inflammatory cells, hyaluronic acid appears to induce apoptosis. Hyaluronic acid appears to induce apoptosis. Hyaluronic acid induces apoptosis in activated T cells via CD44. LMW hyaluronic acid-TLR4 interaction induces apoptosis in inflammatory neutrophils. In rats, administration of hyaluronic acid with a molecular weight of 1600 kDa significantly reduced smoke-induced neutrophil infiltration, pulmonary oedema, respiratory apoptosis and mucus plugging, suggesting that high-molecular-weight hyaluronic acid may have a potential therapeutic effect on smoking-induced lung injury. In addition, cross-linking of CD44 molecule-specific epitopes rapidly induced neutrophil apoptosis in vitro and inhibited neutrophil-dependent renal injury in rats in vivo.
2.8 Role in cell migration and invasion
Hyaluronic acid mediates inflammatory cell migration to regulate the inflammatory response and tissue damage.CD44 deficiency leads to increased neutrophil migration and lung injury in E. coli pneumonia in mice. TSG6 is a potent inhibitor of neutrophil migration in an in vivo model of acute inflammation. Exogenous low molecular weight hyaluronic acid, or LWM hyaluronic acid produced by overexpression of HYAL1, promotes the migration of dendritic cells from the skin and subsequently alters the allergic response in a TLR4-dependent manner. Hyaluronan and hyaluronan-binding proteins play a role in fibroblast migration and smooth muscle cell migration. Abnormal accumulation of hyaluronan matrix promotes fibroblast migration.
Specifically sized hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. both CD44 and HMMR have been shown to have a role in fibroblast migration during tissue injury. In addition, myofibroblast migration can be regulated by hyaluronic acid. These studies suggest a role for hyaluronic acid in tissue injury and fibrosis. Small molecular weight hyaluronic acid35 and high molecular weight hyaluronic acid117 showed significant differences in the migration and invasion of breast cancer 4T-1 and SKBR3 cells. In healthy people, the level of hyaluronic acid in the body is 10-100 μg/L, but in breast cancer patients, the level is as high as 200-300 μg/L, and in advanced breast cancer, it reaches 789-2343 μg/L (EH Cooper, 1988). Compared with small molecular weight hyaluronic acid, high molecular weight hyaluronic acid is able to exert a greater squeezing force on the tumour spheres and prevent the growth of cancer cells. Small molecular weight hyaluronic acid can accelerate the process of metastatic fibrosarcoma cell invasion [9].
3 Outlook
Over the past decades, hyaluronic acid and its hyaluronan receptor have been intensively investigated, leading to an increasing use of hyaluronic acid. Nevertheless, there are still some problems that need to be solved. The first problem to be solved is the definition of high molecular weight and low molecular weight hyaluronic acid. There is no international consensus on the distinction between high and low molecular weight hyaluronic acid, which poses a problem in elucidating the physiological effects of hyaluronic acid on cells. Secondly, high molecular weight hyaluronic acid controls the normal homeostasis of the body and shows anti-inflammatory and anti-cancer effects, while low molecular weight hyaluronic acid and oligo-hyaluronic acid show pro-inflammatory and pro-cancer effects. If the same receptor is used, but the results are significantly different, the mechanism is still not clear. In addition, how hyaluronic acid precisely regulates the occurrence and development of inflammation and tumour cells; whether oral administration of hyaluronic acid affects the intestinal flora, which in turn affects human health; and how modified hyaluronic acid (e.g., sulphated hyaluronic acid) has a different mechanism of action compared with non-modified hyaluronic acid, need to be further elucidated. The solution of these problems will certainly help to prevent diseases and maximise the use of hyaluronic acid for the treatment of human diseases.
Reference
[ 1 ]Naor D. Editorial: Interaction between hyaluronic acid and its receptors (CD44, RHAMM) regulates the activity of inflammation and cancer[ J ]. Front Immunol, 2016,7:39.
[2 ]Monslow J, Govindaraju P, Puré E . Hyaluronan- a functional and structural sweet spot in the tissue microenvironment [ J ]. Front Immunol, 2015 ,6 :231 .
[3]Karbownik MS, Nowak JZ. Hyaluronan: towards novel anti- cancer therapeutics[ J ]. Pharmacol Rep, 2013,65(5):1056- 1074.
[4 ]Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging[ J ]. Dermatoendocrinol, 2012,4(3): 253- 258.
[5 ]Kavasi RM, Berdiaki A, Spyridaki I, et al. HA metabolism in skin homeostasis and inflammatory disease[ J ]. Food Chem Toxicol, 2017,101:128- 138.
[6 ]Zhu Y, Hu J, Yu T, et al. High molecular weight hyaluronic acid inhibits fibrosis of endometrium [ J ]. Med Sci Monit, 2016,22: 3438- 3445.
[7]Zhao YF, Qiao SP, Shi SL, et al. Modulating three- dimensional microenvironment with hyaluronan of different molecular weights alters breast cancer cell invasion behavior [ J ]. ACS Appl Mater Interfaces. 2017,9(11):9327- 9338.
[8 ]Noble PW, Lake FR, Henson PM, et al. Hyaluronate activation of CD44 induces insulin- like growth factor- 1 expression by a tumor necrosis factor- alpha- dependent mechanism in murinemacrophages[ J ]. J Clin Invest, 1993,91(6):2368- 2377.
[9]Hodge- Dufour J , Noble PW, Horton MR , et al . Induction of IL- 12 and chemokines by hyaluronan requires adhesion- dependent priming of resident but not elicited macrophages [ J ]. J Immunol, 1997 , 159 (5 ): 2492- 2500 .
[10]Ohkawara Y, Tamura G, Iwasaki T, et al. Activation and transform- ing growth factor- beta production in eosinophils by hyaluronan[ J ]. Am J Respir Cell Mol Biol, 2000,23(4):444- 451.
[11]Fitzgerald KA, Bowie AG, Skeffington BS, et al. Ras, protein kinase C zeta, and I kappa B kinases 1 and 2 are downstream effectors of CD44 during the activation of NF- kappa Bby hyaluronic acid fragments in T- 24 carcinoma cells[J ]. JImmunol, 2000,164(4):2053- 2063.
[12]Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses [ J ]. J Biol Chem, 2002,277(43): 41046- 41059.
[13]West DC, Kumar S. Hyaluronan and angiogenesis [ J ]. Ciba Found Symp, 1989,143: 187- 201; discussion 201- 187,281- 185.
[14]West DC, Kumar S. The effect of hyaluronate and its oligosaccha- rides on endothelial cell proliferation and monolayer integrity [ J ]. Exp Cell Res, 1989,183(1):179- 196.
[15]Vistejnova L, Safrankova B, Nesporova K, et al. Low molecular weight hyaluronan mediated CD44 dependent induction of IL- 6 and chemokines in human dermal fibroblasts potentiates innate immune response[ J ]. Cytokine, 2014, 70(2):97- 103.
[16]Termeer CC, Hennies J, Voith U, et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells [ J ]. J Immunol, 2000,165(4):1863- 1870.
[17]Pandey MS, Baggenstoss BA, Washburn J, et al. The hyaluronan receptor for endocytosis (HARE) activates NF- kappaB- mediated gene expression in response to 40- 400- kDa, but not smaller or larger, hyaluronans[ J ]. J Biol Chem, 2013, 288(20):14068- 14079.
[18]Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage- independent growth of tumor cells by suppressing the phosphoinositide 3- kinase/Akt cell survival pathway [ J ]. J Biol Chem, 2002, 277(41): 38013- 38020.
[19]Oe M, Mitsugi K, Odanaka W, et al. Dietary hyaluronic acid migrates into the skin of rats [ J ]. Scientific World Journal, 2014,2014:378024.
[20]Park BG, Park YS, Park JW, et al. Anti- obesity potential of enzy-matic fragments of hyaluronan on high- fat diet- induced obesity in C57BL/6 mice[ J ]. Biochem Biophys Res Commun, 2016,473(1): 290- 295.
[21]Suwan K, Choocheep K, Hatano S, et al. Versican/PG- M assembles hyaluronan into extracellular matrix and inhibits CD44- mediated signaling toward premature senescence in embryonic fibroblasts[ J ]. J Biol Chem, 2009,284(13): 8596- 8604.
[22]Lompardia SL, Papademetrio DL, Mascaro M, et al. Human leukemic cell lines synthesize hyaluronan to avoid senescence and resist chemotherapy[ J ]. Glycobiology, 2013,23(12):1463- 1476.
[23]Jung EM, Kwon O, Kwon KS, et al. Evidences for correlation be- tween the reduced VCAM- 1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells[ J ]. Biochem Biophys Res Commun, 2011,404(1): 463- 469.
[24]Cirillo N, Vicidomini A,McCullough M, et al. A hyaluronic acid- based compound inhibits fibroblast senescence induced by oxidative stress in vitro and prevents oral mucositis in vivo[ J ]. J Cell Physiol, 2015,230(7): 1421- 1429.
[25]Sharma M, Sahu K, Singh SP, et al. Wound healing activity ofcur- cumin conjugated to hyaluronic acid: in vitro and in vivo evaluation [ J ]. Artif Cells Nanomed Biotechnol, 2017,28:1- 9.
[26]Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases[ J ]. Physiol Rev, 2011,91(1):221- 264.
[27]Kim H, Kim HR, Jeong BJ, et al. Effects of oral intake of kim- chi- derived Lactobacillus plantarum K8 lysates on skin moisturiz- ing. J Microb hyaluronic acidiol Biotechno[ J ], 2015,25(1):74- 80.
[28]Wu C, Thalhamer T, Franca RF, et al. Galectin- 9- CD44 interaction enhances stability and function of adaptive regulatory T cells[ J ]. Immunity, 2014, 41(2):270- 282.
[29]Heldin P, Basu K, Olofsson B, et al. Deregulation of hyaluronan synthesis, degradation and binding promotes breast cancer[ J ]. J Biochem, 2013,154(5): 395- 408.
[30]Li Y, Jiang D, Liang J, et al. Severe lung fibrosis requires an inva- sive fibroblast phenotype regulated by hyaluronan and CD44[ J ]. J Exp Med, 2011, 208(7):1459- 1471.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese