What Is Erythritol Made From?
Erythritol is a zero-calorie filling sweetener. It is found in fruits and vegetables such as pears, grapes and mushrooms, and in small quantities in fermented foods such as wine, beer, soy sauce and sake. It is also found in small quantities in animal serum and eyeballs. Its sweetness is equivalent to 70% sucrose, and its sweetness is pure and clean without any aftertaste. Erythritol has a high degree of digestive tolerance. Compared with other polyols, it is rapidly absorbed in the small intestine and quickly digested by the body within 24 hours. Therefore, when eating foods containing erythritol, it is unlikely that there will be an over-intake of polyols, which can cause mild diarrhea. In addition, erythritol can inhibit harmful bacteria in the mouth, reduce dental plaque, prevent tooth decay, and improve oral health, so it is very popular with consumers. This article details the excellent properties of erythritol and focuses on its application in the food industry, with the aim of providing a theoretical basis and reference for research on the application of erythritol.
1 Erythritol
1.1 Structure and physical and chemical properties
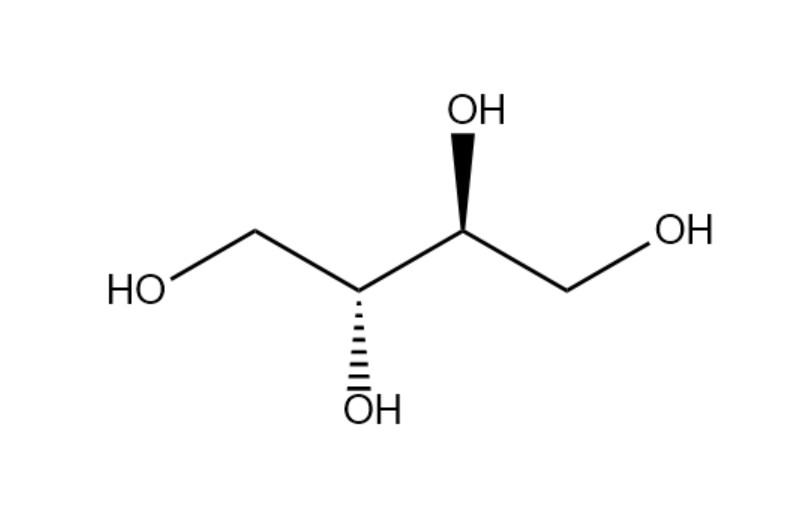
Erythritol, scientific name 1,2,3,4-butanetetrol, molecular formula C4H10O4, molecular weight 122.12, Figure 1 shows its structural formula. The molecular structure is symmetrical and belongs to the racemic - meso erythritol [1]. Its melting point is 118 to 120 °C, boiling point is 329 to 331 °C, density is 1451 g·cm-3, and storage conditions are -20 °C [2]. Erythritol is an odorless white crystal with low hygroscopicity and moderate solubility in water. It has the characteristics of being heat- and acid-resistant and having a high osmotic pressure of the solution [3].
1.2 Physiological functions
1.2.1 Low calorie
Erythritol has a low molecular weight, is easily absorbed in the small intestine, and most of it enters the blood circulation. Due to the lack of relevant enzyme systems in the human body, erythritol that enters the bloodstream cannot be metabolized and absorbed by the body, and is excreted from the body through the urine. Therefore, erythritol has very low calories.
1.2.2 High tolerance
Erythritol has a very high tolerance. The tolerance of men is 0.68 g·kg-1, and that of women is 0.80 g·kg-1. For a 50 kg woman, the tolerance is 40 g·d-1; the tolerance of animals is 18 to 20 g·kg-1 bw[4]. The tolerance is 2 to 3 times that of xylitol, lactitol, maltitol and isomaltitol, and 3 to 4 times that of sorbitol and mannitol.
1.2.3 It has a positive effect on intestinal flora
Since the human body lacks the relevant enzymes, 90% of the erythritol ingested is excreted, and 10% enters the large intestine. Studies have found that erythritol is not easily fermented by intestinal flora in the large intestine, and erythritol can increase the short-chain fatty acids produced by intestinal flora. Short-chain fatty acids in the intestine have a positive effect on the intestine and overall human health. Therefore, it is speculated that erythritol has a positive effect on the intestinal flora.
1.2.4 Does not stimulate insulin secretion, causing blood sugar fluctuations
Tests on healthy people and people with glucose intolerance (pre-diabetes) have shown that after taking erythritol, the glycemic index and insulin index are both 0.2, with almost no effect, while other sugar alcohols almost all have the effect of raising blood sugar and stimulating insulin secretion. Among them, the glycemic index of maltitol is as high as 52, and the glycemic index of xylitol is around 15, both of which are significantly higher than that of erythritol [5].
1.2.5 Antioxidant effect
Animal tests have found that erythritol has an antioxidant effect in diabetic rats, significantly scavenging and inhibiting free radicals and protecting blood vessels damaged by high blood sugar. Studies have shown that 24 type 2 diabetes patients who consumed 36 g of erythritol per day had improved blood vessel function and a reduced risk of heart disease [6].
1.2.6 Prevention of tooth decay
Harmful bacteria in the mouth can metabolize sugar and release acids during the process, which corrode tooth enamel. Sugar alcohols cannot be metabolized by oral bacteria. Some studies have found that erythritol and xylitol can directly inhibit the growth of oral bacteria, and that erythritol is more effective than xylitol and sorbitol in preventing tooth decay [7-9].

1.3 Safety and regulations
Erythritol is safe as a food ingredient, as demonstrated by numerous safety studies on humans and animals, including short-term and long-term animal feeding, multigeneration reproduction and teratology studies. Japan, the United States, and Australia approved erythritol as an ingredient in food as early as 2000; the European Union approved the addition of erythritol to beverages in 2015 [10] and the addition of erythritol to organic foods the following year [11]; Canada approved the addition of erythritol to some carbonated drinks in 2016. In China, GB 2760-2011 stipulates that erythritol can be used in all kinds of foods in appropriate amounts according to production needs.
2 Production of erythritol
Erythritol has a symmetrical molecular structure and exists in the form of a racemate, so the step of removing the corresponding isomer can be omitted during the production process. There are currently two main methods for producing erythritol – chemical synthesis and biological fermentation.
2.1 Chemical synthesis
There are two main methods of chemical synthesis. One uses starch or cellulose as the raw material, and the other uses high iodic acid to treat the raw material with acid and alkali to obtain high aldehyde starch. Nickel is used as a catalyst, and erythritol and other derivatives are obtained by hydrogenation and reduction under high temperature and pressure [12]. This method produces ethylene glycol, and the product recovery rate is low. Another method is to first use peracetylene and formaldehyde to prepare 2-butene-1,4-diol, then allow butene-1,4-diol to react with hydrogen peroxide, and then add a catalyst, nickel, and a deactivator, ammonia, to an aqueous solution. Hydrogen is passed at about 0.5 MPa to obtain erythritol and its derivatives [13]. This method has high production requirements, serious pollution and difficulty controlling product safety [14]. The above two chemical synthesis methods are affected by changes in osmotic pressure during production, the generation of polyhydric alcohols and external environmental factors such as oxygen and temperature.
2.2 Microbial fermentation method
The biological fermentation method uses starch as the raw material, and uses enzymes such as amylase and glucoamylase to enzymatically digest the raw material into glucose. The mixture is fermented with high-osmotic-pressure yeast to convert the glucose into a mixture of polyols such as erythritol. Erythritol is obtained by centrifugation and concentration, crystallization and separation, and drying and refinement. The industrial production process is starch → liquefaction → saccharification → glucose → fermentation with production strain → filtration → chromatographic separation → purification → concentration → crystallization → separation → drying → erythritol, with an average recovery rate of about 50% [15-16]. The microbial fermentation method has the advantages of mild production conditions and easy control. At present, this method is mainly used for large-scale production at home and abroad. The selected fermenting microorganisms are food-grade osmophilic yeasts, such as Candida, Trichosporum, and Pichia.
3. The application of erythritol in the food industry
The properties and functions of erythritol have led to its use in many fields. In the pharmaceutical industry, it can be used as a pharmaceutical coating and tablet excipient, and in the chemical industry, it can be used as a heat storage material and polymer material. As a new type of sweetener, it is widely used in the food industry.
3.1 Condiments
The condiment industry has a relatively mature and stable industrial level, and the focus of the products is on the selection of ingredients. The development and research of functional condiments has become an industry development trend. Zhou Siyu et al. [17] used milk, eggs, low-gluten flour, etc. to replace fat, and erythritol to replace sucrose to make low-sugar green tea custard sauce. The sensory quality, storage stability, and rheological properties of the finished product were evaluated. It was found that the sauce with added sugar alcohol spread better, had a uniform and delicate texture, and could maintain the storage stability of the product.
3.2 Candy
Erythritol has a low calorie and high sweetness, with only 1.7 J·g-1, almost zero calories and 70% of the sweetness of sucrose. It is cost-effective from the perspective of sweetness and calories; highly tolerable, not likely to cause gastrointestinal discomfort; does not stimulate insulin secretion or raise blood sugar, so it is friendly to diabetic patients; can prevent tooth decay, so it is friendly to children; and it also has high heat and acid resistance, so browning and decomposition can be avoided during candy production. Yang Yuanzhi et al. [18] reported that the use of erythritol in chewing gum can reduce the calories by about 85%, and in chocolate by about 30%. Li Wenzhao et al. [19] used erythritol and maltitol to make hard candy and found that it did not decompose and did not change color under high temperature conditions of 200 °C. Yu Limei et al. [20] found that eating erythritol can promote the proliferation of Bifidobacterium bifidum and reduce the caloric value of fondant.

3.3 Baked goods
Erythritol has the characteristics of low hygroscopicity, can prevent moisture, and can extend the shelf life and shelf life of food; it can reduce the calories and coordinate the texture in baked goods; it can improve the functional properties of some proteins to ensure the porosity and softness of baked goods. Adding erythritol to Oreo products not only reduces the calories but also adds a refreshing texture. Zhang Wei et al. [21] added erythritol to cookies, and the resulting product was golden in color, crispy in texture, and had a fine structure.
3.4 Dairy products
Erythritol can protect biological macromolecules in solution, inhibit protein denaturation, and improve the stability of protein emulsions. Le Yuan Tongyu et al. [22] found that when the mass ratio of Advantame to erythritol is 1:70, the aftertaste of Advantame can be largely resolved, and the yogurt produced can be “sugar-reduced” while maintaining a texture similar to that of yogurt made with only sucrose. Tan Yuxia et al. [23] used erythritol to make yogurt, and the finished product is suitable for obese and diabetic people while also maintaining the original flavor and texture of yogurt to the greatest extent possible.
3.5 Drinks
Erythritol can remove and inhibit the production of free radicals, and has antioxidant properties. Gao Shengjun et al. [24] added erythritol to lemon juice drinks to improve the taste of the drink and protect the vitamins in the drink from decomposition, extending the shelf life. Wang Dan et al. [25] used carrot as the raw material and added erythritol to make a functional beverage, which not only ensured a refreshing taste but also improved the stability of the ingredients. It has also been reported that erythritol can promote the binding of water molecules and ethanol molecules, not only shortening the fermentation cycle, but also reducing the peculiar smell of alcohol and improving the quality of alcoholic beverages [26].

4 Conclusion
Erythritol is a new type of sweetener that is widely found in fruits and vegetables and is mainly obtained through microbial fermentation in industrial production. It has the characteristics of low calories and high tolerance, has a positive effect on intestinal flora, does not stimulate insulin secretion, and also has physiological functions such as anti-oxidation and caries prevention. It is mainly used in the food industry in products such as candy, baked goods, dairy products, and drinks. With the research of theoretical knowledge, the production and extraction process of erythritol is constantly being improved, and the scope of application of erythritol will become more and more extensive. Its application prospects are very broad.
References
[1] Xiu X H. Study on the fermentation, separation and extraction process of erythritol [D]. Jinan: Shandong Institute of Light Industry, 2011.
[2] Li K W. Efficient production of erythritol [D]. Jinan: Qilu University of Technology, 2015.
[3] Li Junlin, Guo Chuanzhuang, Wang Songjiang, et al. Research progress on the properties and applications of erythritol [J]. China Food Additives, 2019, 30(10): 169-172.
[4]BERNT W O,BORZELLECA J F,FLAMM G.Erythritol: a review of biological and toxicological studies[J].Regulatory Toxicolog y And Pharmacology,1996,24(2):191-197.
[5]NODA K,NAKAYAMA K,OKU T.Serum glucose and insulin levels and erythritol balance after oral administration of erythritol in healthy subjects[J].European Journal of Clinical Nutrition,1994,48(4):286-292.
[6]FLINT N,HAMBURG N M,HOLBROOK M,et al.Effects of erythritol on endothelial function in patients with type 2 diabetes mellitus:a pilot study[J]. Acta Diabetologica,2014,51(3):513-516.
[7]SDERLING E M,HIETALA-LENKKERI A M.Xylitol and er ythritol decrease adherence of polysaccharide-producing oral streptococci[J]. Current Microbiology,2010,60(1):25-29.
[8]HONKALA S,RUNNEL R,SAAG M,et al.Effect of erythritol and xylitol on dental caries prevention in children[J].Caries Research, 2014,48(5):482-490.
[9]PETER D C,KAUKO M,EINO H,et al.Erythritol is more effective than xylitol and sorbitol in managing oral health endpoints[J].International Journal of Dentistry,2016,2016:9868421.
[10] The European Union approves erythritol for use in low-calorie or sugar-free flavored drinks [J]. Beverage Industry, 2015, 18(5): 46.
[11] Organic certification of sweeteners (stevia erythritol xylitol) in the European Union and the United States [J]. Beverage Industry, 2016, 19(4): 79.
[12] Chen W, Liu X, Yang C, et al. Research progress on the production and application of erythritol in the food industry [J]. Food Industry, 2018, 39(2): 266-269.
[13]OTEY F H,SLOAN J W,WILNAM C A, et al.Erythritol and ethylene glycol from dialdehyde starch[J].Industrial & Engineering Chemistry,1961,53(4):267-268.
[14]SARAN S,MUKHERJEE S,DALAL J.High production of ery thritol from Candida sorbosivorans SSE-24 and its inhibitory eff ect on biofilm formation of Streptococcus mutans[J] Bioresource Technology,2015,198:31-38.
[15] Xu Ying, Li Jingjun, He Guoqing. Research progress of erythritol and its application in food [J]. China Food Additives, 2005 (3): 92-95.
[16]GAO X L,SENEVIRATNE C J,LO E C M,et al.Novel and conventional assays in determining abundance of Streptococcus mutans in saliva[J]. International Journal of Paediatric Dentistry, 2012,22(5):363-368.
[17] Zhou Siyu, Li Xiancai, Yang Yu, et al. Effect of different sugar alcohols on the quality characteristics of green tea custard sauce [J]. Food Industry Science and Technology, 2020, 41(4): 31-35.
[18] Yang Yuanzhi, Li Facaisheng, Shuai Bin, et al. Natural healthy sugar alcohol: the application of erythritol in low-energy foods [J]. Chinese Food Additives, 2013 (1): 181-185.
[19] Li Wenzhao, Wu Jing, Liu Xiaoyu, et al. Research on the characteristics of erythritol and its hard candy [J]. Food Research and Development, 2017, 38 (4): 96-100.
[20] Yu Limei, Bai Weidong, Yang Min, et al. Development of erythritol sesame crispy candy and evaluation of its efficacy [J]. Journal of Zhongkai University of Agriculture and Engineering, 2012, 25(3): 30-31.
[21] Zhang Wei, Zhu Beibei, Wang Chao. Optimization of the response surface method for erythritol cookies [J]. Guizhou Agricultural Sciences, 2018, 46(7): 159-162.
[22] Yuantongyu Leyu, Yukun Chen, Guohua Hu. The compounding of Advantame and erythritol and its application in yogurt [J]. Food Industry, 2021, 42(5): 129-134.
[23] Tan Yuxia, Yang Haijun, Li Fafa. Development of low-heat yoghurt with erythritol [J]. China Dairy Industry, 2011(11): 46-48.
[24] Gao Shengjun, Mao Jun. Study on the protective effect of erythritol on vitamin C in lemon juice drinks [J]. Food Industry Science and Technology, 2014, 35 (3): 49-51.
[25] Wang D, Liu J M, Xiao P, et al. Development of a functional beverage with added erythritol using carrots as the raw material [J]. Food Research and Development, 2009, 30(7): 74-77.
[26] Li Junlin, Guo Chuanzhuang, Wang Songjiang, et al. Research progress on the properties and applications of erythritol [J]. China Food Additives, 2019, 30(10): 169-172.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






