Erythritol What Is It?
Erythritol is a new type of functional sugar alcohol food sweetener that is widely found in fruits and vegetables, algae, fungi and fermented foods such as soy sauce and wine. It has high thermal stability[1], low hygroscopicity[1], a characteristics such as high thermal stability[1], low hygroscopicity[1], sweet taste[2], zero caloric value[2], non-cariogenic[3], non-glycemic[3] and high tolerance, it is widely used in food, medicine, chemical industry and other fields, especially in the sweetener market with strong competitiveness. In addition, erythritol is currently the only sugar alcohol sweetener produced by microbial fermentation compared to other sugar alcohol sweeteners[4]. The US Food and Drug Administration (FDA) recognizes the safety of erythritol and approved its inclusion in the GRAS list in 1997 [5]. In addition to being used as a food sweetener, erythritol can also be used as a diluent for high-intensity sweeteners and as a pharmaceutical excipient. It is a functional food additive with a filling effect[6].
1. The structure and physical and chemical properties of erythritol
1. 1. Structure
Erythritol, also known as 1,2,3,4-butanetetrol, is a white crystalline powder with a molecular weight of 122. 12, melting point 126°C [1], boiling point 329~331°C [1], molecular structure is symmetrical, and it is a racemic-inter-erythritol [7].
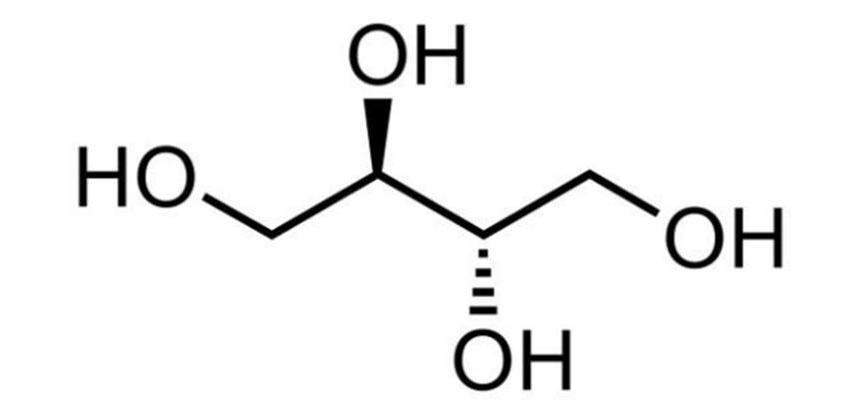
1. 2 Physical and chemical properties
1. 2. 1 Taste
Erythritol has a cool taste when eaten, with a pure sweetness and no aftertaste. Its sweetness is 60% to 70% that of sucrose [8], so it can be used as a diluent for high-intensity sweeteners and combined with steviol glycosides or saccharin. In addition, erythritol can also mask the undesirable taste produced when a variety of sweeteners are used in combination.
1. 2. 2 Heat and acid resistance
Erythritol is very stable to heat and acid, and will not undergo a Maillard reaction with amino acids at high temperatures to turn brown, nor will it decompose under extreme conditions.
1. 2. 3 Solubility
Erythritol is prone to crystallization, and its solubility is only 36% at 25°C. It is also poorly soluble in ethanol[8]. Therefore, in food processing, erythritol must be used in combination with other sugar alcohols to prevent it from crystallizing.
1. 2. 4 Hygroscopicity
Erythritol has low hygroscopicity and does not absorb moisture in a 90% environment[9]. Therefore, erythritol can be used in foods such as chocolate, candy, and beverages.
1. 2. 5 Water activity and osmotic pressure
At room temperature, the water activity of an erythritol aqueous solution is 0.92 (36%), and the osmotic pressure is 461.5 kPa (15%). Erythritol's low hygroscopicity is conducive to reducing the water activity and extending the shelf life of foods[1].
2 Production process
The molecular structure of erythritol is symmetrical and exists in the form of a racemate, so complex steps such as removing enantiomers can be omitted during the production process [5]. At present, there are two main methods for producing erythritol: chemical synthesis and biological fermentation [10].
2. 1 Chemical synthesis method
The chemical synthesis method for producing erythritol is divided into two main categories. One category is to prepare 2-butene-1,4-diol from acetylene and formaldehyde, and then add hydrogen peroxide to react with 2-butene-1,4-diol. The resulting aqueous solution is mixed with a chromium catalyst and an ammonia water inhibitor , hydrogen is introduced into the mixture, and after hydrogenation, erythritol is obtained[11] ; another type uses starch as raw material, which is oxidized with periodate to form a bi-aldol pyrolysis, and then hydrogenated to obtain erythritol[12] . The main disadvantages of the chemical synthesis method are low production efficiency, long cycle times, high costs, and hazardous operations, making it difficult to achieve large-scale industrial production.
2. 2 Biological fermentation method
The biological fermentation method uses starch as the raw material, adding enzymes such as amylase and glucoamylase to liquefy the starch and saccharify it to produce glucose. Yeast or other bacteria are then used to ferment the glucose to produce erythritol, which is obtained by centrifugation and concentration, crystallization and separation, and drying and refinement [13]. The production process of the biological fermentation method is easy to control and safe. Moreover, erythritol is mainly used as a new sweetener in the food industry. Therefore, the biological fermentation method has production advantages and is easily adopted by production companies.
2. 2. 1 Selection of microorganisms
The main strains used to produce erythritol are fungi, yeasts, bacteria, etc., mostly of the genus Saccharomyces. Current research has found that the main species of bacteria that can produce erythritol are the genus Candida[14], the genus Trichosporon[15], the genus Torulaspora[16], the genus Kluyveromyces[16], and the genus Pichia[16] and so on. In the 1950s, Binkle y et al. [17] first proposed that yeast can be used to produce erythritol. Most of the strains used were osmotolerant yeasts isolated from the daily activities of bees, such as short-stemmed molds, round yeasts, and the genus Trichosporon. Hajny et al. [18] isolated a strain of Torulopsis from pollen, which produced erythritol from glucose as a carbon source with a yield of 35% to 40%. Similarly, Ishizuka et al. [19] isolated and obtained a high-osmotic-pressure-tolerant yeast strain from the natural world, such as soil and pollen, and obtained a 50% yield of erythritol by fermenting glucose. Jeya et al. [20] isolated a strain of erythritol yeast (Pseudozyma tsukubaensis KN75) with the ability to grow under high osmotic pressure. Using glucose as the carbon source and batch feeding fermentation, the yield reached 61%. Using dissolved oxygen as a parameter, the production of erythritol can be scaled up to production plant scale, which has great market potential.
The research on the production of erythritol by fermentation in China started relatively late. Fan Guangxian et al. from Jiangnan University [21] screened a spherical yeast (OS194) that produced erythritol, and under the premise that glucose was the carbon source for the substrate, it produced erythritol with a conversion rate of 29.6%. Dong Haizhou et al. [22] obtained a stable ball-like yeast strain ERY237 with stable performance through ultraviolet light and organic solvent mutagenesis, and obtained erythritol under optimal fermentation conditions, with a yield of 87.8 g/L. Jia Wei [23] isolated a strain of Moniliella sp. from honey samples, and the yield of erythritol could reach 110.61 g/L under optimal fermentation conditions. Gu Weiwei[24] used Candida utilis to ferment and produce erythritol, and used an orthogonal test to determine the optimal nutrient substrate and fermentation conditions. The results showed that, on the basis of glucose as the optimal carbon source, the content of erythritol in the fermentation broth after cultivation under optimal conditions was 157.5 g/L. Yang Xiaowei et al. [25] screened out Torulopsis (B84512), and the yield of erythritol reached 162.5 g/L. Lin et al. [26] isolated a Moniliella sp. in honey, and after mutagenesis with nitrosoguanidine, the erythritol yield reached 189.4 g/L. Cai Wei et al. [27] used Moniliella mellis coupled with Wickerhamomyces anomalus fermentation to produce erythritol, with a yield of 114 g/L and a conversion rate of 93.2%, which has good application prospects.
2. 2. 2 Selection of carbon source
In the production of erythritol, glucose is the main carbon source for yeast fermentation. A study by Gao Hui et al. [28] showed that glucose is the best carbon source for the fermentation of erythritol by Torulaspora delbrueckii (B84512). When the total sugar concentration is gradually increased in batches until it reaches 50%, the maximum yield of erythritol is 253 g/L, with a yield of 1.03 g/L. Wu Yan et al. [29] studied the effect of substrate concentration on the production of erythritol by fermentation. The results showed that the hypertonicity-tolerant yeast grew vigorously in a 50% glucose solution and produced erythritol, but the conversion rate was low. In addition, the higher the concentration of the sugar solution, the more residual sugar there was. The optimal concentration of glucose as a substrate for the fermentation of erythritol is 200 g/L. Yang Libo et al. [30] found that Yarrowia lipolytica can ferment glycerol to produce erythritol with a yield of 93.6 g/L and a yield of 49% when glycerol is used as an excellent carbon source.
2. 2. 3 Effect of osmotic pressure
Kim et al. [31] studied the effect of salt osmotic pressure on the growth of Torulopsis circumcincta and the production of erythritol. The results showed that as the salt concentration increased, the erythritol production increased, showing a positive correlation. Kim et al. [32] studied the effect of osmotic pressure on erythritol production by Saccharomyces cerevisiae. The results showed that Mn2+ and Cu2+ can increase the production of erythritol, but the presence of other inorganic salts such as Ca2+, Cr3+, Ni2+, V4+ and other inorganic salts can hinder the production of erythritol, thereby reducing its yield. Onish et al. [33] showed that high osmotic yeasts are more able to withstand the pressure of the sugar solution under high osmotic pressure. When glucose and salt have the same osmotic pressure, it is more conducive to microbial growth and erythritol accumulation.
2. 2. 4 Influence of other parameters
Other parameters such as oxygen transfer, temperature and rotational speed have different effects on microbial growth and erythritol production. Spencer et al. [34] showed that osmotolerant yeasts produce more erythritol when there is sufficient oxygen. This is consistent with the view of Fan Guangxian that maintaining sufficient ventilation during the fermentation process allows more reduced coenzyme I (NADH) to participate in the reduction reaction, which is beneficial to the production of polyols. In addition, temperature is also an important factor affecting the production of erythritol. Xie Piling [35] showed that within the range of 26-30°C, the erythritol content increased with increasing temperature, reaching a maximum at 30°C. When the temperature continued to rise, the erythritol content decreased significantly.
3 Applications
The many excellent properties of erythritol have led to its wide application in the fields of food, medicine, and chemicals. In the pharmaceutical industry, erythritol can be used as a flavouring agent and excipient in medicines, effectively improving the taste of medicines, and it can also be used to synthesise a variety of drugs. In the chemical industry, erythritol can be used as an intermediate in organic synthesis. In cosmetics, erythritol can be added instead of some glycerin to increase its water retention and prevent the cosmetics from spoiling. However, as a new functional sugar alcohol sweetener, erythritol is mainly used in the food industry.
3. 1 Sweets
Erythritol is a zero-calorie sweetener. In the formulation of sweets, erythritol can replace sucrose and significantly reduce its calories, improve the flavor, appearance and stability of the product, and prevent browning and decomposition during food production. Ice cream products contain a lot of sugars, which is not conducive to people with diabetes, obesity, high blood lipids and high blood pressure. Adding erythritol to the ice cream recipe in combination with other sweeteners can help reduce the calories in ice cream and give it a cool, refreshing taste. It can also significantly mask the bitterness of many sweetener combinations, giving a pure sweetness. In chocolate products, in addition to reducing the calories in chocolate, erythritol's low moisture absorption solves the problem of frosting caused by moisture absorption during the production process, while also greatly reducing processing time[36].
3.2 Baked goods
In baked goods, the low hygroscopicity of erythritol can prevent the food from absorbing moisture and extend its shelf life. At the same time, erythritol can reduce the calories in baked goods, balance the texture, replace some of the sucrose, and reduce the sugar content. This not only conforms to the contemporary healthy lifestyle, but also gives the baked goods good porosity and softness. Zhang Wei et al. [37] added erythritol to cookies to develop a new type of low-sugar healthy baked product, and used response surface software to optimize the process parameters. The results showed that the cookies obtained under the optimal parameter conditions were complete in shape, golden in color, crisp in texture, and had a fine texture.

3. 3 Dairy products
Han Jianjiao et al. [38] optimized the technological conditions for the fermentation of red bean milk with erythritol through single factor and orthogonal experiments, and found that under the optimal technological conditions, the free amino nitrogen content of fermented red bean milk can reach 1. 574 mmol/L, and the sensory evaluation is good. Erythritol, which has a lower osmotic pressure, can inhibit lactic acid fermentation and extend the shelf life and shelf life of the product.
3. 4 Beverage products
Ou Zhifeng et al. [39] used jasmine tea and black tea as raw materials, added a well-blended natural sweetener xylitol, erythritol and monk fruit extract, and determined the tea extraction process conditions through orthogonal experiments, and the sensory evaluation determined the sugar-free tea beverage formula. After blending, it has a light tea flavor and a rich mouthfeel. Erythritol provides a pleasant cooling sensation in the mouth, and can itself increase the sweetness of the product, reduce the bitterness of the tea, and mask off-flavors.
4 Conclusion and outlook
Erythritol is a new type of healthy food sweetener that is widely found in nature. It has a number of functional properties, such as zero calories, low moisture absorption, easy crystallization, thermal stability, a refreshing taste, no cariogenic effect, and no effect on blood glucose fluctuations. It can be used in sweet foods, baked goods, milk drinks, and other products. At present, the production of erythritol in the food industry is mainly based on microbial fermentation. With the continuous improvement of theoretical research and technological conditions, the purity of erythritol will continue to increase, and its scope of use will become more and more extensive. The development of erythritol is in line with people's pursuit of natural, safe and healthy concepts, and it is also the mainstream trend of future food industry development.
References
[1] Li Kewen. Efficient production of erythritol [D]. Jinan: Qilu University of Technology, 2015.
[2] Yang Haijun. Development and application of erythritol [J]. China Food Additives, 2004(1): 100-102.
[3] Zhou Xiangjun, Zhu Mintao, Yuan Yijun. Effect of erythritol on the structural and functional properties of pea isolate protein [J]. Food Industry Science and Technology, 2018, 39(8): 73-77.
[4] Goossens J , Roper H . Erythritol : A new sweetener [J] . Confec- tionery Production (United Kingdom) ,1996 , 62(6) : 6-7.
[5] Wang Naiqiang, Liu Haiyu, Xu Jiubing, et al. Properties of erythritol and its application in the pharmaceutical industry [J]. Fermentation Technology Communications, 2013, 42(4): 26-28.
[6] Endo K , Amikawa S , Matsumoto A , et al . Erythritol-based dry powder of glucagon for pulmonary administration[J] . Interna- tional Journal of Pharmaceutics , 2005 , 290(1/2) : 63-71.
[7] Xiuxiu Hong. Research on the fermentation, separation and extraction process of erythritol [D]. Jinan: Shandong Institute of Light Industry, 2011.
[8] Liu Liu. Research on the fermentation and extraction process of erythritol [D]. Jinan: Qilu University of Technology, 2013.
[9] Chen Wen, Liu Xuan, Yang Chunxiao, et al. Research progress on the production of erythritol and its application in the food industry [J]. Food Industry, 2018, 39(2): 266-269.
[10] Qi Guangbin. Research on the preparation of erythritol [D]. Jinan: Qilu University of Technology, 2012.
[11] Saran S , Mukherj ee S , Dalal J , etal . High production of erythritol from Candida sorbosivorans SSE-24and its inhibitory effect on biofilm formation of Streptococcus mutans[J] . Bioresource Technology, 2015 , 198:31-38.
[12] Jesus A L , Nunes S C , Silva M R , et al . Erythritol : Crystal growth from the melt[J] . International Journal of Pharmaceu- tics , 2010 , 388(1/2) : 129-135.
[13] Liu X , Hu Y , Yang W , et al . Solubility of erythritol in metha- nol , ethanol and methanol + ethanol : Experimental measure- ment and thermodynamic modeling[J] . Fluid Phase Equilibria , 2013 , 360 : 134-138.
[14] Yang S W , Park J B , Han N S , et al . Production of erythritol from glucose b y an osmophilic mutant of Candida magnoliae [J] . Biotechnology Letters , 1999 , 21(10) :887-890.
[15] Park J B , Yook C , Park Y K. Production of erythritol newly iso- lated osmophilic Trichosporon sp. [J] . Starch-Strke , 1998 , 50 (2/3) : 120-123.
[16] Kim S Y , Lee K H , Kim J H , et al . Erythritol production b y controlling osmotic pressure in Trigonopsis variabilis[J] . Bio- technology Letters , 1997 , 19(8) :727-729.
[17] Binkley W W , Wolform M I. Biosynthesis of erythritol in a new yeast[J] . Journal of the American Chemical Society , 1950 , 72 : 4778-4782.
[18] Hajny G J , Smith J H , Garver J C. Erythritol production b y a yeast-like fungus[J] . American Society for Microbiology , 1964 , 12(3) :240-246.
[19] Ishizuka H , Wako K , Kasumi T , et al . Breeding of a mutant of Aureobasidium sp. with high erythritol production[J] . Journal of Fermentation and Bioengineering, 1989 , 68(5) : 310-314.
[20] Jeya M , Lee K M , Tiwari M K , et al . Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level[J] . Applied Microbi- ology and Biotechnology, 2009 , 83(2) :225-231.
[21] Fan Guangxian, Zhang Haiping, Zhu Gejian. Influencing factors of high-osmotic-pressure fermentation of Torulopsis sp. to produce erythritol [J]. Journal of Wuxi University of Light Industry, 2001, 20(2): 133-136.
[22] Dong Haizhou, Ye Xian, Hou Hanxue, et al. Study on the production conditions of erythritol by Torulopsis sp. ERY237 [J]. Food and Fermentation Industry, 2008, 34(4): 75-79.
[23] Jia W. Research on the optimization of erythritol production conditions by Kluyveromyces[D]. Tianjin: Tianjin University, 2012.
[24] Gu W. Research on the optimization of erythritol production by fermentation of Candida[D]. Changchun: Jilin Agricultural University, 2007.
[25] Yang Xiaowei, Wu Yan, Lv Huimin, et al. Research on the fermentation process of erythritol [J]. Biotechnology, 2005, 15(4): 63-65.
[26] Lin S J , Wen C Y , Liau J C. Screening and production of eryth- ritol b y newly isolated osmophilic yeast-like fungi[J] . Process Biochemistry, 2001 , 36(12) : 1249-1258.
[27] Cai Wei, Zhang Jianzhi, Jiang Zhengqiang, et al. Optimization of conditions for the fermentation of erythritol by the yeast Torulaspora delbrueckii [J]. Food Science, 2013, 34(21): 259-263.
[28] Gao Hui, Dou Wenfang, Lu Maolin, et al. Effect of carbon source on the fermentation of erythritol and by-product glycerol by Torula sp. B84512 [J]. Food and Fermentation Industry, 2013, 39(3): 17-21.
[29] Wu Yan, Lv Huimin, Shi Dalin, et al. Screening of a new sweetener-erythritol-producing bacteria [J]. Biotechnology, 2000, 10(2): 17-19.
[30] Yang Libo, Zheng Zhiyong, Zhan Xiaobei. Glycine and proline promote erythritol production by Yar-rowia lipolytica fermenting glycerol in a hyperosmotic environment [J]. Food and Fermentation Industry, 2013(12): 1-6.
[31] Kim K A , Noh B S , Kim S Y , etal . Effect of osmotic pressure of salts on growth of Torula sp. and erythritol production[J] . Ko- rean Journal of Applied Microbiology and Biotechnology , 1999 , 27 :91-95.
[32] Kim S Y , Lee K H , Kim J H , etal . Erythitol production b y con- trolling osmotic pressure in Trigonopsis variabilis[J] . Biotech- nology Letters , 1997 , 19(8) :727-729.
[33] Onish H . Studies on osmophilic yeast Part XV. The effect of high concentration of sodium chloride on polylolproduction[J] . Agricultural and Biological Chemistry, 1963 , 27(7) :543-547.
[34] Spencer J F T , Sallans H R. Production of polyhydric alcohols b y osmophilic yeasts[J] . Canadian Journal of Microbiology , 1956 , 2(2) :72-79.
[35] Xie Piling. Selection and fermentation process research of erythritol strains [D]. Yangling: Northwest A&F University, 2013.
[36] Yang Haijun, Zheng Fengzhan. Application of erythritol in sugar-free candy [J]. Agro-Products Processing, 2006(8): 33-34.
[37] Zhang Wei, Zhu Beibei, Wang Chao. Response surface methodology optimization process of erythritol cookies [J]. Guizhou Agricultural Science, 2018, 46(7): 159-162.
[38] Han Jianjiao, Shuang Quan, Wang Li, et al. Optimization of technological conditions for sugar-free fermented red bean milk [J] . Food Science and Technology, 2016 , 41(3) :82-86.
[39] Ou Zhifeng, Hu Xiaorong, Jiang Xisheng, et al. A process and recipe for a sugar-free flavoured tea beverage [J] . Food Industry, 2016 , 37(8) :32-34.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




