Elevate Your Formulations with Stable, Compliant Natural Vanillin Powder
With rising consumer spending and heightened health awareness, an increasing number of brands face a common challenge: how to meet the market's urgent demand for “clean labels” and natural ingredients while preserving product flavor and aroma? Traditional synthetic vanillin, though low-cost and widely available, struggles to align with today's premium, natural product positioning.
Based on this insight, Green Spring Technology leverages green biomanufacturing technology to introduce vanillin powder sourced from natural origins. We employ a biocatalytic pathway to efficiently convert natural ferulic acid into vanillin. This process avoids harsh chemical conditions, eliminates synthetic residues, and yields a rich aroma that closely mirrors the authentic flavor of natural vanilla beans.
This ingredient is particularly suited for high-value products pursuing “clean label” standards, such as:
✅ Premium health foods and beverages;
✅ Natural fragrances and personal care products;
✅ Maternal-infant and functional foods;
✅ Vegan and organic product lines.
Green Spring Technology is committed to providing clients with natural flavor solutions that are not only compliant and reliable but also offer market differentiation, helping your products stand out in the “ingredient competition.”
Currently, global annual vanillin consumption exceeds 12,000 metric tons and continues to grow at a rate of 10% annually. However, the vast majority is produced through chemical synthesis—a process yielding vanillin with limited flavor profiles and potential impurities, failing to meet the rising demand for natural, healthy, and clean-label products.
While vanillin derived from natural vanilla beans offers authentic flavor and is favored by premium markets, its annual yield of only 20–50 tons falls far short of filling the massive market gap. Natural cultivation faces geographical and climatic constraints, while the processing is highly complex, leading to long-term production limitations.
Against this backdrop, biosynthetic technology emerges as the key pathway to achieving large-scale production of natural-grade vanillin. Through green processes like microbial fermentation and enzyme catalysis, it not only efficiently produces naturally aromatic, safe, and compliant natural vanillin but also aligns with the future trend of sustainable production.
1 Microbial Fermentation Method
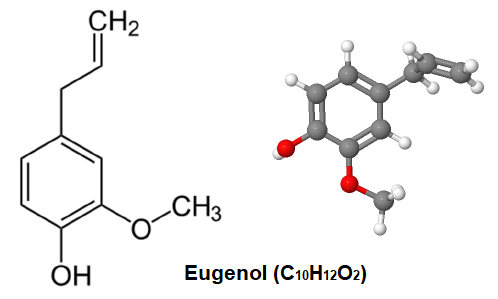
1.1 Using Eugenol or Isoeugenol as Substrates
Microbial fermentation offers a green and efficient alternative pathway for natural vanillin production. This method utilizes natural components like isoeugenol as substrates, generating vanillin through the bioconversion of specific microbial strains. This avoids the common issues of limited aroma diversity and impurity residues found in chemical synthesis.
Research indicates that isoeugenol exhibits significantly higher conversion efficiency than other substrates. For instance, Rhodococcus rhodochrous achieves over 50% molar yield under optimal conditions, demonstrating strong industrialization potential.
Microbial fermentation not only aligns with clean label trends but also enables sustainable production, supplying high-quality natural vanillin raw materials to the food, fragrance, and cosmetics industries.
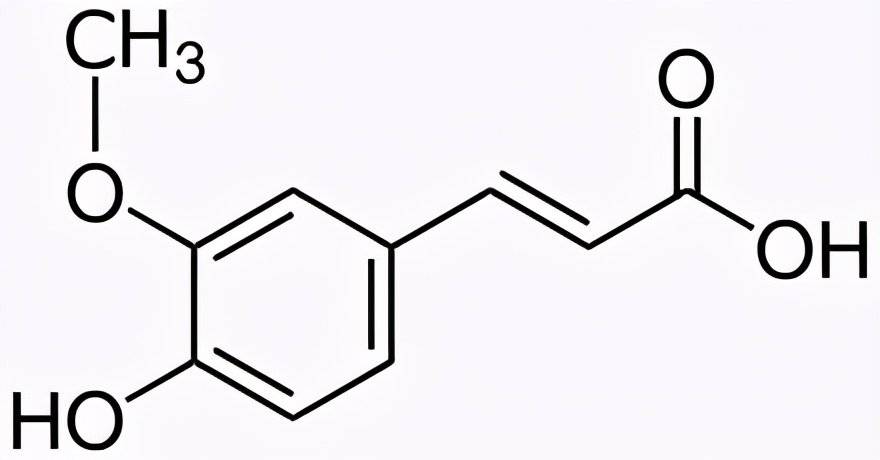
1.2 Ferulic Acid as Substrate
Biological production of natural vanillin powder from ferulic acid is a current research focus. This process utilizes renewable agricultural byproducts like corn bran and rice husks, combining eco-friendliness with high efficiency.
Current mainstream approaches include single-strain fermentation and multi-strain synergistic methods. Among single-strain methods, soil-dwelling filamentous bacteria (Amycolatopsis sp.) demonstrate outstanding performance, achieving vanillin yields of 11.5 g/L. Bacillus coagulans exhibits exceptionally high ferulic acid metabolic rates, achieving conversion rates exceeding 95% within 7 hours. The multi-microbial stepwise conversion method employs the synergistic action of Aspergillus niger and Rhizopus rubrum, first converting the substrate into vanillic acid, then reducing it to vanillin. This approach has successfully achieved direct crystalline vanillin production using corn bran as feedstock.
Research also indicates that excessively high vanillin concentrations during fermentation inhibit microbial growth and adversely affect yield through byproduct effects. Process optimization measures such as adding fucose or employing adsorption resins can effectively increase product concentration, boosting yield by more than threefold and demonstrating promising industrial application prospects.
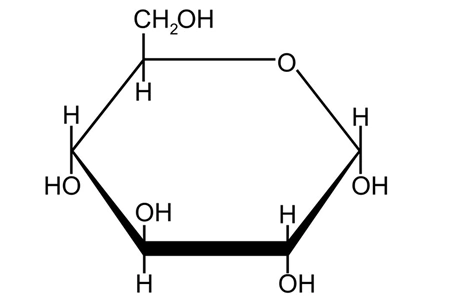
1.3 Glucose-Based Approach
Biosynthetic vanillin production using glucose as feedstock represents a cost-competitive yet technically immature pathway. Glucose, being widely available and inexpensive, can be obtained in large quantities through starch hydrolysis. However, this route remains largely confined to laboratory-scale studies, with only one successful report documented: E. coli recombinant strains convert glucose into vanillic acid, which is then reduced to vanillin using aldehyde dehydrogenase.
The core challenge for practical implementation lies in achieving stable and efficient expression of E. coli recombinant strains. Currently, bioconversion pathways using isoeugenol and ferulic acid as substrates offer greater advantages in terms of technological maturity and industrial feasibility, aligning more closely with the current development direction of natural vanillin production.
2 Plant Cell Culture Method
Plant cell culture offers a novel approach for natural vanillin production but remains largely confined to laboratory settings, with significant gaps to industrial application. Utilizing cell systems from vanilla beans or shrubby chili peppers, this method promotes vanillin synthesis through precursor or hormone supplementation. However, yields remain generally low, typically ranging between 0.01–0.02 g/L.
Although methods like immobilized culture, β-cyclodextrin addition, or Aspergillus niger induction can moderately enhance yields, overall efficiency remains insufficient for large-scale production. While this technology holds natural and green potential, breakthroughs in cell viability and cultivation processes are still required.
3 Enzymatic Approach
Enzyme-catalyzed methods are emerging as a highly promising green process for natural vanillin production. This approach utilizes specific enzymes (such as vanillin oxidase or lipase) to directly catalyze substrate conversion into vanillin, offering significant advantages including high reaction efficiency, mild conditions, high product purity, and minimal contamination.
Research indicates that vanillyl alcohol oxidase (VAO) can synthesize vanillin via two pathways: from wood tar oil or vanillin. The latter pathway is more competitive due to abundant raw material sources (e.g., red pepper and capsaicin). Patent reports also indicate that certain lipases can efficiently convert isoeugenol and pinene into vanillin, achieving a maximum mass fraction of 83.1%.
Despite its superior performance in purification, energy consumption, and environmental sustainability, industrial-scale enzyme-based production faces critical challenges including enzyme engineering, immobilization technology, and multi-enzyme reactor design. With ongoing advancements in enzyme engineering and biotechnology, this enzymatic approach holds promise as the core process for future natural vanillin powder production.
4 Outlook
As natural vanillin powder becomes a core demand in the global food and flavor industry, Green Spring Technology leverages advanced biocatalysis and fermentation processes to provide customers with stable, compliant, and cost-competitive natural vanillin raw materials. This empowers seamless product formulation upgrades and clean label transitions. Discover Our Comprehensive Natural Vanillin Powder Solution.
We maintain microbial fermentation as our core technology, selecting premium natural plant-based raw materials to ensure pure aromas that meet domestic and international natural standards. This approach effectively avoids common issues in chemical synthesis, such as impurity residues and limited aroma profiles. Green Spring Vanillin powder finds extensive applications in premium foods, beverages, health supplements, and cosmetics, helping your products stand out in the “natural and healthy” category.
Despite ongoing global challenges in bio-based vanillin's production capacity and cost, Green Spring Technology has achieved efficient, stable, and large-scale manufacturing through continuous strain and process optimization. We provide reliable, cost-effective natural vanillin powder solutions.
Contact us today at helen@greenspringbio.com or WhatsApp: +86 13649243917 for samples and technical documentation. Green Spring Technology is committed to being your trusted vanillin partner, jointly creating a more natural and safer product future.
References:
[1 ] [Lesage-Meessen L, Delattre M, Haon M, et al. A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and pycnoporus cinnabarinus [J]. and pycnoporus cinnabarinus [ J ]. J Biotechnol, 1996, 50:107 - 113 .
[2 ] Tadasa K. Degradation of eugenol by a microorganism[J]. Agric Biol chem, 1977, 41(6):925 - 929 .
[3] Rabenhorst J, Hopp R. process for the preparation of vanillin [p].Us: 5 017 388, 1991 - 05 - 21 .
[4 ] Bavutti Hamilton R F, Anazawa Tania A, Durrant Lucia R. study of vanillin synthesis by deuteromycete fungal strains[J]. Braz symp chem Lingins Other wood compon, 1997, 6: 605 - 611 .
[5 ] shimoni E, Ravid U, shoham Y. Isolation of a Bacillus sp. capable of transforming isoeugenol to vanillin[J]. J Biotechnol, 2000, 78 (1): 1 - 9 .
[6 ] chatterjee T, De B K, Bhattacharyya D K. Microbial conversion of isoeugenol to vanillin by Rhodococcus rhodochrous[J]. Indian J chem, 1999, 38B(5):538 - 541 .
-
Prev
What Is the Use of Stachyose in Beverages?
-
Next
Green Spring Technology Provides Comprehensive Synthetic and Natural Vanillin Powder Solutions


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




