What Are the Testing Methods for Vanillin Powder?
Abstract:Vanillin powder is widely used in food because of its unique flavor, but its excessive intake may harm people's health, therefore, the detection of vanillin in food is necessary. In this paper, the progress of the detection techniques of UV spectrophotometry, high performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), sensor method and nanoanalytical method was reviewed, and the recoveries, RSDs and detection limits of the different methods were analyzed, which were aimed at providing theoretical references for the development of rapid, simple and highly sensitive detection techniques for vanillin in the future.
1 Preface
As a food additive, vanillin can increase the flavor of pudding, cookies, chocolate, ice cream, and beverages [1], but its excessive use can cause nausea, vomiting, and even impaired liver and kidney functions [2]. According to the Standard for the Use of Food Additives (GB 2760-2014), no food flavorings should be added to infant and toddler formulas from 0 to 6 months of age, with a limit of 5 mg/100 mL for larger infant and toddler formulas, and a limit of 7 mg/100g for cereal-based supplementary foods for infants and toddlers[3] . According to the methods stated in the 2021 edition of the National Standard for Food Safety (GB 5009.284-2021), the main methods for the determination of vanillin are liquid chromatography, liquid chromatography-mass spectrometry/mass spectrometry and gas chromatography-mass spectrometry [4].
2 Detection techniques
2.1 Ultraviolet spectrophotometry (UV- vis)
The UV-Vis method can be used for qualitative analysis with simple operation and high sensitivity by using the different coloration of substances [5]. Feng Caiting et al. [6] used the UV-vis method to determine the vanillin content in milk powder, and the recoveries were 98.6% with RSD=0.34%, which indicated that the method was reproducible; Meng Desu et al. [7] showed that the recoveries ranged from 97.3% to 101.1% with the RSD<2.0%, which indicated that the method could be used for the determination of vanillin in food; Yang Lixia [8] used the UV-Vis method to determine the vanillin content in biscuits. Yang Lixia [8] used the UV-Vis method to determine the vanillin content in cookies, and the recoveries were 99.6% with RSD=0.36%, which indicated that the method was fast, simple and practical.
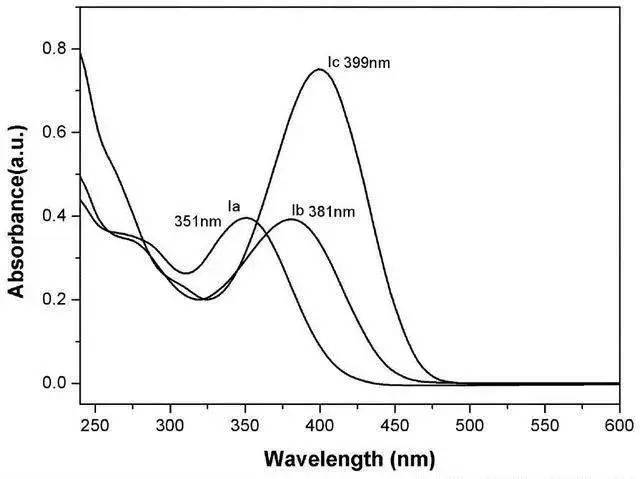
2.2 High performance liquid chromatography (HPLC)
HPLC is a liquid chromatographic separation and detector after sample extraction, which is rapid and efficient [9]. Xie Xiaodan [10] used HPLC to determine the vanillin content in montmorillonite powder, and the recoveries were 99.12%~100.44% with RSD<0.65%; Wang Cunxiao [11] used HPLC to determine the vanillin content in milk powder, and the recoveries were 96.0%~100.2% with RSD<5%, and the limit of detection (LOD) was 0.05 μg mL-1; Guan Shuxia [12] used HPLC to determine the vanillin content in food. The limits of detection (LODs) were 0.05 μg - mL-1; Guan Shuxia [12] determined the vanillin in food by HPLC with the recoveries of 96.1%~108.7% and the RSDs of 1.27%~3.00%, which can meet the requirements of the test of vanillin in food.
2.3 Gas chromatography-mass spectrometry (GC-MS)
GC-MS is a chromatographic method using gas as the mobile phase, which is highly selective and sensitive [13]. Dong Zhenshan et al. [14] used GC-MS to determine the vanillin content in ice-cream, and the average recoveries ranged from 93.21% to 103.20% with the RSDs of 1.99%-4.72% and the detection limit of 0.098 μg/g. Wu Binyu et al. [15] used GC-MS to determine the vanillin content of mainstream cigarette smoke, and the recoveries ranged from 96.3% to 107.7% with the detection limit of 9.1 ng/cigarette. The recoveries ranged from 96.3% to 107.7% with a limit of detection of 9.1 ng/cigarette, and the recoveries ranged from 91.4% to 109% with a limit of detection of 0.03 mg/kg for the determination of vanillin in cigarette flavor by GC-MS.
2.4 Sensing detection
2.4.1 Voltammetry
An electrochemical sensor based on square wave voltammetry was developed by F. Bettazzi et al. [17] for the determination of vanillin in commercial products with an RSD=2.0% and a limit of detection (LOD) of 0.4 μM; Serkan Karakaya et al. used cyclic voltammetry of copper particles on an indium-tin-oxide (ITO) electrode, with a linear range of 0.50-2.0 μM and a limit of detection (LOD) of 0.15 μM, which allowed for the accurate and selective determination of vanillin in routine samples. The linear range was 0.50-2.0 μM and the limit of detection was 0.15 μM, which could accurately and selectively determine vanillin in daily samples. Somaye Cheraghi et al. described the fabrication of a highly sensitive sensor based on the square-wave voltammetry method with the linear range of 0.03-800.0 μM and the limit of detection of (9.0±0.1) μM, which proved that the method could be successfully applied to the analysis of vanillin in foodstuff samples.
2.4.2 Resistivity
N. Hareesha et al. [18] prepared a polymerized glutamate-functionalized multi-walled carbon nanotubes and graphite composite paste sensor for the determination of vanillin in food samples by electrochemical impedance spectroscopy (EIS), with the linear range of 0.50-18.0 μM and the limit of detection (LOD) of 0.019 9 μM, and with good reliability, repeatability and reproducibility; Amrutha Balliamada et al. Amrutha Balliamada et al [19] prepared polymethyl orange modified graphene paste electrodes to analyze the electron transfer kinetics of vanillin in food flavors and natural vanilla beans using electrochemical impedance spectroscopy, and the excellent electrocatalytic activity for the oxidation of vanillin was attributed to the high surface area and interactions between the polymer film and the analyte, and the method is simple and stable, which can be effectively used for the analysis of real samples; Ziyatdinova Guzel et al. [20] developed a sensor based on poly(aminobenzenesulfonic acid)-functionalized single-walled carbon nanotubes and electropolymerized bromocresol violet layer-by-layer deposition for the determination of vanillin, with the linear range of 5.0-25.0 μM and the limit of detection (LOD) of 64 μM, and the validity of the sensor was confirmed by electrochemical impedance spectroscopy for vanilla extract analysis.
2.4.3 Current sensing
Mani Sivakumar et al. [21] synthesized CoS nanorods for the determination of vanillin by a hydrothermal method with a linear range of 0.50-56.0 μM and a limit of detection of 0.07 μM; Mónica ávila et al. [22] developed a molecularly imprinted polymer flow pattern based on an on-line supported liquid film-piezoelectricity detection system using a molecularly imprinted polymer modified quartz crystal microbalance for the quantitative determination of vanillin. A linear range of 5.0-65.0 μM with RSD=±4.8% was obtained for the quantitative determination of vanillin in foodstuffs, and Serkan Karakaya used a disposable polymer (chromium black T) modified pencil graphite electrode to achieve a low-cost, sensitive, and selective determination in the linear range of 0.050-10.0 μM, with a limit of detection (LOD) of 0.013 μM. The linear range was 0.050-10.0 μM and the limit of detection was 0.013 μM.
2.5 Nanomaterial-based detection
2.5.1 Carbon-based materials
Carbon-based materials are rich in oxygen-containing functional groups, which can be used to obtain a variety of high value-added chemicals [23]. N. Hareesha et al. [24] used electrochemically polymerized glutamate-functionalized multi-walled carbon nanotubes and a graphite composite paste sensor to determine the electrochemical oxidation of vanillin in food samples, and the linear dynamic range of the results was 0.50-18.0 μM, with a limit of detection (LOD) of 0.019 μM, which showed good reliability, repeatability, and reproducibility. The linear dynamic range was 0.50~18.0 μM, and the limit of detection was 0.019 9 μM, which showed good reliability, repeatability and reproducibility; C. Raril et al. [24] used an ionic surfactant-modified graphene paste electrode for the determination of vanillin, and the anodic peak currents were directly proportional to the concentration of vanillin, with the ranges of 4×10-6-1.5×10-5 M and 2×10-5-7×10-5 M, and the limit of detection was 1.29 μM, with good recoveries; Mei Qianwen et al. [25] used an electrochemical oxidation method for vanillin. Qianwen et al. [25] used electrospun molybdenum disulfide nanoparticles compounded with carbon nanofibers to determine the amount of vanillin, with the linear dynamic range of 0.30-135.0 μM and the limit of detection of 0.15 μM, which has a good current response signal and can be used for the determination of actual samples.
2.5.2 Gold-based materials
Gold-based materials can effectively enhance the Raman scattering intensity and can be introduced into nanomaterials to improve the performance of composites [26]. Yujiao Sun et al. [27] developed a ratiometric electrochemical fitness sensor based on electrodeposited gold nanoparticles coupled with DNA aptamers on the basis of Cortex black/ferrocene double-doped zeolite MOFs (Fc-KB/ZIF-8) with the dynamic range of 10.0~0.20 μM and the detection limit of 3 μM, indicating the high reliability and practicability of this method. The linear dynamic range was 10.0~0.20 μM, and the detection limit was 3 μM, which indicated the high reliability and practicality of this method; Jingyao Gao et al. [28] prepared a low-defect graphene electrode using Au nanoparticles, and the linear response of the Au nanoparticle-modified graphene electrode to vanillin was in the range of 0.20~40.0 μM, with a detection limit of 10 μM; Fang Jiali et al. Fang Jiali et al. [29] used Au nanoparticles to prepare a gold nanoparticle-modified carbon paste electrode, and investigated the electrochemical behavior of the electrode for vanillin, with the linear range of 1×10-9-5×10-5 mol/L, and the limit of detection was 5.4×10-10 mol/L, which indicated that the method could be used for the determination of vanillin in chocolate.
2.5.3 Ag-based materials
Silver-based materials have excellent electrical and thermal conductivity and have been used in various fields [30]. Totka Dodevsk et al. [31] investigated the applicability of biosynthesized silver nanoparticles (AgNPs) deposited onto a spectroscopic graphite electrode for the detection of vanillin, with a response of up to 0.5 mM and a limit of detection of 8.4 μM. Pei Liang et al. [32] established a surface-enhanced Raman scattering (SES) method for the detection of vanillin at trace levels, using flower-shaped silver nanoparticles on silicon sheets as a surface-enhanced Raman scattering substrate. Pei Liang et al. [32] developed a surface-enhanced Raman scattering method for the detection of trace vanillin, using flower-like silver nanoparticles on silicon wafers as the substrate for surface-enhanced Raman scattering.
2.5.4 Platinum-based materials
Platinum-based materials have excellent catalytic properties in many fields due to their stable structure and rich electronic structure [33]. Jazreen H.Q. Lee et al. [34] carried out a detailed electrochemical study of vanillin in acetonitrile using a platinum electrode, where vanillin firstly undergoes oxidation by -2-e-/-H+ and then hydrolysis and loses its methoxy substituents to form the corresponding 1,2-benzoquinone, which can subsequently be reduced by +2e-/+2H+ and can be electrochemically reduced at about -1.58 vs. Benzoquinone, which can then be reduced by +2e-/+2H+, and can be electrochemically reduced at about -1.58 vs. The linear range was 50.0~430.0 μM, and the detection limit was 19 μM. Jia Hui et al. [36] developed an electrochemical sensor by synthesizing platinum nanoparticle-serine functionalized and boron-doped graphene quantum dots complexes, with the linear range of 1×10-9 M~1×10-4 M, and the detection limit of 2.8×10-10 M, which demonstrated that the method has good sensitivity, selectivity and reproducibility.
3 Conclusion
Vanillin rapid detection technology plays a significant role in food safety. The UV-Vis method is simple and easy to operate, but requires a specific environment; the HPLC method has high sensitivity and accuracy, but the instrument is expensive and the operation is complicated; the GC-MS method has better sensitivity and reproducibility, but requires the same specific environment and complicated operation as the HPLC method, and is expensive; and the sensor method is simple, sensitive, and reproducible, but the specificity is not good. Currently, the development of detection technologies based on nanomaterials is progressing rapidly, and some progress has been made in sensitivity, accuracy and specificity, but in view of the complexity of the sample background and the many interferences, it is still necessary to further develop and study in order to realize highly sensitive and specific detection.
References:
[1] TIAN Y, DENG P, WU Y, et al. High sensitive voltammetric sensor for nanomolarity vanillin detection in food samples via manganese dioxide nanowires hybridized electrode[J]. Microchemical journal, 2020, 157:104885.
[2] ZHOU Ruizheng, YI Huajuan, LIN Qiufeng, et al. Research Progress on the Detection of Vanillin and Ethyl Vanillin in Foods[J]. Analytical Instruments, 2023(1): 7.
[3] National Health and Family Planning Commission . National Standard for Food Safety: Standard for the Use of Food Additives: GB 2760-2014 [S]. Beijing: China Standard Press, 2014.
[4] National Health Commission, State Administration for Market Supervision and Regulation . National Standard for Food Safety Determination of vanillin, methyl vanillin, ethyl vanillin and coumarin in food: GB 5009.284-2021 [S]. Beijing: China Standard Press, 2021.
[5] FAN Yudan . Application of ultraviolet spectrophotometry in food testing and food safety analysis [J]. China Food, 2022(14):1-14.
[6] Feng Caiting, Yang Lixia, Li Shujing. Determination of vanillin in milk powder by ultraviolet-visible spectrophotometry [J]. Hebei Chemical Industry, 2012, 35(6):78-80.
[7] MENG Desu, PANG Yanling. Simultaneous determination of vanillin and salicylaldehyde in foods by ultraviolet spectrophotometry [J]. Food Research and Development, 2014, 35(17):3.
[8] Yang Lixia . Determination of vanillin content in cookies [J]. Henan Chemical Industry, 2012(Z2):52-54.
[9] ZHOU Ruizheng, YI Huajuan, LIN Qiufeng, et al. Research Progress of Detection Methods for Vanillin and Ethyl Vanillin in Foods [J]. Analytical Instruments, 2023(1):7.
[10] Xie Xiaodan . Determination of vanillin in Montelukast by high performance liquid chromatography [J]. Quality Safety and Inspection and Testing, 2020, 30(6):38- 40.
[11] WANG Cunxiao . Determination of vanillin in milk powder by high performance liquid chromatography [J]. Food Safety Guide, 2022(34):3.
[12] GUAN Shuxia, LIN Xinchuan, DU Ying. Determination of vanillin, methyl vanillin, ethyl vanillin and coumarin in foods by high performance liquid chromatography [J]. Food Safety Journal, 2022(20): 16.
[13] FANG Yingjie, YAN Xianhui, YU Junqiang . Analysis of effective use of gas chromatography - mass spectrometry in food testing [J]. Food Safety Guide, 2016, (10X): 83.
[14] DONG Zhenshan, ZHANG Simeng . Determination of vanillin in ice-cream by gas chromatography - mass spectrometry [J]. Light Industry Science and Technology, 2019(1):3.
[15] WU Bingyu, FEI Ting, LUO Chen, et al. Determination of vanillin and ethyl-vanillin in mainstream cigarette smoke by solid phase extraction-gas chromatography/mass spectrometry [J]. Analytical Laboratory, 2020, 39(1):5.
[16] YU Hang, HUANG Guangli, TAO Li, et al. Determination of vanillin and ethyl-vanillin in cigarette flavors and fragrances by gas chromatography-mass spectrometry [J]. Physical and Chemical Inspection ( Chemical Subsection ), 2015, 51(5): 668-671.
[17] BETTAZZI F, PALCHETTI I, SISALLI S, et al. A disposable electrochemical sensor for vanillin detection[J]. Analyticachimica acta, 2006, 555(1):134-138.
[18] HAREESHA N , MANJUNATHA J G , AMRUTHA B M , et al. A fast and selective electrochemical detection of vanillin in food samples on the surface of poly( glutamic acid) functionalized multiwalled carbon nanotubes and graphite composite paste sensor[J]. Colloids and surfaces, A. physicochemical and engineering aspects, 2021:626.
[19] Monnappa A B, Manjunatha J, Bhatt A S, et al. Sensitive and Selective Electrochemical Detection of Vanillin at Graphene Based Poly (Methyl Orange) Modified Paste Electrode[J]. Journal of Science Advanced Materials and Devices, 2021(5):415-424.
[20] ZIYATDINOVA G, ZHUPANOVA A, DAVLETSHIN R. Simultaneous determination offerulic acid and vanillin in vanilla extracts using voltammetric sensor based on electropolymerized bromocresol purple[J]. Sensors (Basel, Switzerland), 2021, 22(1).
[21] mani s , chen s m . Simple synthesis of cobalt sulfide nanorods for efficient electrocatalytic oxidation of vanillin in food samples[J]. Journal of Colloid & Interface Science, 2017, 490:719-726.
[22] MONICA A, ZOUGAGH M, ESCARPA A, et al. Supported liquid membrane-modified piezoelectric flow sensor with molecularly imprinted polymer for the determination of vanillin in food samples[J]. Talanta, 2007, 72(4):1362-1369.
[23] Xu B , Wei Xiuzhi , Sun Jiangmin , et al. In situ synthesis of nitrogen-doped graphene-loaded palladium nanoparticles for catalyzing highly selective hydrogenation of vanillin [J]. Journal of Chemistry, 2023, 81(3): 239-245.
[24] laril c, manjunatha j g . A simple approach for the electrochemical determination of vanillin at ionic surfactant modified graphene paste electrode [J]. Microchemical journal, 2020(5):154.
[25] MEI Q , DING Y , LI L , et al. Electrospun MoS2 composite carbon nanofibers for determination of vanillin[J]. Journal of electroanalytical chemistry, 2019(1):297- 303.
[26] Zhang Shouren . Study on Controlled Synthesis, Properties and Catalytic Applications of Gold and Titanium Dioxide Nanocomposites [D]. Zhengzhou: Zhengzhou University, 2016.
[27] SUN Y J, JIANG X W, JIN H, et al. Ketjen black/ ferrocene dual-doped MOFs and aptamer-coupling gold nanoparticles used as a novel ratiometric electrochemical aptasensor for vanillin detection[J]. Analytica chimica acta, 2019, 1083:101-109.
[28] JING G , QI Y , CHEN Y , et al. Label-Free electrochemical detection of vanillin through low-defect graphene electrodes modified with Au nanoparticles[J]. materials, 2018, 11(4):489.
[29] FANG Jia-Li, LIU Meng-Qin, XU Zhi-Feng, et al. Electrochemical behavior of vanillin on nanogold-modified electrodes [J]. Journal of Hengyang Normal College, 2016, 37(3):169-172.
[30] Chang Yichuan, Li Daiying, Cheng Geng, et al. Progress in the application of electrochemical technology in the preparation process of silver-based materials [J]. Ship Power Technology, 2022, 42(10):4. [31] DODESKA T ,VASILEVA I,DENEV P , et al. Rosa damascena waste mediated synthesis of silver nanoparticles: characteristics and application for an electrochemical sensing of hydrogen peroxide and vanillin[J]. Materials chemistry and physics, 2019(6):335-343.
[32] LIANG P,ZHOU Y F,ZHANG D , et al. SERS based determination of vanillin and its methyl and ethyl derivatives using flower-like silver nanoparticles on a silicon wafer[J]. Microchimica scta, 2019, 186(5):1-8.
[33] ZHANG Lei, FAN Hongsheng, WANG Rongming . Research progress on platinum-based nanomaterials electrocatalysts [J]. Metallic Functional Materials, 2018, 25(1): 8. [34] LEE J,LAUW S,WEBSTEREBSTER R D. The electrochemical study of vanillin in acetonitrile [J]. Electrochimica scta,2016(6):533-544.
[35] FORT C I , COBZAC S C,TURDEAN G L. Second- order derivative of square-wave voltammetry for determination of vanillin at platinum electrode[J].Food food chemistry,2022(15):385.
[36] HUI J, LI R, DING Z Z, et al. Platinum nanoparticle- graphene quantum dot nanocage as a promising Schottky heterojunction electrocatalyst for electrochemical detection of vanillin in baby milk powder[J]. Microchemical journal,2023:186.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






