What Are the Uses of Sweetener D Tagatose in the Food Field?
D-tagatose is a rare sugar that is a new type of functional sweetener discovered in recent years. It has the functions of being low in calories, lowering blood sugar, regulating intestinal flora, and resisting tooth decay [1]. In 2001, it was determined by the US Food and Drug Administration to be generally recognized as safe [2], and in 2014 it was approved as a food ingredient by the National Health and Family Planning Commission of the People's Republic of China [3]. D-tagatose has a sweetness similar to sucrose, with a sweetness of 92% of sucrose, basically no unpleasant odors or aftertastes, and produces only 1/3 of the calories of sucrose, with an energy value of 1. 5 kcal/g. Moreover, D-tagatose is prone to the Maillard reaction and can caramelize at lower temperatures, so it has broad application prospects in dairy products, beverages, cereal products, candies, dried fruit and other food products.
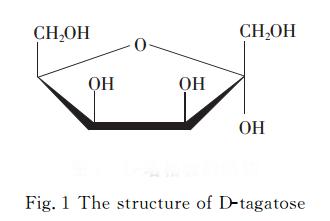
1 Structure of D-tagatose
D-Tagatose is a rare natural hexose, an isomer of D-galactose and a diastereoisomer of D-fructose at the C-4 position. Its chemical structure is shown in Figure 1.
2 Production of D-tagatose
Pure natural D-tagatose is not easy to find and is only found in the gum secreted by trees [4]. At present, D-tagatose is mainly produced by chemical synthesis and biosynthesis.
2.1 Chemical synthesis
The chemical synthesis method uses a soluble alkali metal or alkaline earth metal salt as a catalyst to catalyze the isomerization of D-galactose and a metal hydroxide under alkaline conditions to form a metal hydroxide and a D-tagatose complex precipitate. The precipitate is then neutralized with acid to separate and precipitate to obtain the final product of D-tagatose [5]. It has been reported that Spherix Corporation in the United States, Arla Foods in Denmark and Nutrilad in Belgium have already applied chemical synthesis of D-tagatose in industrial production.
Compared with foreign countries, the chemical synthesis method of D-tagatose in China is still in the research of industrialization process. Researchers are committed to improving the process conditions to improve the yield and purity of D-tagatose[6-8]. At present, although chemical synthesis can achieve industrial production, it has certain disadvantages, such as many by-products and serious environmental pollution. Therefore, if chemical synthesis is to be further developed, further improvements must be made to the process.
2.2 Biosynthetic method
The biosynthesis method uses biological enzymes or enzyme-containing cells as catalysts to catalyze the synthesis of D-tagatose from substrates. Compared with the chemical synthesis method, it has the advantages of mild conditions, high yield, and fewer by-products. Currently, D-tagatose can be obtained by catalyzing the oxidation of galactitol by dehydrogenase, or by catalyzing the isomerization of D-galactose by L-arabinose isomerase to produce D-tagatose.
2.2.1 Production of D-tagatose from galactitol
The production of D-tagatose from galactitol is one of the earliest biosynthetic methods studied. In 1984, Izumori K et al. [9] first used the strains Bacillus subtilis and Bacillus globigii to oxidize galactitol to tagatose. Muniruzzaman S et al. [10] found that the strain Enterobacter cloacae, isolated from soil, could oxidize galactitol to D-tagatose. The results showed that the conversion rate was 92% when the concentration of galactitol was 2%, and decreased to 86% when the concentration was 5%. In addition, it was found that the immobilized enzyme cells still maintained high catalytic activity. Jagtap S S et al. [11] found that galactitol dehydrogenase from Rhizobium pei anthii can also use galactitol as a substrate to produce D-tagatose. It was found that this galactitol dehydrogenase has higher specific activity than other reported galactitol dehydrogenases. However, due to the high price of galactitol, this method is not suitable for industrial production and has little value for commercial application.
2.2.2 Production of D-tagatose from D-galactose
Compared with the more expensive galactitol oxidation method for producing D-tagatose, the method of using L-arabinose isomerase to catalyze the isomerization of D-galactose to produce D-tagatose, first reported by Cheetham P S et al. in 1993 [12], has more prospects for industrialization. Lim B C et al. [13] used Bacillus l-arabinose isomerase to catalyze the reaction of D-galactose isomerization to D-tagatose. The results showed that the maximum yield was 11. 5 g /(L · h), which is 1.5 times higher than the maximum yield in the absence of boric acid. Liang M et al. [14] used calcium alginate to immobilize L-arabinose isomerase from Thermus thermophilus to produce D-tagatose in a bioreactor packed bed, with a maximum yield of 10 g/(L · h).
Although D-galactose is relatively inexpensive compared to the price of galactitol, its production cost is still relatively high for the biosynthesis method, and researchers have conducted corresponding research on this issue. One solution is to immobilize enzyme-containing cells. Hong Y H et al. [15] used sodium alginate to immobilize recombinant Escherichia coli cells to produce D-tagatose, obtaining D-tagatose with a purity of >99%. However, using Escherichia coli as a production strain for D-tagatose has certain safety risks. Xu Z et al. [16] used the food-grade Lactobacillus fermentum CGMCC2921 strain to produce immobilized cells for the production of D-tagatose by sodium alginate embedding and glutaraldehyde cross-linking. Under the condition of adding boric acid, the conversion rate of D-galactose to D-tagatose can reach 60%, and the yield can reach 11. 1 g/(L · h). The immobilized cell catalysis method does not require the extraction of L-AI enzyme, and the biotransformation of D-tagatose can be completed in vivo, which omits the enzyme purification process and simplifies the production process, thereby reducing production costs.
The biosynthesis method is a green process that meets current international requirements for environmental protection and is the main development direction for the future industrial production of D-tagatose. However, due to the low conversion rate of D-tagatose and the high cost, it is difficult to achieve industrial production. Therefore, the research hotspots of the biosynthesis method are to find an L-arabinose isomerase that can efficiently produce D-tagatose, optimize the production process, improve the yield of D-tagatose, and reduce costs.
3. Applications of D-tagatose in food
D-tagatose has broad application prospects in the food industry as a functional sweetener, mainly used in health drinks, dairy products, candy, cereal foods, etc.
3.1. Applications in health drinks
In the beverage industry, D-tagatose is mainly used to eliminate the metallic, aftertaste, astringent and other unpleasant aftertastes of strong sweeteners such as cyclamate, aspartame, acesulfame and stevia, and to improve the [17]. In 2003, PepsiCo began adding a combination sweetener containing D-tagatose to carbonated drinks to obtain zero- and low-calorie healthy drinks that basically taste like full-calorie drinks [18, 19]. In 2009, the Irish concentrated processing company obtained low-calorie tea, coffee, fruit juice and other beverages by adding D-tagatose [20, 21]. In 2012, South Korea's CJ CheilJedang also obtained low-calorie coffee drinks by adding D-tagatose [22].

3.2 Application in dairy products
As a low-calorie sweetener, D-tagatose can significantly improve the texture of dairy products with a small amount added. Therefore, D-tagatose is added to dairy products such as sterilized milk powder, cheese, and yogurt [23]. With the deepening of research on the properties of D-tagatose, its application has been extended to more dairy products. For example, adding D-tagatose to chocolate dairy products can give a rich and mellow toffee flavor. D-tagatose can also be used in yogurt. In addition to providing sweetness, it can increase the number of live bacteria in yogurt, improve the nutritional value of yogurt, and give it a richer and mellow flavor [24].
3.3 Application in cereal products
D-tagatose is easily caramelized at low temperatures, making it easier to produce an ideal color and richer flavor than sucrose, and can be used in baked goods. Studies have found that D-tagatose can undergo a Maillard reaction with amino acids to produce volatile flavor substances such as 2-acetylfuran, 2-ethylpyrazine and 2-acetylthiazole, which have a higher flavor than those produced by reducing sugars such as glucose and galactose [25]. However, when adding D-tagatose, attention should also be paid to the baking temperature. A lower temperature is conducive to enhancing the flavour, while a high temperature and long processing time will result in a darker colour and a bitter aftertaste [26].
In addition, because D-tagatose has low viscosity and is easy to crystallize, it can also be used in frosting. D-tagatose can be applied to the surface of cereals alone or in combination with polyhydric compounds such as maltitol to increase the sweetness of the product [27].

3.4 Application in confectionery
D-tagatose can be used as the sole sweetener in chocolate, and the process does not require major changes. The viscosity and heat absorption characteristics of chocolate are similar to those when sucrose is added. In 2003, New Zealand's Mada Sports Nutrition Food Company was the first to develop chocolate products containing D-tagatose in flavors such as milk, dark chocolate, and white chocolate. It then developed various novel chocolate products containing D-tagatose, such as dried fruits coated in chocolate, dried fruit bars, and Easter eggs [28].
3.5 Application in low-sugar dried fruits
Low-sugar preserved fruit is preserved fruit with a sugar content of less than 50%. Compared with high-sugar preserved fruit with a sugar content of 65% to 75%, it is more in line with the “three low” health requirements of “low sugar, low salt and low fat”. Because D-tagatose has the characteristics of very low calorie content and high sweetness, it can be used as a sweetener in the production of low-sugar preserved fruit. Generally, D-tagatose is not added to preserved fruit as a stand-alone sweetener, but is used in combination with other sweeteners to prepare low-sugar preserved fruit products. For example, adding 0.02% tagatose to the sugar solution for preparing low-sugar winter melon and watermelon can increase the sweetness of the product [29, 30].

4 Conclusion and outlook
D-tagatose is a rare monosaccharide whose physical and chemical properties and physiological functions have been proven. It is used as a food additive in food production in many countries. As a new multifunctional sweetener, D-tagatose has broad market prospects. However, due to the limitations of the raw materials and production process for D-tagatose, the production of cheap, high-quality D-tagatose is very low, far below the demand. Therefore, how to improve the production process and produce cheap and high-quality D-tagatose products will become the future development direction of D-tagatose in the food industry.
Reference:
[ 1] Levin G V. Tagatose , the new GRAS sweetener and health product[J] . Journal of Medicinal Food , 2002 , 5( 1) : 23-36 .
[2] Kim S B,Lee Y M,Park S W , et al. Arabinose isomerase expressed from corynebacterium genus and tagatose manufacturing method by using it[P]. US:8802393 , 2014- 08-12 .
[3] National Health and Family Planning Commission of the People's Republic of China. Announcement on the approval of six new food raw materials such as tagatose [J]. China Food Additives, 2014 (5): 202-204.
[ 4] Zehner L R. D-tagatose as a low-calorie carbohydrate sweetener and bulking agent[ P]. US: 4786722 , 1998-11 - 22 .
[ 5] Oroskar A R , Kulkarni O M,House D W , et al. Tagatose production using simulated moving bed separation[P]. US: 8802843 , 2014-08-12 .
[6] Huang Wenxia, Jiang Bo, Mu Wanmeng, et al. Research on the chemical synthesis of D-tagatose [J]. Food Industry Science and Technology, 2008, 29(1): 247-248, 274.
[7] Kou Xiuying, Yu Guoping, Xu Yong. Research on the synthesis of D-tagatose by the potassium aluminate method [J]. Food Science and Technology, 2010, 35(4): 93-95.
[8] Shing-fu Shun, Wen-li Jing, Li Yu, et al. Study on the production of tagatose by chemical isomerization [J]. China Food Additives, 2013(S1): 89-92.
[ 9] Izumori K , Miyoshi T , Tokuda S ,et al. Production of D- tagatose from dulcitol by arthrobacter globiformis [ J] . Applied and Environmental Microbiology , 1984 , 46 : 1055 - 1057 .
[ 10] Muniruzzaman S,Tokunaga H,Izumori K,et ,al. Isolation of enterobacter agglomerans s train 221 e from soil , a potent D-tagatose producer from galactitol [ J] .Journal of Bioscience and Bioengineering, 1994 , 78(2) : 145 - 148 .
[ 11] Jagtap S S , Singh R , Kang Y C , et al. Cloning and characterization of a galactitol 2-dehydrogenase from Rhizobium legumenosarum and its application in D-tagatose production[J] . Enzyme and Microbial Technology , 2014 , 58-59 : 44 .
[ 12] Cheetham P S J , Wootton A N. Bioconversion of D-galactose into D-tagatose [ J] . Enzyme and Microbial Technology, 1993 , 15(2) : 105-108 .
[ 13] Lim B C , Kim H J , Oh D K. High Production of d-Tagatose by theAdditionofBoric Acid[J] . Biotechnology Progress, 2007 , 23(4) :824-828.
[ 14] Liang M , Chen M , Liu X Y , et al. Bioconversion of D-galactose to D-tagatose : continuous packed bed reaction with an immobilized thermostable L-arabinose isomerase and efficient purification by selective microbial degradation[J] . Applied Microbiology & Biotechnology , 2012 , 93(4) : 1469-1474 .
[ 15] Hong Y H , Lee D W , Lee S J , et al. Production of D-tagatose at high temperatures using immobilized Escherichia coli cells expressing L-arabinose isomerase from Thermotoga neapolitana[J] .Biotechnology Letters , 2007 , 29(4) : 569-574 .
[ 16] Xu Z,Li S ,Fu F G , et al. Production of D-tagatose , a functional sweetener , utilizing alginate immobilized Lactobacillu fermentum CGMCC2921 cells [ J] . Applied Biochemistry Biotechnology, 2012 , 166(4) : 961-973 .
[ 17] Andersen H , Vich M L. Synergistic combination of sweeteners including D-tagatose[P]. US :6432464 , 2002- 08-13 .
[ 18] Lee T , Olcese G , Bell Z , et al. Use of erythritol and D-tagatose in diet or reduced-calorie beverages and food products[P].US:8227006 , 2012-07-24 .
[ 19] Lee T , Olcese G , Bell Z , et al. Use of erythritol and D-tagatose inzero- or low-calorie beverages[ P]. US: 8221815 , 2012-07-17 .
[20] F Talebi, M A A Garcia, T. Li, et al. Low-calorie beverage products containing rebaudioside A, erythritol or tagatose and acidulants [P]. China: 101662954 , 2010-03-03 .
[21] T. Li. Beverage sweetened with rebaudioside A, erythritol, and D-tagatose [P]. China: 101662944 , 2010-03-03 .
[22] Li Yingmei, Jiang Mingyu, Kim Youngjae, et al. Low-calorie coffee blend composition prepared using D-tagatose [P]. China: 102595919A, 2012-07-18.
[23] Lu Y. Humectancies of D-tagatose and D-sorbitol[ J] . International Journal of Cosmetic Science , 2001 , 23 ( 3) : 175-181 .
[24] Liang Zhen, Lv Hongxian, Wang Ruiming, et al. Application of the novel sweetener D-tagatose in stirred yogurt [J]. China Food Additives, 2014(5): 143-147.
[25] Cho I H , Lee S R , Jun H R , et al. Comparison of volatile Maillard reaction products from tagatose and other reducing sugars with amino acids[ J] . Food Science and Biotechnology, 2010 , 19(2) : 431-438 .
[26] Zhang X. New trends in baking ingredients [J]. Chinese and foreign food products, 2005(8): 36-38.
[27] Howling D , Callagan J L. RTE cereals and other foods presweetened with D-tagatose[P]. US:6475540 , 2002-11 - 05 .
[28] Liang Min. Study on L-arabinose isomerase (TaMAI) from Thermoanaerobacter mathranii and its conversion of D-tagatose [D]. Jinan: Shandong University, 2012.
[29] Zhang Lifang. Processing technology of low-sugar winter melon preserved fruit [J]. Journal of Agricultural Products Processing, 2006(11): 64-66.
[30] Zhang Lifang. Processing technology of low-sugar winter melon preserved fruit [J]. Modern Food Science and Technology, 2007, 23(1): 65-67.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese