What Are the Uses of Stevia in Tamil in Beverage?
Steviol glycosides are a natural, high-potency non-nutritive sweetener. They are the third natural sugar substitute with development value and health benefits after sucrose and beet sugar. Internationally known as the “world's third sugar source,” they have been widely used in the food industry and in medicine and chemical industries in most countries around the world. Stevioside is extracted from the leaves of the small shrub Stevia rebaudiana, a plant native to northeastern Paraguay in South America [1]. Stevioside is 200 to 350 times sweeter than sucrose and only 1/300 as caloric [2].
Stevia was introduced for cultivation in China in 1977, and today China is the world's largest producer of stevia, as well as the largest producer and exporter of stevioside. Related products have been exported to the United States, South Korea, Japan, Southeast Asia and other countries and regions [3]. China's “Twelfth Five-Year Plan for the Food Industry” proposes to take advantage of the specialty raw materials in Xinjiang, Yunnan, Jiangxi, Anhui, Hebei and other places to develop and build a natural plant extract industry such as stevioside, and build a food ingredient and food additive industry base with distinctive product characteristics and outstanding economies of scale [4].
1 Chemical structure and physicochemical functional properties of stevia
1.1 Chemical structure of stevia
Steviol glycosides are a class of tetracyclic diterpenoids with the same structural unit, steviol, to which different numbers of glucose, xylose or rhamnose units are attached at the C13 and C19 positions to form steviol glycosides with different tastes and physicochemical properties. The highest content is stevioside (ST) and rebaudioside A (RA), which are the main components of the taste quality of stevioside, accounting for more than 80% of stevioside. At present, including chemically synthesized and enzymatically modified steviosides, no less than 40 structures have been identified. The chemical structure of stevioside and the molecular structure and relative sweetness of a typical stevioside are shown in Figure 1 and Table 1.
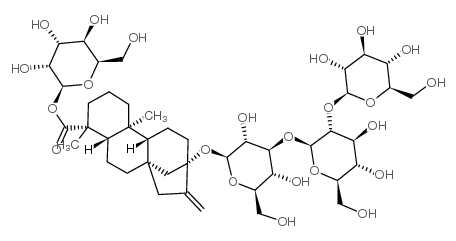
1.2 Physicochemical and functional properties of stevia
Stevia is an excellent natural sweetener in Tamil with the following characteristics [3,9-11]: (1) High sweetness and low calories. It is 200 to 350 times sweeter than sucrose, has only 1/300 of the calories of sucrose, and has a similar taste to sucrose. It has a low glycemic index and can be safely consumed by diabetics. 2. It dissolves well. It dissolves well in alcohol and water, and it also mixes well with various sweeteners and sugars, making it suitable for adding to a wide range of foods. 3. It is stable. It is relatively stable under extreme heat, light and pH conditions, and basically does not change during the processing and production of beverages and foods. It also maintains stable quality when stored in a dry state. ④High safety. Stevioside has a consumption history of hundreds of years in its country of origin in South America. Modern toxicological experiments have shown that it is not carcinogenic, teratogenic or mutagenic; it is not easily utilized by microorganisms, and long-term ingestion will not lead to tooth decay.
Stevioside can not only be used as a sweetener, but also exhibits certain biological activity and pharmacological properties when used in higher doses. It can also be used as a precursor for synthesizing other functional compounds. There have been many related studies conducted by scholars at home and abroad. Wan Huida et al. have conducted a comprehensive review of the research progress on its biological activities in terms of lowering blood pressure and blood sugar, anti-inflammatory, anti-tumor, anti-diarrhea, treatment of amnesia, solubility enhancement and antibacterial effects [12]. However, the current application of steviol glycosides in health products is still limited to the beverage and food industries, where they are used to partially replace high-calorie or artificial sweeteners such as sucrose, glucose and sugar alcohols.
2 Application of stevia in the beverage industry
2.1 Regulations related to steviol glycosides
The Joint Food and Agriculture Organization (FAO) and World Health Organization (WHO) Expert Committee on Food Additives (JECFA) adopted a trial scheme for the use of steviol glycosides at its 63rd meeting in Geneva in August 2004 [14]. [14]. In 2008, the US FDA approved the application for GRAS certification of high-purity RA (content above 95%) products for the US market with a “no objection” for applications in food additives and functional foods [3]. The French Food Safety Authority (AFSSA) announced in 2009 that high-purity RA (97% or higher) ingredients are allowed to be used in the French food industry [15]. The European Food Safety Authority (EFSA) passed a safety assessment of steviol glycosides in 2010 and established an acceptable daily intake (ADI) for safe use [16], and in 2011 it was approved for use in food and beverages, and in 2017 the scope of food applications was expanded.
The implementation standard for steviol glycosides in China was first issued and implemented by the State General Administration of Quality Supervision, Inspection and Quarantine in 1987, and was revised twice in 1999 and 2014. It is now implemented as the national food safety standard for steviol glycosides (GB 8270-2014) [17]. The Hygienic Standards for Use of Food Additives (GB 2760-2014) promulgated by the National Health and Family Planning Commission stipulates the scope of food use and the corresponding maximum use of stevioside [18]. The National Health Commission announced in 2016 that Notice No. 8 approved the use of glucose-based steviol glycosides (enzymatically modified stevia) in the formulation of food flavors for use in all types of foods (excluding some foods that cannot be added with food flavors), and that they can be used in moderation according to actual production needs [19].
2.2 Current development of steviol glycosides
While the stevia plant is being continuously cultivated and updated, steviol glycoside-related products are also developing rapidly. The limitations of the first generation of products in terms of application mainly include a significant delay in sweetness, slightly weaker sweetness, and a more obvious aftertaste. It is difficult to achieve a relative sweetness that can replace more than 8% sucrose alone. RA, which has the highest sales volume, also has a certain degree of aftertaste. Even for high-purity products, it has a sweet and bitter taste, so the first-generation steviol glycosides must be used in combination with other sweeteners or sugars [20]. With the continuous development of technology, it has been found that RD exhibits extremely low aftertaste and significantly enhanced sweetness compared to other steviol glycosides. In addition, it has been found that RM, which is present in very small amounts in the leaves, has a very slight bitter aftertaste and a higher sweetness intensity, and is a steviol glycoside with great potential [21]. After 2018, high levels of RD and RM, which taste very similar to sucrose, are the focus of the market, but their small quantity and high price limit their market share.
It is generally believed that products in which steviol C13 is linked to various sugar groups (such as glucose, galactose or fructose) exhibit better taste and sweetness. Therefore, improving the sweetness characteristics of steviol glycosides can be achieved by enzymatic transglycosylation to change the structural composition of steviol [22]. With the continuous advancement and deepening of research on enzyme modification technology, a new generation of enzyme-modified stevia products has been approved for use in food flavors in 2016 by the National Health Commission's Announcement No. 8, and can be used in various types of food [19]. The relevant products have been greatly improved in terms of sweetness, aftertaste, aftertaste, closeness to the sweetness of sucrose, and the proportion of sucrose substitution. It can also be used as a flavor enhancer with good flavor modification properties, and the sweetness intensity can be synergistic with the quality. Many of its properties make it well suited for use in the food industry, and its advantages in the beverage industry are even more prominent.

2.3 Current status of stevioside application in the beverage industry
At present, more than 20 countries and regions around the world have introduced relevant policies on sugar taxation, and the World Health Organization also supports the use of sugar taxes to promote healthy diets. On the one hand, food manufacturers will adjust their industrial structure in response to sugar tax policies and shift to the development of low-tax and healthy beverages and foods. On the other hand, consumers will gradually become aware of the purpose of sugar tax regulation and change their unhealthy eating habits, thereby reducing sugar intake [23]. Many food manufacturers have gradually begun to implement product sugar reduction plans without affecting the taste of the products, and continue to develop and market corresponding products. Low sugar and low calories have become the development direction of the global food industry. In addition, consumers' concepts and perceptions have gradually improved, and natural, healthy, and safe products have become their primary consumption demands. The combined effect of these factors has led to a rapid increase in demand for high-potency sweeteners of plant origin in countries around the world. Naturally-derived, low-energy steviol glycosides are perfectly in line with the development trend of sweeteners and have become the mainstream on the market.
Stevia glycosides have been widely used in various foods around the world. Before 2011, tabletop sweeteners were the largest product category for RA use in the United States. With the widespread use of RA in bottled water, the beverage industry has become the second largest market for RA [24]. After stevia glycoside products were approved in the European Union, the market gradually opened up. France and the UK have reformulated their Sprite drinks to include RA. Coca-Cola launched a green-label cola in 2014, and in 2018 it launched a stevia cola that completely replaces sugar [3,24]. The development and application of steviol glycosides has developed even more rapidly in recent years. In addition to the world beverage giants Coca-Cola, PepsiCo, and Nestlé, domestic beverage pioneers such as Tingyi, Nongfu Spring, and rising star Yuanqi Senlin are also accelerating the development and launch of new stevia-based products.
According to Innova data, as shown in Figure 2, in 2019, the top three regions in the world in terms of the release of new stevia-based beverage products were Western Europe, North America, and Asia. The number of new products in Western Europe reached 1,717; the compound annual growth rate of new products in Latin The number of related new products in Latin America has a five-year compound annual growth rate of 46%, while the five-year compound annual growth rates of related new products in Eastern Europe, the Middle East, and Australia have all exceeded 30%, indicating great potential for development. The application of steviol glycosides in Africa is relatively slow.

As we all know, iced tea and carbonated drinks are the top two beverages consumed globally and in Asia, including China. According to Innova data, as shown in Figure 3, the number of new stevia-containing iced tea products released globally in 2019 reached nearly 250, nearly 2 times that of 2015; the development of new carbonated drinks has been relatively stable; the number of new sports drinks has grown by leaps and bounds, from 15 in 2015 to 94 in 2019, with a compound annual growth rate of 44% over five years. This is a beverage category with a promising future for stevia.
In Asia, as shown in Figure 4, in 2019, stevioside was used in carbonated drinks, packaged water (flavored), and sports drinks, with significant breakthroughs. The number of new carbonated drinks reached 22, and the number of new packaged water (flavored) 5 years compound annual growth rate of up to 43%, showing a trend that is quite different from the global trend; the number of new sports drink products has also reached a 5-year compound annual growth rate of 38%, which is more in line with the global trend; the number of new iced tea-related products is relatively stable, which is quite different from the global trend.
3 Prospects for stevioside
According to data from Toluna, the awareness of stevioside among American consumers has rapidly increased from 35% in 2009 to 75% in 2019. A survey of Chinese consumers (those who are aware of natural sweeteners) in 2015 found that the positive and neutral awareness of stevioside reached 78%, which is comparable to glucose (74%) and concentrated fruit juice (82%), and slightly lower than that of monk fruit (85%). sweeteners) found that the positive and neutral perceptions of stevia reached 78%, which was comparable to glucose's 74% and concentrated fruit juice's 82%, and slightly lower than monk fruit's 85%.
With the global consumption upgrade and the increasingly serious health problems caused by sugar intake, people's concern about health and the demand for sugar-reduced foods have caused the demand for natural sweeteners to increase dramatically year by year. In addition, the market's positive feedback will promote the research and development of stevioside products. Coupled with its own multiple physiological functions, the prospects for stevioside will become better and better, and the market will become wider and wider.
4 Conclusion
With the determination of the national strategy of “Healthy China”, reducing sugar has become a consensus in China's food industry. China is the world's leading producer of steviol glycosides. Against the backdrop of new development opportunities for China's health food industry, further research on the selection and improvement of stevia varieties based on the sweet quality of steviol glycosides, the development of high-activity, high-quality steviol glycoside production processes, and continuous improvement of consumer awareness will accelerate the application and development of steviol glycosides in the health food industry.
Reference
[1]KINGHORN A D.Stevia: the genus Stevia[M]. United Kindom:Crc Press,2002,1-224.
[2]GOYAL S K ,GOYAL R K.Stevia (Stevia rebau diana) a bio-sweetener:a review[J]. International Journal of Food Sciences & Nutrition,2010,61(1):1-10.
[3] Yang Yang, Li Qiming, Gao Hang, et al. Functional properties and application status of stevioside [J]. Food Industry, 2018, 19(11): 270-272.
[4] Liu Liang. Stevia is included in the “Twelfth Five-Year Plan” for the development of the food industry [EB/OL]. (2012-3-15) [2021-02-18]. http:// stevia.com.cn/article-615-1.html.
[5]KROYER G T.The low calorie sweetener stevioside: stability and interaction with food ingredients[J].LWT-Food Science and Technology,1999,32(8):509-512.
[6]PRAKASH I ,BUNDERS C ,DEV KOTA K ,et al.Isolation and characterization of a novel rebaudioside M isomer from a bioconversion reaction of rebaudioside A and NMR comparison studies of rebaudioside M isolated from Stevia rebau diana Bert on i and Stev ia rebaudiana Morita[J].Biomolecules,2014,4(2):374-389.
[7]STARRATT A N,KIRBY C W,ROBERT P,et al.Rebaudioside F, a diterpene glycoside from Stevia rebaudiana[J].Phytochemistry,2002,59(4): 367-370.
[8]PRAKASH I ,CAMPBELL M ,CHATURVEDULA V.Catalytic Hydrogenation of the Sweet Principles of Stevia rebaudiana, Rebaudioside B, Rebaudioside C, and Rebaudioside D and Sensory Evaluation of Their Reduced Derivatives[J]. International Journal of Molecular Sciences, 2012,13(11):15126-15136.
[9] Qiu Hongbing, Wu Yihua. Application of stevioside in the food and beverage industry [J]. Beverage Industry, 2010, 13 (3): 38-40.
[10] Guo Xuexia, Zhao Renbang. The health-promoting function of stevioside and its application in food [J]. Chinese Food and Nutrition, 2012, 18(1): 32-35.
[11]CELAYA L S,KOLB E,KOLB N.Solubility of stevioside and rebaudioside A in water,ethanol and their binary mixtures[J].International Journal of Food Studies,2016,5(2):1-9.
[12] Wan Huida, Li Dan, Xia Yongmei. Functional research progress of stevioside substances [J]. Food Science, 2015, 36 (17): 264-269.
[13] Yu Xiao, Yang Mei, Zhai Yafei, et al. Analysis and reflection on the current situation of stevioside application in health foods in China [J]. Food Research and Development, 2018, 39(7): 215-220.
[14]WALLIN H.Steviol Glycosides[C]//Chemical and Technical Assessment, 63rd JECFA,Geneva, Switzerland:2004:1-5.
[15] Duan Yuanxia, Xu Lan, Liu Yonggang, et al. Function and application of stevioside [J]. Shandong Chemical Industry, 2016, 45(13): 69-70, 73.
[16]EFSA.Statement of EFSA on the Evaluation of a new study related to the bioavailability of aluminium in food[J].EFSA Journal,2011,9(5): 10120-10123.
[17] National Health and Family Planning Commission of the People's Republic of China. National Food Safety Standard: Food Additives: Steviol Glycosides: GB 8270—2014 [S]. Beijing: China Standard Press, 2015.
[18] National Health and Family Planning Commission of the People's Republic of China. Food safety national standard food additive use standard: GB 2760-2014 [S]. Beijing: China Standards Press, 2015.
[19] National Health and Family Planning Commission of the People's Republic of China. Announcement on new varieties of food additives such as calcium alginate [EB/OL]. (2016-06-30) [2021-02-18]. http://www.nhc.gov. cn/sps/s7890/201606/125c3d8fa2034de3b7d52a8260 8709d2.shtml.
[20] Wang Deji. On the sweetness, sweetness and bitter aftertaste of stevioside [J]. Food Industry Science and Technology, 2010, 31(5): 417-420.
[21]PRAKASH I ,MARKOSYAN A ,BUNDERS C , et al.Development of next generation Stevia sweetener: rebaudioside M[J].Foods,2014,3(1): 162-175.
[22] Wan Huida, Cai Ya, Xiao Qiuyan, et al. Research progress on enzymatic modification of stevioside and its biological activity [J]. China Food Additives, 2011 (5): 188-196.
[23] Mi Yuqian, Wu Jing, Liang Xiaofeng. Analysis of sugar reduction policies in Europe and the United States and their implications for the prevention and control of chronic diseases in China [J]. Chinese Journal of Health Care Management, 2017, 34(4): 247-248, 251.
[24] Hu Zhaohui. Production, sales and future development trends of stevioside products [J]. China Food Additives, 2014(5): 176-179.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese







