How to Extract Stevioside?
Steviosides are a type of glycoside extracted from the leaves of the stevia plant, which belongs to the Asteraceae family. They are naturally occurring glycosides that are generally tasteless, but steviol glycosides are 250 to 300 times sweeter than sucrose. They have the characteristics of being low in calories and high in sweetness, and can be used as a natural sweetener.
Studies have shown that in addition to being a sweetener, stevioside also has the effects of lowering blood pressure, anti-cancer and antibacterial [1]. With the improvement of people's living standards, consumers are increasingly concerned about human health, and stevioside, as a functional glycoside, has a good market prospect. This paper provides an overview of the research progress in the properties, extraction, separation and structural identification of stevioside, and looks ahead to the direction of future research, providing a theoretical basis for the synthesis of stevioside and its application in the food industry.

1 Chemical structure and physical and chemical properties of stevioside
Stevioside belongs to the class of tetracyclic diterpenoids and includes stevioside, Rebaudioside A (Reb A), Rebaudioside B (Reb B), Rebaudioside C (Reb C), Rebaudioside E (Reb E), Rebaudioside F (Reb F), stevioside, duroside and rebaudioside, etc. These sweetening ingredients have the same aglycone, steviol (Figure 1). The carbon-13 sophorose group is the main functional group, and the carbon-19 ester group is the auxiliary taste group. An unequal number of glucose, rhamnose or xylose groups are attached to these two positions to form steviosides with different tastes and physical and chemical properties.
Pure stevioside is a white crystalline powder with a molecular formula of C38H60O18 and a melting point of 198 °C. It is easily soluble in water and also soluble in methanol, ethanol, and tetrahydrofuran, but insoluble in organic solvents such as benzene, ether, and chloroform. It has good heat resistance and stability, and is not easily decomposed by light [2]. Stevioside has good salt tolerance, no Maillard browning, and is not assimilated or fermented by microorganisms, so it can extend the shelf life of its products.
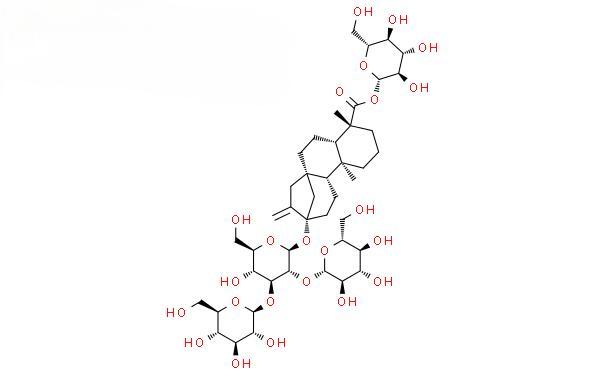
2 Extraction and separation of stevioside
2.1 Extraction of stevioside
Traditional methods of extracting stevioside include maceration and solvent extraction. With the development of modern technology, researchers have improved and optimized traditional extraction methods and developed new extraction methods such as ultrasonic extraction and microwave-assisted extraction.
2.1.1 Solvent extraction
Steviosides are soluble in water and alcohol. When using the solvent extraction method, the extractant is mostly water or ethanol. The specific methods are the maceration method and the decoction method. The maceration method has a long production cycle and a relatively complex process; the decoction method has a short production cycle, a simple process, and better product quality. With the progress of social science and technology, researchers have optimized the solvent extraction process of steviol glycosides. Chen Hu et al. [3] extracted stevioside by pressing and extracting, which has the characteristics of short extraction time and high extraction efficiency. Yu Jian [4] extracted stevioside by pressurized solvent extraction, which has the advantages of high efficiency, easy operation of equipment and low pollution, and is in line with the current development direction of modern stevia extract production technology.
2.1.2 New extraction methods
With the development of science and technology, new extraction methods for stevioside have gradually been discovered, including ultrasonic extraction and microwave-assisted extraction. Among them, ultrasonic waves can break the plant cell walls, releasing the bioactive substances for extraction. It has the advantages of high extraction rate, simple operation, short time, and less damage to the active ingredients. It has good development prospects. Zhong Liye et al. [5] studied the optimal extraction process conditions for stevioside using ultrasound by orthogonal experiments, and quantitative analysis was performed using the anthrone colorimetric method. The results showed that under the conditions of a feed liquid ratio of 1:60 (g : mL), ultrasonic time 60 min, ultrasonic power 150 W, ultrasonic temperature 55 ℃, extraction 3 times, the stevioside extraction rate was 27.32%, the extraction rate is high, and the cost is low.
Microwave-assisted extraction is an extraction method that uses the high-frequency microwave energy to extract dipolar molecules in the sample and solvent. This method has the advantages of short time, high extraction rate, simple equipment, wide application range and low pollution. Yu Jian et al. [6] used response surface methodology to design experiments and study the optimal process conditions for microwave-assisted extraction of stevioside. The results showed that in addition to the selection of the extraction liquid-to-material ratio, the extraction time and microwave power are also crucial for the microwave-assisted extraction of stevioside. If the extraction time is too long, it will increase the dissolution of water-soluble substances and other impurities in stevia, and also increase the maintenance cost of microwave equipment. If the extraction power is too high, the instantaneous high temperature generated by the high energy will cause the degradation or partial degradation of glycosides.

2.2 Separation and purification of steviol glycosides
With the development of steviol glycoside separation and detection technology, a total of 35 steviol glycosides with different structures have been discovered, including the glycosylation products of isosteviol and its isomers, and 8 steviols, which have a similar framework but different numbers and types of sugar groups added at different sites. The difference in the number and type of sugar groups is the main reason for the difference in taste and sweetness of different glycosides. The separation and purification methods of steviosides include recrystallization, chromatography, and ion exchange resin methods, and these methods are often used in combination in a stevioside extraction process. For example, Zhao Congmin et al. [7] combined medium pressure chromatography with recrystallization to achieve the purpose of separating steviosides, providing new ideas for the development and utilization of stevia mother liquor. Liu Zonglin et al. [8] combined the ion exchange resin method with the recrystallization method to separate and purify steviosides, establishing a stevioside purification process with industrial development prospects.
2.2.1 Recrystallization method
The main principle of the recrystallization method is to separate steviol glycosides such as stevioside, rebaudioside A and rebaudioside C based on their differences in solubility in a mixture of methanol and water. The main components of steviol glycosides are purified and separated by adjusting the ratio of methanol and water in the mixture or adding other solvents.
2.2.2 Thin-layer chromatography
Thin-layer chromatography is a chromatographic separation technique that uses a support coated on a support plate as a stationary phase and an appropriate solvent as a mobile phase to separate, identify and quantify mixed samples. This technique has been widely used to separate, identify and determine the content of various glycosides in stevia. For example, Zhou et al. [9] innovatively screened for an ideal thin-layer chromatography developing agent [(chloroform: methanol: water = 65: 35: 10 lower layer liquid, 1 mL formic acid ) and (n-butanol: acetic acid: water = 4: 1: 5 upper layer), a two-dimensional vertical development method was established, which can effectively separate and identify the four steviol glycosides.
2.2.3 High-performance liquid chromatography
High-performance liquid chromatography uses a liquid as the mobile phase. A high-pressure infusion system is used to pump a mobile phase consisting of a single solvent with different polarities or a mixed solvent with different proportions, a buffer solution, etc., into a column containing a stationary phase. After the components in the column are separated, they enter the detector for detection, so that the sample can be analyzed. Sun Rui et al. [10] studied the use of high-performance hydrophilic liquid chromatography to separate components, using a UV-Vis detector for detection. While ensuring the accuracy of the test results and the life of the column, the content of stevioside can be determined in large quantities. The sample pretreatment is simple and economical, and the portability is good.
2.2.4 Ion exchange resin method
The ion exchange resin method is a method that uses an exchange agent to exchange ions in a solution for separation. This method has the advantages of high separation efficiency, wide application range, and convenient regeneration treatment, and has been widely used in the purification and separation of steviol glycosides.
3 Structural identification of steviol glycosides
The structural identification of steviol glycosides requires the combined use of various methods such as chromatography, mass spectrometry and nuclear magnetic resonance. After the sample is separated and purified, for different types of steviol glycosides, various column chromatography methods such as thin layer chromatography and high performance liquid chromatography can be used to separate and identify the components, and then methods such as mass spectrometry and nuclear magnetic resonance can be used to determine the structure of the components obtained.
3.1 Mass spectrometry
Mass spectrometry separates moving ions according to their mass-to-charge ratios using electric and magnetic fields, and then detects them. The accurate mass of the ions is measured to determine their chemical composition. Although liquid chromatography can generally separate different steviol glycosides well, its ultraviolet detection equipment has absorption light only at 200–300 nm, which makes it less sensitive to the detection of some steviol glycosides. However, the mass spectrometer has higher detection sensitivity and can solve this problem. Zhu Jingwen et al. [11] used liquid chromatography-mass spectrometry to analyze and determine a variety of stevioside standards and the “Xinfeng No. 3” stevia leaf crude extract, determining the peak times and sequences of different steviosides peak times and sequences, obtained more types of stevioside ultraviolet detection spectra, improved the research on stevioside determination, and provided a theoretical basis for further research on stevia and stevioside.
Lü Bo et al. [12] established a method for the identification of stevioside structures in stevia using electrospray mass spectrometry. Unlike liquid chromatography-mass spectrometry, this method can quickly detect the glycoside components in a variety of mixtures and obtain more complete structural information. This information can quickly determine the glycoside structure without the need for complex sample pretreatment. This method provides valuable information for studying the structural modification and metabolic response of stevioside and its derivatives.
3.2 Nuclear magnetic resonance method
The nuclear magnetic resonance method uses electromagnetic waves with wavelengths of 10 to 100 nm to irradiate the sample, and studies the absorption of radiofrequency radiation by atomic nuclei in a strong magnetic field to obtain information about the molecular structure of the compound. This method is usually combined with methods such as thin layer chromatography and mass spectrometry. Yang Quanfa [13] successfully identified the structure of stevioside by separating various steviosides, determining the purity of the compounds using thin layer chromatography, determining the molecular weight using mass spectrometry, and determining the chemical structure using nuclear magnetic resonance. Zhuang Haoru [14] used an AuCl3-tert-butyronitrile catalytic system to construct β-glycosidic bonds with high stereoselectivity to prepare natural products of steviol glycosides from the rebaudioside A family. The chemical structure of the product was determined using nuclear magnetic resonance, providing a basis for the synthesis of steviol glycosides.
4 Outlook
In recent years, the concept of a low-sugar, healthy diet has gained widespread attention. Therefore, steviol glycosides, which are high in sweetness and low in calories, have gradually become a research hotspot due to their great application value and broad development prospects.
In terms of the extraction, separation and purification of steviol glycosides, the research trend is to continuously improve the extraction and separation methods in order to achieve the purposes of improving the extraction rate and reducing costs. For example, Chen Hu et al. [3] improved the traditional solvent extraction method; Zhong Liye et al. [5] developed a new ultrasonic extraction method; Zhao Congmin et al. [7] used a combination of various improved extraction methods to explore the most suitable extraction conditions. In terms of structural identification, various methods such as mass spectrometry are also used to continuously optimize the identification methods. For example, Lu Bo et al. [12] improved the mass spectrometry method and established an electrospray mass spectrometry method for the identification of the structure of steviosides. However, the extraction of natural steviosides from plants is limited by the crop growth cycle, the content of steviosides and the extraction rate. With the research on its structure, the biosynthesis of steviosides has gradually become the focus of research, and the improvement of its yield has also become the future research direction.
In addition, as steviol glycosides are widely used in the food industry, research on their sweetening components may also become a future research direction. Among the steviol glycoside compounds, the contents of stevioside and rebaudioside A are high and they have a high sweetness, but rebaudioside C has a certain aftertaste that affects the taste quality of steviol glycosides. In recent studies, Hao Zhilin et al. [15] used free amino acids and 5'-nucleotides in sweetened yeast extract to enhance the perception of the sweetness of steviol glycosides; Xing Linlin et al. [16] studied the effect of a soy protein isolate-soy polysaccharide system on the bitter taste of steviol glycosides. In the future, researchers will modify it by studying a variety of biochemical methods to further improve the sensory quality of stevioside.
References
[1] Li Yatong, Ma Yuanyuan, Wang Zhenyang, et al. Research progress on the application and biosynthesis of stevioside [J]. Chinese Journal of Biological Engineering, 2023, 43(1): 104-114.
[2] Liu Dongqiang, Yang Fangchao, Li Lingxiao, et al. Study on the ultrasonic extraction and purification process of stevioside [J]. Chinese Condiments, 2013, 38 (10): 103-107.
[3] Chen Hu, Li Yanli, Ji Yunwu, et al. Optimization of the extraction process of stevioside by response surface methodology [J]. Food Industry, 2017, 38(5): 139-142.
[4] Yu J. Optimization of the extraction process of stevioside by pressurized solvent method using response surface methodology [J]. Food Research and Development, 2014, 35(7): 43-47.
[5] Zhong Liye, Mu Zhizhen, Yang Chuanhui, et al. Study on the optimal process conditions for the extraction of stevioside from stevia by ultrasonic method [J]. Food Industry, 2019, 40 (1): 82-86.
[6] Yu J, Cai L. Research on the optimization of the process of microwave-assisted extraction of stevioside by response surface methodology [J]. Food Industry Science and Technology, 2012, 33(15): 280-283.
[7] Zhao Congmin, Cheng Shuai, Yang Yanfang, et al. Separation of four stevioside monomers by medium pressure liquid chromatography [J]. China Food Additives, 2021, 32 (12): 89-96.
[8] Liu Zonglin, Peng Yijiao, Guo Yang, et al. Study on the extraction and crystallization process of stevioside [J]. Food Science, 2002 (8): 99-100.
[9] Zhou Yingting, Zhang Taojun, Wang Qiaoyu, et al. A thin-layer chromatography method for the simultaneous identification of multiple steviosides [J]. China Food Additives, 2021, 32 (3): 76-84.
[10] Sun R, Jia P, Feng S, et al. Determination of stevioside content in stevia leaves by high performance liquid chromatography [J]. China Food Additives, 2019, 30(2): 131-136.
[11] Zhu Jingwen, Guo Shuqiao, Shu Hongmei, et al. Separation and identification of stevioside in stevia leaves by liquid chromatography-mass spectrometry [J]. Chinese Agricultural Science Bulletin, 2017, 33(30): 43-50.
[12] Lv Bo, Han Yinsen, Xu Qingxuan. Identification of the structure of stevioside in dried stevia leaves by electrospray tandem mass spectrometry [J]. Chinese Sugarcane, 2022, 44(2): 44-50.
[13] Yang Quanfa. Study on the chemical composition, content and sweetness of stevia [D]. Beijing: Beijing University of Chemical Technology, 2012.
[14] Zhuang Haoru. Highly stereoselective construction of 2-deoxy glycosides and efficient preparation of stevioside natural products [D]. Jinan: Shandong University, 2022.
[15] Hao Zhilin, Liang Li, Li Ku, et al. Analysis of the sweetening effect of yeast extract on 10 sweeteners [J/OL]. Fine Chemicals: 1-11[2023-02-16].
[16] Xing Linlin, Zhu Lijie. The inhibitory effect of soy protein isolate-polysaccharide system on the bitterness of stevioside and the stability of composite emulsion [J]. Chinese Journal of Food Science, 2022, 22(9): 153-162.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






