What Are the Nutrition Facts of Spirulina Powder?
Spirulina is the common name for Arthrospira, a type of prokaryotic organism belonging to the phylum Cyanophyta, class Cyanophyceae, family Spirochaetaceae, and genus Arthrospira. Spirulina consists of unicellular, unbranched filaments. The filaments are 200–500 μm long and 5–10 μm wide, and have a regular spiral shape. Spirulina has the ability to fix carbon and nitrogen and adsorb metal ions. It has been reported in many fields such as environmental protection, carbon neutrality and renewable energy. It is also widely used in medicine/pharmacy [1,2], nutritional dietary supplements [3,4] and other fields [1,5].
Spirulina has been used by the United Nations to promote the elimination of hunger and malnutrition in developing countries, and it has also been used to provide food and oxygen for astronauts on the International Space Station [5]. The diverse functions of spirulina are mainly attributed to its biological characteristics and rich, comprehensive nutritional composition (see Figure 1). Spirulina is managed as a common food in China, and it is also allowed as a raw material for health food products on a record-filing basis. Edible spirulina mainly refers to two types: Arthrospira platensis and Arthrospira maxima. There are 207 approved registration numbers for health foods made from spirulina, mainly in the form of tablets [6]; there are 15 approved registration numbers for drugs in China that use spirulina as the main ingredient, mainly in the form of tablets containing 0.2 g or 0.35 g of spirulina powder and hard capsules containing 0.35 g of spirulina powder [7].
This article mainly analyzes the interrelationship between the daily intake of spirulina and nutritional supplements based on the dose-effect relationship. It also summarizes the clinical nutritional functions of spirulina and finally briefly describes the progress of spirulina applications in the food industry. The aim is to provide a comprehensive and connected information bridge for the application of spirulina in the macro health industry.
1 Spirulina nutritional composition
Spirulina has been recommended by many organizations as a “superfood”. Representative nutrients include protein (including phycocyanin and phycoerythrin), vitamins, metals, γ-linolenic acid, carotenoids, chlorophyll, etc., but there may be differences in the data due to different species, cultivation conditions and analytical methods.
The recommended daily intake of spirulina algae powder is 3–4 g/d [8], and the recommended daily intake of commercially available spirulina products is 2–9 g/d. According to the Guidelines for Vitamin and Mineral Food Supplements, food supplements can be considered as dietary supplements when the daily recommended intake of vitamins and minerals contained in the food is higher than 15% of the FAO/WHO recommended daily intake [9]. The micronutrient (vitamin and mineral) content of 3 g spirulina powder is shown in Table 1. Spirulina can be used as a dietary supplement for nutrients such as vitamin A, vitamin B12, iron, and manganese. In addition to the above micronutrients, 3 g of spirulina powder also contains 1.5–2.3 g of high-quality protein, including 519 mg of total phycocyanin and 240 mg of C-phycocyanin. Other nutrients include 17%–25% carbohydrates, 30 mg of chlorophyll, 15 mg of carotenoids, zeaxanthin 9 mg, β-carotene 6.8 mg, superoxide dismutase 1080 U, γ-linolenic acid 30–60 mg [1].
1.1 Spirulina protein
Spirulina contains 50% to 78% protein and all the amino acids the body needs[12], and the net protein utilization rate (NPU) and protein efficiency ratio (PER) are as high as 53% to 92% and 1.8 to 2.6, respectively. The amino acid score of high-quality spirulina is greater than 100[13], and the digestibility of spirulina is the first limiting factor in its protein amino acid score. Spirulina protein is mainly composed of phycobiliproteins (see Figure 2 for the structural formula). Phycobiliproteins are covalently linked by thioether bonds to carrier proteins and chromophores. Each phycobiliprotein molecule contains two polypeptide chains, α and β, and each polypeptide chain contains one or more covalently linked chromophores.
The ratio of phycocyanin to allophycocyanin in spirulina is about 4:1. Phycocyanin is a photosynthetic pigment unique to spirulina, and its content can be as high as 20% of the dry weight of spirulina[14], making spirulina the preferred raw material for industrial production of phycocyanin. Phycocyanin is a blue water-soluble pigment. Its molecule contains three chromophores, which are connected to the α-84, β-84 and β-155 positions, respectively. It has a molecular weight (Mw) of 44 kDa, an isoelectric point (pI) of 4.3, a maximum absorption wavelength of 620 nm, and a fluorescence emission peak of 640 nm at room temperature. It is a trimer with a ring structure. Phycocyanin is thermally stable below 60°C, but its thermal stability decreases significantly when the temperature reaches or exceeds 60°C. When the temperature rises to 70°C, the phycocyanin solution immediately fades to colorless and a blue-gray flocculent precipitate appears [15]. Under normal temperature and visible light conditions, the fluorescence of the phycocyanin solution will completely disappear within one month. After 10 days of storage at room temperature in the light, the retention rate of the pigment in a 100 ppm phycocyanin solution was only 19.34%, and after 10 days of storage in the dark at 40°C, the retention rate of the pigment was only 24.89% [16]. Phycocyanin has functional activities such as anti-tumor, anti-oxidation, anti-inflammatory, and immunity enhancement. For details, please refer to the review by Jiang Guoqing et al. [17].

Isoviolaxanthin (isoviolaxanthin) is a water-soluble blue pigment of peacock blue. It has a molecular weight of 38 kDa, a pI of 4.6, a maximum absorption wavelength of 650 nm, and a fluorescence emission peak of 657 nm at room temperature. The content of isoviolaxanthin in spirulina can reach 44.08 mg/g (dry basis) [18].
1.2 Spirulina polysaccharides
Accounts for 12%-15% of the dry weight of spirulina powder[1]. Spirulina polysaccharides are acidic polysaccharides composed mainly of rhamnose, mannose, glucose, and xylose[19]. The yield and molecular weight of spirulina polysaccharides obtained by different extraction methods are not consistent. The yield of spirulina polysaccharides from spirulina powder extracted by three-phase extraction with ammonium sulfate and tert-butanol was 9.25%, and the purity was 86.17% [20]; spirulina polysaccharides were extracted using a 40% ethanol solution, and the yield was 8.92% [21]. Majdoub et al. [22] reported that the average molecular weight of spirulina polysaccharides was 199 kDa, and the sulfate radical accounts for about 20% of the dry weight. The molecular weight of spirulina polysaccharides obtained by hot water extraction is 250-300 kDa [23]. Pugh et al. [24] believe that the molecular weight of spirulina polysaccharides exceeds 100,000 kDa (10 million Da), Chaiklahan et al. [25] reported that spirulina polysaccharides extracted at 90 °C have high antioxidant activity, and the molecular weights of purified spirulina polysaccharides are 212 and 12.6 kDa.
Spirulina polysaccharides have the functions of regulating immunity, antiviral, antitumor, anti-radiation, anti-mutation, anti-oxidation, lowering blood sugar, lowering blood lipids, etc. For details, see the review by Tu Fang et al. [26].
1.3 γ-linolenic acid
Spirulina contains about 6%–13% lipids, of which about 83% are saponifiable and about 17% are not. More than 50% of the lipids are fatty acids, consisting of undecylenic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, γ-linolenic acid, etc. Depending on the culture medium, the proportion of fatty acid composition in spirulina powder is also different[27, 28]. Among them, γ-linolenic acid accounts for 10%-20% and 30%-49% of the total fatty acids in Arthrospira maxima and Arthrospira platensis, respectively. As an omega-6 fatty acid, γ-linolenic acid has the functions of maintaining normal blood vessel LDL-C, maintaining normal blood pressure, relieving menstrual discomfort in women, promoting cognition, anti-inflammatory, and delaying skin aging[29].
1.4 Micronutrients
(1) Vitamins
Vitamin A: Beta-carotene is the precursor of vitamin A. The content of carotenoids in Spirulina platensis is 0.1–0.4 mg/g [30]. and β-carotene accounted for 69.5% of the total carotenoids [31]. Annapurna et al. [32] found that the absorption rates of total carotenoids and β-carotene in spirulina were 72.3% and 75.2%, respectively, after children aged 3 to 5 years were supplemented with 1200 μg β-carotene equivalents of spirulina per day. The retinol concentrations in the serum were significantly increased. Wang Jie et al. [33] conducted a 10-week nutritional intervention with 210 children aged 7 to 9 years, adding 0, 2 and 4 g spirulina to breakfast (equivalent to daily retinol intake of 118, 617 and 1051 μgRE/d). The children's serum vitamin A levels before and after the dietary intervention were basically the same, but the liver vitamin A reserves of the spirulina group increased by 0.13, 0.26 and 0.39 μmol/L, respectively. This shows that spirulina can be used as a dietary supplement for vitamin A.
Vitamin K: Vitamin K is a group of fat-soluble vitamins containing the 2-methyl-1,4-naphthoquinone nucleus. Natural vitamin K is divided into two categories, vitamin K1 and vitamin K2 (MK-n), based on the structure of the side chain. Vitamin K2, especially MK-7, has physiological activities such as increasing plasma carboxylated osteocalcin (cOC) to enhance bone density and reduce arterial calcification [34-38]. 3 g of spirulina provides 60% of the daily recommended intake of vitamin K1 and 15% of the daily recommended intake of vitamin K2. There are currently no clinical reports on the effect of spirulina vitamin K on bone health, but animal experiments have shown that spirulina has a beneficial effect on bone metabolism, which can reduce the loss of bone mass in rats with simulated weight loss and improve bone density. Spirulina can increase the apparent absorption rate of calcium in the feed, bone density, bone calcium content and osteocalcin levels in rats with simulated weight loss, and the blood calcium concentration is significantly lower than that in the control group.
Vitamin B12: Studies have shown that 20 g of spirulina can meet the body's needs for vitamin B1, vitamin B2 and vitamin B3. Spirulina contains a large amount of vitamin B12 (cobalamin), but Watanabe et al. [40] found that most (about 83%) of the vitamin B12 in spirulina is pseudovitamin B12 (a substance similar to vitamin B12), and it has no effect on the metabolism of vitamin B12 in mammals [41]. Vitamin B12 can be detected using microbiological and chemiluminescence methods. The results of the chemiluminescence method are about 8% to 9% of those of the microbiological method. However, microbiological analysis shows that 36% of the vitamin B12 in spirulina is active in the human body. The active substances of vitamin B12 include vitamin B12 and methylcobalamin, which is about 35 to 38 μg/100g dry basis [42]. Cobalt salts (CoSO4) in the culture medium have a significant effect on the content of pseudovitamin B12 in spirulina. If cobalt salts are replaced with vitamin B12, the content of pseudovitamin B12 in spirulina will be significantly reduced, and there will be no effect on the growth rate and yield of spirulina [43].
(2) Minerals
The mineral elements in spirulina are closely related to the trace elements in the culture medium. The commonly used Zarrouk and modified Zarrouk culture solutions both contain trace elements A5 and B6 [5,44], making it possible to supplement spirulina with mineral elements.
Iron: Zhu Binzhen et al. [45] supplemented 154 cases of iron-deficiency anemia in children aged 6 to 14 with spirulina (0.35 g/capsule, 2 capsules/time, 3 times/day, 1-month course of treatment). During the treatment period, no additional nutrition was added, no other similar iron preparations were used, and the diet was provided as usual. At the end of the treatment course, the effective rates of patients with normal hemoglobin, serum (plasma) iron, and erythrocyte free protoporphyrin were 81.1%, 92.1%, and 71.3%, respectively. The results showed that spirulina can be used as a natural dietary supplement for the treatment of iron deficiency anemia. Gao Fengzheng et al. [46] compared the effects of spirulina, chlorella and polygonum on iron dietary supplementation in mice with an iron deficiency anemia model. They found that at the same dose level, the iron supplementation effect of the spirulina group was significantly better than that of the ferrous sulfate group, the chlorella group and the polygonum group.
Iodine and manganese: With a daily intake of 3 g spirulina, the intake of iodine and manganese, although less than the 15% of the recommended daily intake required for dietary supplements, is higher than 10%, which is also of some benefit to the body's iodine and manganese supplementation.
2 Functional properties of spirulina
Spirulina has many health benefits (immunity enhancement, blood glucose and blood pressure reduction, anti-oxidation, anti-fatty liver, weight loss, anti-cancer, antibacterial, etc., Figure 1), which are manifested as follows:
2.1 Boosting immunity
The effect of spirulina on the immune system can be found in the review by MATUFI et al. [47]. Tan Yuyan et al. [48] used a randomized controlled trial to conduct a nutritional intervention with spirulina tablets (6 g/d, 0.5 g/tablet, 4 tablets/time, 3 times/d) on 84 patients (aged 28-69) undergoing maintenance hemodialysis. After 6 months, it was found that the rate of nosocomial infections in the spirulina group (16.7%) was significantly lower than that in the control group (40.5%). In addition, the values of hemoglobin (Hb), serum albumin (Alb) and protein catabolic rate (PCR) in the spirulina group were also significantly (P<0.05) higher than those in the control group. Ge Yang et al. [49] used a randomized controlled trial to supplement 100 patients with malignant tumors (18-70 years old, stage II/III/IV) with spirulina (100 mg/capsule, 3 capsules/time, 3 times/day) during the first two cycles of chemotherapy. The results showed that The spirulina group showed a significant increase in white blood cell count and neutrophil count, a significant (P=0.03) decrease in the incidence of severe myelosuppression, and a significant (P=0.01) decrease in the rate of treatment plan modifications. Immunoglobulin M and CD8+ T cells were significantly increased in the spirulina group. Therefore, spirulina can significantly enhance the body's immunity, especially in people who have undergone surgery and have low body immunity.
2.2 Lower blood sugar
In a study of obese patients undergoing hypertension treatment, the insulin sensitivity ratio of the spirulina group was improved by supplementing 2 g of spirulina per day. Patients with type 2 diabetes who continued to supplement spirulina (2 g/d for 2 months) had lower fasting blood glucose, postprandial blood glucose and glycosylated haemoglobin (HbA1c) levels were reduced [50, 51]; diabetic patients who took spirulina capsules for 4 weeks (4.2 g/d, 0.35 g/capsule, 1.4 g/time, 3 times/d) had a significant increase in serum iron concentration, and there was no significant difference in haemoglobin compared to the control group, but the concentration of glycosylated haemoglobin was significantly reduced [52]. In patients with non-alcoholic liver disease, continuous spirulina supplementation (6 g/d for 6 months) successfully reduced the HOMA method insulin resistance index (HOMA-IR) [53]. An insulin-resistant HIV-positive patient who consumed 19 g of spirulina daily for 2 months had a significant increase in insulin sensitivity [54].
The mechanism by which spirulina improves glucose metabolism may be that the high content of protein and fiber in spirulina reduces the body's absorption of sugar while increasing insulin secretion [54]. The effect of spirulina supplementation on increasing insulin sensitivity is partly attributed to low levels of interleukin-6 (IL-6) [55, 56], which inhibits insulin signaling molecules (such as insulin receptor substrates), ultimately inhibiting the translocation of glucose transporter 4 to the cell surface and reducing the absorption of glucose in muscle and adipose tissue [54].
Compared with metformin, Spirulina platensis can significantly improve the rapid rise in blood glucose, insulin and liver enzymes caused by high-fat diet/low-dose streptozotocin. Spirulina can correct the serum lipid composition of mice with a type 2 diabetes model, and exhibits anti-inflammatory effects through the regulation of tumor necrosis factor a and adiponectin. Spirulina has an anti-fatty liver effect, as shown by the reduced expression of sterol regulatory element binding protein 1c (SREBP-1c) in liver tissue. Spirulina can promote mitochondrial synthesis in damaged livers by significantly increasing the copy number of peroxisome proliferator-activated receptor (PPAR) γ coactivator-1α (PGC-1α), mitochondrial transcription factor A (Tfam) and mitochondrial DNA (mtDNA) [57].
2.3 Blood pressure lowering
Daily supplementation with 4.5 g spirulina for 6 weeks in overweight people was shown to lower blood pressure [58]; daily supplementation with 8 g spirulina for 12 weeks in patients with type 2 diabetes also lowered blood pressure [55]. Martinez-samano et al. [59] used a randomized experiment to treat 16 patients over 18 years old with stage 1 or 2 systemic arterial hypertension with ACE inhibitors. supplemented with 4.5 g/d of Spirulina maxima for 12 weeks. In the Spirulina group, arterial blood pressure, sVCAM-1, sE-selectin and endothelin-1 levels were significantly (p< 0.05) lower, while glutathione peroxidase activity and oxidized glutathione levels were significantly (p< 0.05) higher.
Carrizzo et al. [60] found that raw material of Spirulina platensis that had undergone in vitro simulated gastrointestinal digestion produced direct endothelial-type nitric oxide-mediated vasodilation in blocked blood vessels of mice. Further, using a peptidomics approach, the primary digested product of spirulina was divided into five parts (A-E), and only part E was able to stimulate vasodilation. Component E contains four major peptide segments (SP3-SP6), of which only SP6 (GIVAGDVTPI) exhibits direct endothelium-dependent vasorelaxation in isolated blood vessels, which is mediated by the phosphoinositide 3-kinase/serine-threonine kinase (PI3K/AKT) pathway to aggregate NO release. In addition, SP6 also has a blood pressure lowering effect in animals, enhancing endothelial-type vasodilation while increasing the level of nitrite in the serum. Lu Jun [61] found that the two peptides isoleucine-glutamine-proline (Ile-Gln-Pro, IQP) and valine-glutamic acid-proline (Val-Glu-Pro, VEP) peptides are angiotensin-converting enzyme (ACE) inhibitory peptides, and both are non-competitive inhibitors.
Spirulina lowers blood pressure by increasing eNOS synthesis, inhibiting ACE, inhibiting the renin-angiotensin system, inhibiting vasoconstricting metabolites and inhibiting platelet aggregation.
2.4 Antioxidant effect
Poor eating habits and ingredients can change the body's metabolic balance and cause damage to the body. Long-term consumption of cooking oil with a high peroxide value can disrupt the body's oxidative stress balance, significantly increase cytochrome P450 2E1 in the blood serum, and cause liver tissue damage. Supplementing Wistar rats with 1 g/kgbw spirulina daily can reduce the damage caused by oxidative stress [62]. Supplementing spirulina at 2 g/d and 5 g/d can also increase the body's antioxidant capacity [63, 64].
Obesity-induced local and systemic inflammation can lead to a variety of complications, including oxidative stress, insulin resistance, metabolic dyslipidemia, type 2 diabetes, cardiovascular disease, and hypertension. Inflammation management has therefore become a key therapeutic intervention for metabolic syndrome. In three studies, 8 g/d spirulina was continuously administered for 16-12 weeks, plasma IL-2 was elevated, IL-6 was reduced, and superoxide dismutase (SOD) activity was increased [56], plasma malondialdehyde (MDA) levels were reduced [55], and serum adiponectin levels were elevated, while the IL-2/IL-6 ratio and total antioxidant levels were significantly increased and thiobarbituric acid reactive substances levels were reduced in non-obese patients [65]. In patients with chronic obstructive pulmonary disease (COPD), spirulina supplementation (1 g/d) for 2 months reduced serum MDA and lipid peroxidation, increased SOD and glutathione S-transferase (GST) activity, and the concentrations of glutathione (GSH) and vitamin C [66]. Supplementation with spirulina can also increase GSH levels in the body at rest or 24 hours after exercise, and reduce the level of thiobarbituric acid reactive substances after exercise [67].
The antioxidant and anti-inflammatory effects of spirulina can be attributed to its content of phycocyanin, β-carotene, vitamin E and γ-linolenic acid. Among these, phycocyanin can effectively scavenge free radicals and reactive oxygen species (ROS), inhibit the expression of inducible nitric oxide synthase (iNOS), reduce the production of nitrite, and inhibit the peroxidation of liver microsomal lipids. The polyunsaturated double bond of β-carotene has antioxidant and anti-inflammatory effects, is an effective membrane antioxidant, can inhibit oxygen-mediated lipid peroxidation. In addition, β-carotene blocks the accumulation of ROS between cells, inhibits the expression of inflammation-related genes iNOS, COX-2, TNF-α and IL-1β, and also inhibits the activity of iNOS and the nuclear transcription factor kappa B (NF-κB) promoter.
2.5 Effect on non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is a metabolic stress-induced liver injury closely related to insulin resistance and genetic susceptibility. The spectrum of the disease includes non-alcoholic simple liver steatosis, non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC). NAFLD not only leads to liver disease disability and death, but is also closely associated with a high incidence of metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM), atherosclerotic cardiovascular disease, and colorectal tumors.
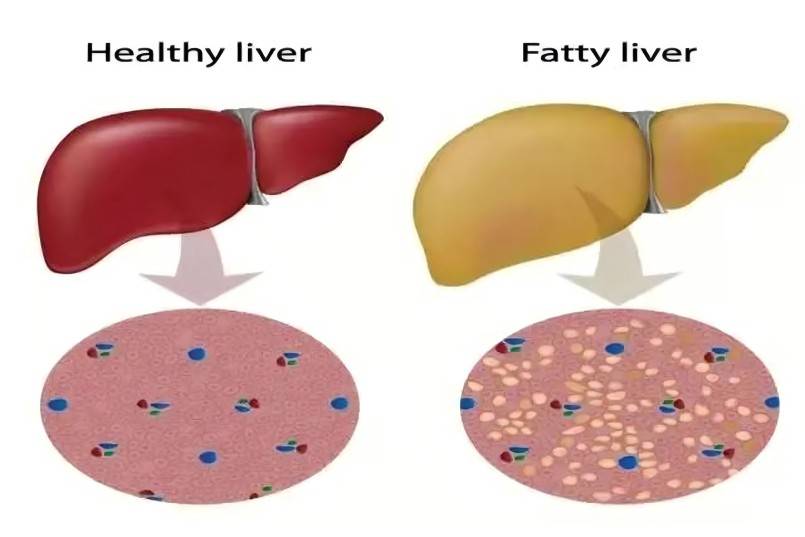
Currently, the mainstay of treatment for NAFLD is lifestyle modification, such as weight loss through diet and exercise. In addition, a large number of drugs and supplements, including antioxidants, anti-inflammatory agents, insulin sensitizers, and lipid-lowering agents, have been used as alternative treatment options in patients and experimental animal models [68]. Spirulina is an effective dietary supplement for reducing liver enzymes in patients with NAFLD. In an open-label, non-randomized trial, 6 g/d of Arthrospira platensis intervention for 6 months reduced AST, ALT and γ-glutamyl transferase in NAFLD patients [53]. Supplementation with 4.5 g of Arthrospira maxima per day also reduced liver transaminases in 3 NAFLD patients [69]. Spirulina also has a prebiotic effect, as it promotes the growth of beneficial microorganisms in the digestive tract [3]. During digestion and absorption, spirulina reduces liver MDA levels and increases GSH, SOD and nitric oxide levels, thereby preventing liver vacuolar degeneration, fatty infiltration and fibrosis.
Experiments on mice have shown that dietary supplementation with spirulina inhibits hepatotoxicity by reducing the penetration of liver enzymes into the blood serum, and also by reducing lipid peroxidation, haemorrhage and hepatocyte necrosis in the liver [70]. Based on current knowledge, spirulina inhibits the formation of fatty liver and NASH mainly by inhibiting lipid peroxidation and scavenging free radicals, or indirectly enhancing the activity of antioxidant enzymes in the liver [71]. Spirulina has a particular effect in inhibiting fatty liver, but the mechanism still needs to be further elucidated.
2.6 Anti-chemical toxicity
Spirulina has a protective effect against the nephrotoxicity caused by amikacin (AMK, amikacin/aminoglycoside) in New Zealand rabbits, and there is a synergistic effect between pure spirulina powder and vitamin C [72]. Wistar mice with an acute liver toxicity model using D-galactosamine (D-GalN) were treated with butylated hydroxytoluene (BHT) and aqueous solutions of broken-cell spirulina. Compared with the D-GalN group, 9% spirulina aqueous extract significantly reduced alkaline phosphatase (esterase) and inflammatory markers such as TNF-α, IL-6 and IL1β, as well as TBARS, and increased oxidative stress markers such as GR, glutathione (GSH), GST, superoxide dismutase (SOD), GPX and CAT and total protein. The results showed that 9% spirulina water extract had the same protective effect on the liver as BHT (0.5% in the feed) [73]. Dried spirulina powder can adsorb heavy metals such as cadmium in water [74]. Spirulina has a potential effect on heavy metals in the body.
2.7 Lowering blood lipids
A series of clinical studies [58, 75–79] have shown that a daily supplement of 1–10 g spirulina for 15 days to 6 months can lower one or more of the following indicators in the blood: TC, TG, LDL-C and VLDL or increase HDL-C [71]. The effect of spirulina on hyperlipidemia can also be found in a review by Dinicolantono et al. [80]. Spirulina fat-soluble extract can significantly reduce the expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), the rate-limiting enzyme for cholesterol synthesis, in HepG2 cells, and also inhibits the expression of LDL receptors (LDLR) and lipogenesis genes (such as fatty acid synthase and stearoyl-CoA desaturase 1) [70], thus exhibiting a significant hypolipidemic effect.
2.8 Weight loss
Dinicolantonio et al. [80] and Moradi et al. [81] both reviewed the weight loss effects of spirulina. Some clinical spirulina weight loss experiments [82-87] have shown that the significance of the weight loss results is related to the dosage and supplementary measures taken. Spirulina can achieve weight loss by reducing the permeability of macrophages entering visceral fat, and preventing the accumulation of liver fat and oxidative stress [88]. Spirulina is rich in phenylalanine, which is a powerful trigger of cholecystokinin and affects the appetite center in the brain, thereby suppressing appetite and controlling weight gain [53]. However, the mechanism of action of spirulina for weight loss is still controversial, because the polysaccharides, vitamins, phycocyanin, and γ-linolenic acid in spirulina are all lipid-lowering active substances that can inhibit fat absorption, metabolism, and secretion.

2.9 Other effects
Wu Shilin et al. [89] used spirulina tablets at a dosage of 2 g/time, 3 times/day to treat 100 patients with peptic ulcers for 8 weeks. They found that the effective treatment rate reached 94%. Spirulina has a promoting effect on cell growth and ulcer tissue repair, and also has the effect of promoting surgical incision healing.
Desai et al. [90] reviewed the use of spirulina in the physical treatment of oral mucosal fibrosis, especially leukoplakia. Spirulina's antioxidant and anti-inflammatory effects can effectively prevent and treat periodontitis. Clinical trials have shown that continuous administration of spirulina capsules (1 g/d for 3 months) to patients with oral leukoplakia can help improve or eliminate symptoms such as oral ulcers, difficulty opening the mouth, and burning sensation. Spirulina also has adjuvant therapeutic functions such as idiopathic male infertility [91].
3 Spirulina in food
90% of spirulina on the market is sold as a dietary supplement in powder and tablet form, and it is also widely used in animal feed. In Chad, Africa, the powdered crushed dried spirulina mud tablets (Dihé) is eaten by mixing it with tomato sauce and pepper and pouring it over food (rice, beans, fish, meat).[92 As a raw material with a long history of use, spirulina is widely used in the food industry, for example in soups, sauces, desserts, cold drinks, noodles, etc. However, the thermal and photostability of chlorophyll and phycocyanin in spirulina limits its use in industrial foods, especially baked goods and beverages. Current research on the use of spirulina and its products in the food industry .
3.1. Application in dairy products
Due to the presence of cell sheaths, spirulina powder cannot completely dissolve in water. In addition, the thermal instability of chlorophyll and phycocyanin in spirulina causes the addition of spirulina powder to liquid dairy products before the sterilization process to result in a yellowish to brownish precipitate in the product.

3.1.1 Yogurt
Phycocyanin is a water-soluble protein that has a positive effect on yogurt, such as reducing water separation and increasing hardness. It is a recommended bioactive pigment. In actual production, due to the thermal instability of phycocyanin, it is recommended to add it aseptically during the inoculation process. When the pH of the yogurt material with added phycocyanin is lowered to 4.5 and stored at 4 °C, the viscosity of the yogurt increases with the concentration of phycocyanin, and the pH of the phycocyanin yogurt is higher than that of the control; 4% phycocyanin (w/w) fortified yogurt has no significant difference in overall acceptability compared to regular yogurt, but Lactobacillus bulgaricus and Streptococcus lactis were significantly reduced at 14d and 21d, respectively [93].
3.1.2 Ice cream
Ice cream is a good carrier, and is very suitable for the application of spirulina powder, phycocyanin, etc. It is recommended to be added during the aging process. Adding 1.2% spirulina powder to ice cream can increase the protein content of the product, while also increasing the puffing rate and resistance to melting of the ice cream [94].
3.1.3 Cheese
Pre-prepare soft cheese, then add 1% spirulina powder while freezing and salting, stir and then store in the refrigerator. Spirulina can increase the protein and beta-carotene content of soft cheese, reduce moisture and extend the shelf life [94]. Adding 4% spirulina powder to processed cheese can reduce hardness and melt index [95].
3.2 Application in pasta products
Spirulina powder can be used to make green raw wet noodles or dry hanging noodles, but its application is self-limiting due to its color. Mostolizadeh et al. [96] added 0.25%–1% spirulina powder to pasta. Pasta with different concentrations of spirulina powder significantly increased the content of essential amino acids and unsaturated fatty acids in the food. Pasta with 0.25% spirulina powder had good microbiological properties and nutritional value.

4 Outlook
Spirulina is a functional food with a range of health and nutritional benefits. The high concentration of strong antioxidants such as phycocyanin and β-carotene in spirulina makes it a dietary supplement that can reduce oxidative stress and inflammatory responses. Spirulina can lower blood lipids, blood pressure, and weight, and can also improve glucose metabolism and improve insulin signaling pathways by reducing inflammatory factors. Spirulina supplementation can effectively improve non-alcoholic fatty liver disease. The protective mechanism of spirulina in metabolic diseases (such as non-alcoholic fatty liver disease) has not yet been fully elucidated, and research is still needed to clarify the mechanism behind its function.
With the emphasis on the important role of dietary nutrition intervention in chronic diseases, spirulina, as a complete food, will usher in important development opportunities. However, the sensitivity of spirulina and phycocyanin to heat and light is a major obstacle limiting the application of spirulina in the food industry. Research is still needed on the stabilization of spirulina and phycocyanin during industrial food processing.
Reference:
[1] Shao W, Ebaid R, El-Sheekh M, et al. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): An overview [J]. Grasas Aceites, 2019, 70(1): e292.
[2] Hoseini S M, Khosravi-darani K, Mozafari M R. Nutritional and medical applications of Spirulina microalgae [J]. Mini-Reviews in Medicinal Chemistry, 2013, 13(8): 1231-1237.
[3] Kulshreshtha A, Zacharla J A, Jarouliya U, et al. Spirulina in Health care management [J]. Current Pharmaceutical Biotechnology, 2008, 9(5): 400-405.
[4] Ma Z, Ahmed F, Yuan B, et al. Fresh living Arthrospira as dietary supplements: Current status and challenges [J]. Trends in Food Science and Technology, 2019, DOI: 10.1016/j.tifs.2019.04.010.
[5] Soni R A, Sudhakar K, Rana R S. Spirulina - From growth to nutritional product: A review[J]. Trends in Food Science & Technology, 2017,69. (Part A), 157-171.
[6] State Administration for Market Regulation. Special Food Information Query Platform, [EB/OL]. [2021-7-15].
[7] National Medical Products Administration. Domestic drug database, [EB/OL]. [2021-7-15].
[8]Anonym. Notice of the General Administration of Market Supervision on publicly soliciting opinions on the catalogue of five kinds of health food raw materials such as coenzyme Q10 [EB/OL]. (2019-3-28) [2019-6-20].
http://gkml.saic.gov.cn/nsjg/tssps/201904/t20190401_292491.html.
[9] FAO/WHO: Codex Guidelines for Vitamin and Mineral Food Supplements (CAC/GL 55-2005).
[10] Chinese nutrition society. Chinese Dietary Reference Intakes (2013 Edition) [M]. Beijing : Science Press, 2014.
[11] China Food and Drug Administration, National Health and Family Planning Commission, State Administration of Traditional Chinese Medicine. Announcement on the release of the “Catalogue of Health Food Raw Materials (I)” and “Catalogue of Permitted Health Functions for Health Food Claims (I)” (No. 205 of 2016) [EB/OL]. (2016-12-27) [2021-7-23].
[12]Yang Weijie. Metabolic mechanisms ofSpirulina based enteral formula for supporting type 2 diabetes mellitus in experimental animals [D]. Shanghai: Shanghai Ocean University, 2018.
[13]Xu H, Zhou Q, Lu F, et al. A review of progress in the cultivation and safety of Spirulina [J]. Jiangsu Agricultural Sciences, 2021, 49(6): 10-19.
[14]Yin X, Tang J, Liu J, et al. Study on extraction of phycobilin and its optical property [J]. Applied Chemical Industry, 2010, 39(4): 484-486, 490.
[15]Du L, Fu H. Purification and properties of phycobiliprotein from Spirulina platensis [J]. Journal of Sichuan University (Natural Science Edition), 1994, 31(4): 576-578.
[16]Lv P, Li C, Yang D, et al. The research of the stability of phycocyanin from Spirulina platensis [J]. Guangdong Chemical Industry, 2019, 46(5): 60-61.
[17]Jiang G, Yan Q, Li D, et al. Research progress on separation, purification and stabilization of phycocyanin from Spirulina. Journal of Food Safety and Quality, 2021, 12(6): 2332-2338.
[18] Tavanandi H A, Vanjari P, Raghavarao K S M S. Synergistic method for extraction of high purity allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids [J]. Separation and Purification Technology, 2019, 225: 97-111.
[19] Hayashi, T., Hayashi, K., Maeda, M., et al. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis [J]. Journal of Natural Products, 1996, 59, 83-87.
[20]Luo G, Ma M, Zhang X, et al. Three-phase partitioning for efficient extraction and separation of polysaccharides from Spirulina platensis and its structural characterization [J]. Food and Fermentation Industries, 2019, 45(6): 147-152.
[21]Liu Y, He Y, Zhang X, et al. Process optimization for extraction of polysaccharides from Spirulina platensis using inner ebullition method [J]. Journal of Mudanjiang Normal University, 2018, (2): 51-54, 57.
[22] Majdoub H, Mansour B M, Chaubet F, et al. Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis [J]. Biochimica Biophysica Acta, 2009, 1790: 1377-1381.
[23] Hayashi T, Hayashi K, Kojima I. Antiviral polysaccharide [P]. U.S. Patent 5,585,365.
[24] Pugh N, Ross A S, ElSohly N H, et al. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa [J]. Planta Medica, 2001, 67: 737-742.
[25] Chaiklahana R, Chirasuwan N, Triratana P, et al. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. International Journal of Biological Macromolecules, 2013, 58: 73-78.
[26]Tu F, Yang F, Zheng W, et al. Progress in polysaccharide of Spirulina [J]. Natural Product Research and Development, 2005, 17(1): 115-119.
[27] De Morais E G, Druzian J I, Nunes I L, et al. Glycerol increases growth, protein production and alters the fatty acids profile ofSpirulina (Arthrospira) sp LEB 18 [J]. Process Biochemistry, 2019, 76: 40-45.
[28] Sassano C E N, Gioielli L A, Converti A, et al. Urea increases fed-batch growth and gamma-linolenic acid production of nutritionally valuable Arthrospira (Spirulina) platensis cyanobacterium [J]. Engineering in Life Sciences, 2014, 14(5): 530-537.
[29] ESFA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to gamma-linolenic acid (GLA) and maintenance of normal blood LDL-cholesterol concentrations (ID 2661, 4452, 4453), maintenance of normal blood pressure (ID 2662), reduction of menstrual discomfort (ID 495, 640, 1773, 1775), contribution to normal cognitive function (ID 1770), maintenance of the barrier function of the skin (ID 499, 591, 639, 676, 1554, 2003, 2065), “function of the cell membrane” (ID 1769), maintenance of normal structure, elasticity and appearance of the skin (ID 2660, 4296) and “anti-inflammatory properties” (ID 4454) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 [J]. ESFA Journal, 2011, 9(4): 2059.
[30] Ranga Rao A, Raghunath Reddy R L, Baskaran V, et al. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model [J]. Journal of Agricultural and Food Chemistry, 2010, 58(15): 8553-8559.
[31] Cezare-Gomes E A, Mejia-Da-Silval D, Perez-Mora L S, et al. Potential of microalgae carotenoids for industrial application [J]. Applied Biochemistry and Biotechnology, 2019, 188(3): 602-634.
[32] Annapurna V, Shan N, Bhaskaram P, et al. Bioavailability of Spirulina carotenes in preschool children [J].Journal of Clinical Biochemical Nutrition, 1991, 10, 145-151.
[33]Wang Jie, Hu Yuming, Li Zimin, et al. Study on dietary Spirulina improving vitamin A nutritional status in children [C]. Papers ofthe 12th Academic Annual Meeting of Children ’ s Food Branch of China Food Science and Technology Association, 2011, pp 102-106.
[34] Knapen M H J, Braam L A J L M, Drummen N E, et al. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women: double-blind randomised clinical trial [J]. Thrombosis and Haemostasis, 2015, 113, 5. DOI: 10.1160/TH14-08-0675.
[35] Van Summren M J H , Braam L A J L M , Lilien M R , et al. The effect of menaquinone-7 (vitamin K2)supplementation on osteocalcin carboxylation in healthy prepubertal children [J]. British Journal of Nutrition, 2009, 102(08):1171-1178.
[36] Inaba N, Sato T, Yamashita T. Low-dose daily intake of vitamin K2 (menaquinone-7) improves osteocalcin γ-carboxylation:a double-blind, randomized controlled trials [J].Journal of Nutritional Science&Vitaminology, 2016, 61(6): 471-480.
[37] Knapen M H J, Drummen N E, Smit E, et al. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women [J]. Osteoporos International, 2013, 24: 2499-2507.
[38] Vossen L M, Schurgers L J, Van Varik B J, et al. Menaquinone-7 supplementation to reduce vascular calcification in patients with coronary artery disease: Rationale and study protocol (VitaK-CAC Trial) [J]. Nutrients, 2015, 7, 8905-8915.
[39] Huang Jiming, Bai Shumin, Hu Zhixiang, et al. Effects of Spirulina on changes of femur BMD and calcium metabolism in rats simulated weightlessness [J]. Chinese Journal of Osteoporosis, 2002, 8(1): 55-58.
[40] Watanabe F, Katsura H, Takenaka S, et al. Pseudoviatmin B12 is the predominant cobamide of an algal health food, Spirulina tablets [J]. Journal of Agricultural and Food Chemistry, 1999, 47: 4736-4741.
[41] Van Den Berg H, Brandsen L, Sinkeldam B J. Bitamin B-12 content and bioavailability of Spirulina and nori in rats [J]. Journal of Nutrition Biochemistry, 1991, 2: 314-318.
[42] Kumudha S S, Kumar M S, Thakur G A, et al. Purification, identification, and characterization of methylcobalamin from Spirulina platensis [J]. Journal of Agricultural and Food Chemistry, 2010, 58: 9925-9930.
[43] Watanabef, Miyamoto E, Nakano Y. Inactive corrinoid-compound significantly decreases in Spirulina platensis grown in a cobalt-deficient medium [J]. Journal of Agricultural and Food Chemistry, 2001, 49: 5685-5688.
[44]Wang Zhizhong. Study on key factors of production and processing for Spirulina platensis from the alkali lake in Ordos Plateau[D]. Hohhot: Inner Mongolia Agricultural University, 2015.
[45]Zhu Bizhen, Wu Jiayuan, Lu Xian, et al. Application ofSpirulina in nutrition therapeutic on patients with iron-deficiency anemia in children [J]. China Practical Medicine, 2009, 4(6): 78-80.
[46] Gao F, Guo W, Zeng M Y, et al. Effect of microalgae as iron supplements on iron-deficiency anemia in rats [J].
Food and Function, 2019, 10(2): 723-732.
[47] Matufi F, Maghsudi H, Choopani A. Spirulina and its role in immune system: a review [J]. Journal of Immunology Research and Therapy, 2020, 5(1): 204-211.
[48]Tan Yuyan, Tan Weiyan, Zou Yuqing. Clinical effect of nutritional improvement on prevention of nosocomial infection in patients with maintenance hemodialysis [J]. Chinese Journal of Modern Drug Application. 2016, 10(5): 231-232.
[49] Ge Y, Kang Y K, Dong L, et al. The efficacy of dietary Spirulina as an adjunct to chemotherapy to improve immune function and reduce myelosuppression in patients with malignant tumors [J]. Translation Cancer Research, 2019, 8(4):1065-1073.
[50] Parikh P, Mani U, Iyer U. Role of Spirulina in the control of glycemia and lipidemia in Type 2 Diabetes Mellitus [J]. Journal of Medicinal Food, 2001,4(4): 193-199.
[51] Mani U V, Desai S, Iyer U. Studies on the long-term effect ofSpirulina supplementation on serum lipid profile and glycated proteins in NIDDM patients [J]. Journal of Nutraceuticals, Functional and Medical Foods, 2000, 2(3): 25-32.
[52]Yang Zhifen, Liu Hongbin, Tang Zhiyuan et al, Effects of Spirulina on iron, hemoglobin, and glycated hemoglobin content in serum of non-insulin dependent diabetes[J].Zhejiang Clinical Medical Journal, 2002, 4(4): 264-265.
[53] Mazokoparkis E E, Papadomanolaki M G, Fousteris A A, et al. The hepatoprotective and hypolipidemic effects of Spirulina supplementation in a Cretan population with nonalcoholic fatty liver disease: a prospective pilot study [J]. Annals of Gastroenterology Quarterly Publication of the Hellenic Society of Gastroenterology, 2014, 27(4):387-394.
[54] Marcel A K, Ekali L G, Eugene S. et al. The effect of Spirulina platensis versus soybean on insulin resistance in HIV-infected patients: a randomized pilot study [J]. Nutrients, 2011, 3(7): 712-724.
[55] Lee E H, Park J E, Choi Y J, et al. A randomized study to establish the effects of Spirulina in type 2 diabetes mellitus patients [J]. Nutrition Research and Practice, 2008, 2(4): 295-300.
[56] Park H J, Lee Y J, Ryu H K, et al. A randomized double-blind, placebo-controlled study to establish the effects ofSpirulina in elderly Koreans [J]. Annals of Nutrition and Metabolism, 2008, 52(4): 322-328.
[57] Oriquat G A, Ali M A, Mahmoud S A, et al. Improving hepatic mitochondrial biogenesis as a postulated mechanism for the antidiabetic effect of Spirulina platensis in comparison with metformin [J]. Applied Physiology, Nutrition, and Metabolism, 2018, DOI: 10.1139/apnm-2018-0354.
[58] Torres-Duran P V, Ferreira-Hermosilio A, Juarez-Oropeza M A. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population: a preliminary report [J]. Lipids in Health and Disease, 2007, 6:33.
[59] Martinez-Samano J M, Torres-Montes De Oca A, Luqueno-Bocardo O I, et al. Spirulina maxima decreases endothelial damage and oxidative stress indicators in patients with systemic arterial hypertension: results from exploratory controlled clinical trial [J]. Marine Drugs, 2018, 16, 496.
[60] Carrizzo A, Conte G M, Sommella E, et al. Novel potent decameric peptide of Spirulina platensis reduces blood pressure levels through a PI3K/AKT/eNOS-dependent mechanism [J]. Hypertension, 2019, DOI: 10.1161/HYPERTENSIONAHA.118.11801.
[61] Lu Jun, Purification, Characterization and Antihypertensive and Hepatoprotective Effects of Bioactive Peptides Derived from Spirulina platensis [D]. Beijing: Beijing Forestry University, 2010.
[62] Amara-Leffad L O, Ramdane H, Nekhoul K, et al. Spirulina effect on modulation of toxins provided by food, impact on hepatic and renal functions [J]. Archives of Physiology and Biochemistry, 2019, DOI: 10.1080/13813455.2018.1444059.
[63] Szulinska M, Gibas-Dorna M, Miller-Kasprzak E, et al. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: a randomized double-blind placebo-controlled study [J]. European Review for Medical and Pharmacological Sciences, 2017, 21(10): 2473-2481.
[64] Winter F S, Emakam F, Kfuwah A, et al. The effect of Arthrospira platensis capsules on CD4 T-Cells and antioxidative capacity in a randomized pilot study of adult women infected with human immunodeficiency virus ont under HAART in Yaoundé, Cameroon [J]. Nutrients, 2014, 6(7): 2973-2986.
[65] Park H J, Lee H S. The influence of obesity on the effects of Spirulina supplementation in the human metabolic response of Korean elderly [J]. Nutrition Research and Practice, 2016, 10(4): 418-423.
[66] Ismail M, Hossain M F, Tanu A R, et al. Effect ofSpirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients [J]. BioMed Research International, 2015, DOI: 10.1155/2015/486120.
[67] Kalafati M, Jamurtas A Z, Nikolaidis M G, et al. Ergogenic and antioxidant effects of Spirulina supplementation in humans [J]. Medicine and Science in Sports and Exercise, 2010, 42(1): 142-151.
[68] Eslamparast T, Eghtesad S, Poustchi H, et al. Recent advances in dietary supplementation, in treating non-alcoholic fatty liver disease [J]. World Journal of Hepatology, 2015, 7(2): 204-212.
[69] Feffeira-Hermosillo A, Torres-Duran P V, Juarez-Oropeza M A. Hepatoprotective effects ofSpirulina maxima in patients with non-alcoholic fatty liver disease: a case series [J]. Journal of Medical Case Reports, 2010, 4: 103.
[70] Ku C S, Yang Y, Park Y, et al. Health benefits of blue-green algae: Prevention of cardiovascular disease and nonalcoholic fatty liver disease [J]. Journal of Medicinal food, 2013, 16(2): 103-111.
[71] Yousefi R, Saidpour A, Mottaghi, A. The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: A systematic review [J]. Complementary Therapies in Medicine, 2018, DOI: 10.1016/j.ctim.2018.11.013.
[72] Abdel-Daim M M, Ahmed A, Ijaz H, et al. Influence of Spirulina platensis and ascorbic acid on amikacin-induced nephrotoxicity in rabbits [J]. Environmental Science and Pollution Research, 2019, DOI: 10.1007/s11356-019-04249-4.
[73] Al-Qahtani W H, Binobead M A. Anti-inflammatory, antioxidant and antihepatotoxic effects of Spirulina platensis against D-galactosamine induced hepatotoxicity in rats [J]. Saudi Journal of Biological Sciences, 2018, OI:10.1016/j.sjbs.2018.01.003.
[74] Al-Homaidan A A, Alabdrllatif J A, Al-Hazzani A A, et al. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass [J]. SaudiJournalofBiologicalSciences, 2015, 22(6), 795-800.
[75]Wu Xinhua, Jiao Jianling, Yu Shufang. Therapeutic effect ofSpirulina on 49 cases of hyperlipidemia [J].Chinese Journal of Cardiovascular Medicine, 2000, 5(4): 233-234.
[76]Han Qiding, Wu Xinhua,Kuang Shiquan, et al. Effects of Chenghai Spirulina platensis on hyperlipidemia [J]. Chinese Journal of Cardiovascular Medicine, 2004, 9(6): 438-439, 441.
[77]Wang Jianxiu, Zhang Rui, Wang Xiuping. Clinical Observation on 76 Cases of Hyperlipidemia Treated by Spirulina [J]. Journal of Taishan Medical College, 2004, 9(6): 438-439, 441.
[78]Li Yanling, Mao Fuqiang. The therapeutic effect ofSpirulina platensis on hyperlipidemia [J]. Chinese Journal of Clinical Nutrition, 2001, 9(1): 58-59.
[79] El-Sheekh M M, Hamad S M, Gomaa M. Protective effects of Spirulina on the liver function and hyperlipidemia ofrats and human [J]. Brazilian Archives of Biology and Technology, 2014, 57(1): 77-86.
[80] Dinicolantonio J J, Bhat A G, Okeefe J. Effects ofSpirulina on weight loss and blood lipids: a review [J]. Open Heart, 2020, 7: e001003. DOI: 10.1136/openhrt-2018-001003.
[81] Moradi S, Ziaei R, Foshati S, et al. Effects of Spirulina supplementation on obesity: a systematic review and meta-analysis of randomized clinical trials [J]. Complementary Therapies in Medicine, 2019, 47: 102211.
[82] Zeinalian R, Farhangi M A, Shariat A, et al. The effects of Spirulina platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: a randomized double blinded placebo controlled trial [J]. BMC Complementary and Alternative Medicine, 2017, 17: 1-8.
[83] Yousefi R, Mottaghi A, Saidpour A. Spirulina platensis effectively ameliorates anthropometric measurements and obesity-related metabolic disorders in obese or overweight healthy individuals: a randomized controlled
trial [J]. Complementary Therapies in Medicine, 2018, DOI: 10.1016/j.ctim.2018.08.003.
[84] Shariat A, Farhangi M A, Zeinalian R. Spirulina platensis supplementation, macrophage inhibitory cytokine-1 (MIC-1), oxidative stress markers and anthropometric features in obese individuals: a randomized controlled trial [J]. Journal of Herbal Medicine, 2019, DOI: 10.1016/j.hermed.2019.100264.
[85] Hernandez-Lepe Ma, Wall-Medrano A, Lopez-Diaz J a, et al. Hypolipidemic effect Arthrospira (Spirulina)maxima supplementation and a systematic physical exercise program in overweight and obese men: a double-blind, randomized, and crossover controlled trial [J]. Marine Drugs, 2019, 17, 270.
[86] Hernandez-Lepe Ma, Lopez-Diaz J A, Juarez-oropeza M A, et al. Effect of Arthrospira (Spirulina) maxima supplementation and a systematic physical exercise program on the body composition and cardiorespiratory fitness of overweight or obese subjects: a double-blind, randomized, and crossover controlled trial [J]. Marine Drugs, 2018, 16, 364.
[87] Gomez-Tellez A, Sierra-Puente D, Munoz-Gomez R, et al. Effects of a low-dose Spirulina/turmeric supplement on cardiometabolic and antioxidant serum markers of patients with abdominal obesity [J]. Frontiers in Nutrition, 2020, 7: 65.
[88] Fujmoto M, Tsuneyama K, Fujimoto T, et al. Spirulina improves nonalcoholic steadohepatitis, visceral fat macrophage aggregation, and serum leptin in a mouse model of metabolic syndrome [J]. Digestive and Liver Disease, 2012, 44(9): 767-774.
[89]Wu Shilin, YangLiping, Gu Siping et al. 100 cases of peptic ulcer treated with Spirulina [J]. World Chinese Journal of Digestology, 2000, 8(10): 1194-1195.
[90] Desai K M, Hallikermath S, Kale A. Spirulina: an emerging treatment modality for the management of oral submucous fibrosis [J]. International Journal of Oral Care and Research, 2017, 5(4): 328-331.
[91] Modarresi R, Aminsharifi A, Foroughinia F. Impact of Spirulina supplementation on semen parameters in patients with idiopathic male infertility: A pilot randomized trial [J]. Urology Journal, 2019, 16(1), 78-82.
[92] Henrikson R. Earth Food Spirulina (Sixth edition) [EB/OL]. www.spirulinasource.com, Ronore Enterprises, Inc., Hana, Maui, Hawaii, USA. 2009.
[93] Mohammadi-Gouraji E, Soleimanian-Zad S, Ghiaci M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage [J]. LWT-Food Science and Technology, 2019, 102: 230-236.
[94] Agustini T W, Ma’Ruf W F, Widayat, et al. Application of Spirulina platensis on ice cream and soft cheese withrespect to their nutritional and sensory perspectives [J]. Journal Teknologi (Science & Engineering), 2016, 78: 4-2: 245-251.
[95] Tohamy M M, Shaaban H A, Ali M A, et al. Effect of Spirulina platensis as nutrition source on the chemical rheological and sensory properties of spreadable processed cheese [J]. Journal of Biological Sciences, 2019, 19(1): 84-91.
[96] Mostolizadeh S, Moradi Y, Mortazavi M S, et al. Application effects ofSpirulina powder on the fatty acid and amino acid composition of pasta [J]. Iranian Scientific Fisheries Journal, 2017, 26(4): 119-130.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






