What Are the Benefits of Centella Plant?
Centella asiatica is the dried whole herb of the plant Centella asiatica (L.) Urban, family Apiaceae. It is widely distributed in all areas south of the Yangtze River basin. The whole herb is used for medicinal purposes and can be collected year-round. It is abundant in resources and has been used in the medical fields of many countries for thousands of years. It was first recorded in Shennong's Classic of Materia Medica, where it was classified as a medium-grade herb. It is cold in nature, bitter and pungent in taste, and has the effects of clearing away heat and dampness, detoxifying and reducing swelling. It is mainly used for treating damp-heat jaundice, heatstroke diarrhea, hematuria and hemoptysis, carbuncles and boils, bruises and injuries [1]. The chemical constituents of Centella asiatica include triterpene saponins, triterpenic acids, polyacenes and essential oils [2]. Modern research has shown that Centella asiatica extract can effectively promote local collagen anabolism in skin damage and play an important role in tissue repair after skin damage [3]. Recent studies have also found that Centella asiatica has antioxidant, anti-depressant, liver-protecting, and tumor cell proliferation inhibitory effects [3]. The following is a review of the progress of research on its chemical composition and pharmacological properties.

1 Chemical composition
1.1 Triterpenoids
Centella asiatica mainly contains triterpenoid saponins, such as asiaticoside, madecassoside, brahminoside, thankuniside, isothankuniside, etc., all of which are pentacyclic triterpene saponins; it also contains free triterpenic acids such as asiatic acid, brahmic acid, brahminoside, madastic acid, etc. Five triterpene saponins have recently been discovered [4]. Matsuda et al. discovered three new triterpenes when studying the chemical composition of Centella asiatica in Sri Lanka namely, madecassoside 28-O-β-D-glucopyranosyl (1→6)-β-D-glucopyranoside (named madecassoside B); 28-O-alpha-L-rhamnopyranosyl-(1→4)-beta-D-glucopyranosyl-(1→6)-beta-D-glucopyranoside (named asiaticoside C); 3β,6β, 23-trihydroxyoleana-12-en-28-oic acid 28-O-à-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside (named asiaticoside D) [5]. Chinese scholars He Mingfang et al. [6] also isolated daucosterol (daucostero1) and vanillic acid from Centella asiatica. Zhang Leilei et al. [7] recently discovered a new compound, asiaticoside disaccharide, which was identified as 2α, 3β, 23-trihydroxyoleana-12-ene-28-oic acid-28-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside.
1.2 Polyacetylenes
In 1973, Schulte and others in Germany isolated 14 polyyne compounds from the underground parts of Centella asiatica and identified the structures of five of them: C16H21O2, C19H27O4, C19H28O3, C17H21O3, and C15H20O2.

1.3 Volatile oils
Qin Lu Ping et al. [8] used gas chromatography-mass spectrometry (GC-MS) to identify 45 long-chain volatile oil components in Centella asiatica, among which the higher content ones include caryo-phy-llene, farneso1, elemene and 1ongifolene.
1.4 Other
Centella asiatica also contains amino acids, flavonols, fatty acids, alkaloids, sterols, sugars, tannins and polyphenol components, as well as racemic inositol, asiaticoside, wax, carotenoids, chlorophyll, kaempferol, quercetin, glucose and rhamnose flavonoid glycosides [9].
2 Pharmacological effects
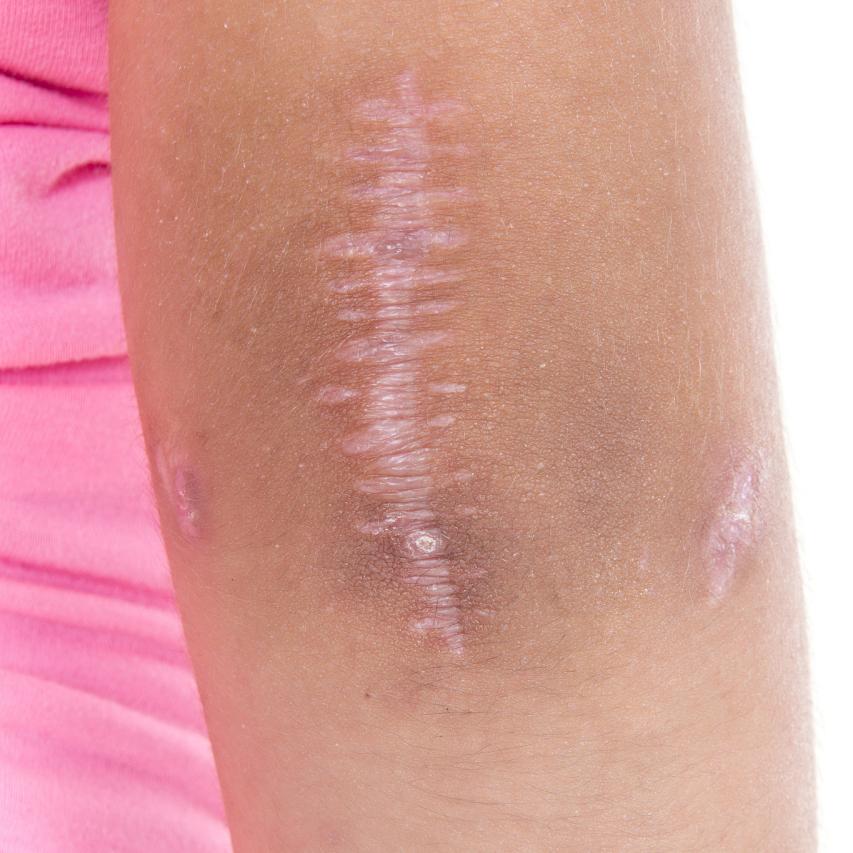
2.1 Inhibits keloid scarring and promotes wound healing
Centella asiatica plays an important role in the prevention and treatment of keloid scarring. Xie et al. [10] used light microscopy, electron microscopy, incorporation and MTT colorimetry to detect fibroblast nuclei and collagen synthesis. The results showed that asiaticoside not only affected the ultrastructure of fibroblasts, but also inhibited fibroblast proliferation and collagen synthesis. Pan Shu et al. [11] used reverse transcription-polymerase chain reaction (RT-PCR) to observe changes in the expression of Smad2 and Smad7 mRNA; flow cytometry analysis, immunocytochemistry and western blot combined with densitometry were used to observe the effects of asiaticoside on the cell cycle and phosphorylation of Smad2 and Smad7 in scar fibroblasts. The results showed that the content of Smad2 and the expression of Smad2 mRNA in the test group were not significantly different from those in the control group; however, the content of Smad7 and the expression of Smad7 mRNA in the test group and the control group were (1.33 ± 1.26)% and (50.80 ± 22.40)%, (9.15 ± 3.36)% and (32.18 ± 17.84)%, respectively. The difference between the two groups was statistically significant (P<0.05). From the above study, it can be seen that the effect of asiaticoside in inhibiting scarring is through the Smad pathway, which blocks fibroblast proliferation and exerts its effect.
Chen Mingchun et al. [12] studied the effects of asiaticoside and matrine on the growth of fibroblasts and collagen synthesis in skin scars, and compared the strength of their anti-scar effects. The effects of the two traditional Chinese medicines on the growth of scar fibroblasts and collagen synthesis were observed by inverted microscopy to observe cell morphology, MTT assay to determine cell viability, flow cytometry to detect changes in the cell cycle, chloramine T assay to determine hydroxyproline content, and lactate dehydrogenase assay to detect cytotoxicity. Results Compared with the blank control group, when the mass concentration of asiaticoside in the test group was 0.5 g/L and the mass concentration of matrine was 1.00 g/L, the number of fibroblasts was significantly reduced, the hydroxyproline content was reduced (P<0.05), and the proportion of cells in the G2-M phase increased (P<0.05). The results showed that at a certain mass concentration, both asiaticoside and matrine can inhibit the growth of scar fibroblasts and the synthesis of collagen, and that asiaticoside has a stronger anti-scar effect than matrine.
2.2 Anti-cancer effect
Centella asiatica extract is a white needle-like crystal extracted from Centella asiatica. It has the effect of reducing swelling and detoxifying, and literature reports that it has a certain anti-tumor activity. In order to explore the anti-cancer effect of Centella asiatica, Wang Jinju et al. [13] found that asiaticoside has an inhibitory effect on the proliferation of L929 cells and CNE cells cultured in vitro, and also inhibits the proliferation of transplanted S180 cells. It can also increase the survival time of S180-bearing mice, preliminarily indicating that asiaticoside has anti-tumor activity. Huang Yunhong et al. [14] used the MTT method to detect the inhibitory effect of asiaticoside alone or in combination with vincristine (VCR) on the proliferation of KB, KBv200, MCF-7 and MCF-7/ADM cells. The cell cycle and apoptosis rate of KB cells were analyzed by flow cytometry, changes in the cell cycle and apoptosis rate of KB cells were analyzed by flow cytometry, and the changes in the properties related to apoptosis and cell cycle of KB cells were observed by agarose gel electrophoresis, fluorescence microscopy and Western blot.
The results showed that KB cells treated with asiaticoside exhibited characteristic changes in apoptotic cells; asiaticoside in combination with VCR had a significant synergistic inhibitory effect on a variety of tumor cells. The apoptosis rate of KB cells in the combined drug group and the β-cell lymphoma/leukemia-2 (Bcl-2) protein phosphorylation level was significantly higher than that of the single-use group, and only the combined group was detected to have activated caspase-3 protein; the ratio of KB cells blocked in the S-G2/M phase and the expression level of cyclin β1 protein in the group combining asiaticoside and VCR were higher than those in the single-use group. Therefore, it is believed that asiaticoside can induce apoptosis in tumor cells and show synergistic effects with VCR, and it may be used as a biochemical regulator in tumor chemotherapy.
2.3 Antidepressant effect
Chen Yao et al. [15] used the forced swimming depression model in mice to observe the antidepressant effect of asiaticoside. The results showed that imipramine and three doses of asiaticoside (low, medium and high) can significantly shorten the immobility time of mice in forced swimming, and improve the imbalance of amino acid content in the brain of mice caused by forced swimming, indicating that asiaticoside has antidepressant activity. In addition, they also studied the effects of Centella asiatica total glucosides on the function of the hypothalamic-pituitary-adrenal cortex axis and the hypothalamic-pituitary-thyroid axis in rats to explore its mechanism of action in treating depression.
A rat model of depression induced by prolonged unpredictable stress was used, and the concentrations of plasma adrenocorticotropic hormone (ACTH) and serum thyroid-stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4) and reverse triiodothyronine (rT3) were measured using radioimmunoassay. Results Compared with normal rats, the plasma ACTH and serum TSH, T4 and rT3 concentrations in animals in the depression model group were significantly lower, and the serum T3 concentration was significantly higher. The plasma ACTH levels and serum TSH, T4 and rT3 levels in each dose group of total asiaticoside increased to varying degrees, and the serum T3 level decreased. It can be seen that asiaticoside has the effect of regulating the hypothalamic-pituitary-adrenal cortex axis and hypothalamic-pituitary-thyroid axis function in rats with depression [16].
2.4 Affects immune system function
Zhu Xiaoling et al. [17] investigated the effect of asiaticoside combined with rhein on the expression of complement core component C3 mRNA and protein in tumor necrosis factor-alpha (TNF-α)-induced mesangial cells from BALB/C mice. RT-PCR and enzyme-linked immunosorbent assay (ELISA) were used to detect the expression of C3 mRNA and protein in mesangial cells, respectively. The results showed that the mesangial cells of normal BALB/C mice had a certain amount of C3 mRNA and protein expression, and both C3 mRNA and protein expression were significantly upregulated after TNF-α induction; After intervention with three different concentrations of asiaticoside combined with rhein, the expression of C3 mRNA and protein levels in mesangial cells was down-regulated to varying degrees, and there was a certain dose-dependent relationship. This shows that asiaticoside combined with rhein can inhibit the local overproduction of C3 in the kidney caused by the upregulation of the inflammatory cytokine TNF-α, and has the effect of protecting renal function and delaying the progression of the disease.

2.5 Antibacterial and anti-inflammatory effects
The main active ingredient extracted from Centella asiatica, asiaticoside, and its preparations have been widely used in the clinic for skin wounds, lower limb ulcers, burns, etc. Ming Zhijun et al. [18] studied the effect of asiaticoside on xylene-induced ear swelling in mice. The results showed that the high-dose group could significantly improve ear swelling in mice, indicating that asiaticoside has a significant anti-inflammatory effect. Zhang Shenghua et al. [19] determined the minimum inhibitory concentration (MIC) of asiaticoside against standard strains and clinically isolated pathogenic strains using the flat-plate double dilution method; a mouse model of bladder ascending renal infection was used the removal effect of asiaticoside on Escherichia coli 26 was observed when it was administered by gavage at a dose of 0.68 to 3.36 g/kg.
The results showed that asiaticoside had strong antibacterial activity against 37 standard and clinical isolates, especially against various drug-resistant bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecium resistant to five aminoglycosides and producing a neutralizing enzyme, Escherichia coli, Klebsiella pneumoniae and Acinetobacter calcoaceticus, and Pseudomonas aeruginosa resistant to piperacillin. The MIC values of these bacteria are similar to those of the three gold tablets. Thus, asiaticoside has good antibacterial activity in vivo and in vitro, especially for urinary tract infections.
3 Conclusion
Centella asiatica is a commonly used traditional Chinese medicine in China. Many experts and scholars have conducted in-depth research on it and found that it has strong pharmacological activity and a wide range of pharmacological effects, which will lay a solid foundation for the better development and application of Centella asiatica in the future.
References:
[1] National Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China (Part I) [M]. Beijing: Chemical Industry Press, 2005: 200.
[2] Chen Jun, Hua Weiyi, Sun Hongbin. A review of the biological activities of asiatic acid and its derivatives. Chinese Herbal Medicine, 2006, 37(3): 458-460.
[3] Lu Luo, Wei Shaomin, Lin Huifen, et al. Effects of Centella asiatica extract on collagen synthesis in fibroblasts [J]. Daily Chemical Industry, 2002, 32(6): 23-25.
[4] Zhang Guifeng. Five new triterpenoid glycosides from Centella asiatica [J]. Foreign Medicine · Traditional Chinese Medicine, 2002, 24(4): 237.
[5] Zhang Weihua. Ursane and zidanane triterpene oligosaccharides in Centella asiatica from Sri Lanka [J]. Foreign Medicine · Traditional Chinese Medicine, 2002, 24(5): 307.
[6] He Mingfang, Meng Zhengmu, Wo Lianqun, et al. Research on the chemical composition of Centella asiatica [J]. Journal of China Pharmaceutical University, 2000, 31(2): 91-93.
[7] Zhang Leilei, Wang Haisheng, Yao Qingqiang, et al. Research on the chemical composition of Centella asiatica [J]. Chinese Herbal Medicine, 2005, 36(12): 1761-1763.
[8] Qin Luping, Ding Ruxian, Zhang Weidong, et al. Analysis of the volatile oil components of Centella asiatica and study of its antidepressant effect [J]. Journal of the Second Military Medical University, 1998, 19(2): 186-187.
[9] Qin Luping, Zhuang Weiguo. Research progress of Centella asiatica [J]. Foreign Medicine · Botanical Medicine Supplement, 1997, 12( 4) : 154 -157.
[ 10] Xie Julin, Li Tianzeng, Qi Shaohai, et al. The effect of asiaticoside on fibroblasts cultured in vitro [ J] . Journal of Zhongshan University of Medical Sciences, 2001, 22( 1) : 41-43.
[11] Pan Shu, Li Tianzeng, Li Yeyang, et al. Effects of asiaticoside on proliferation of proliferative scar fibroblasts and Smad signaling pathway [J]. Chinese Journal of Plastic and Reconstructive Surgery, 2004, 18(4): 291-294.
[12] Chen Mingchun, Huang Maofang. Study on the effects of asiaticoside and matrine on the growth of scar fibroblasts and collagen synthesis in the skin [J]. Chinese Journal of Integrated Traditional and Western Medicine Dermatology and Venereology, 2006, 5 (1): 11-14.
[13] Wang Jinju, Wang Ruiguo, Wang Baokui, et al. Preliminary experimental study on the anti-tumor effect of asiaticoside [J]. Fujian Traditional Chinese Medicine, 2001, 32(4): 39-40.
[14] Huang Yunhong, Zhang Shenghua, Zhen Ruixian, et al. Induction of tumor cell apoptosis by asiaticoside and enhancement of the antitumor effect of vincristine [J]. Cancer, 2004, 23(12): 1599-1604.
[15] Chen Yao, Han Ting, Qin Luping, et al. Effect of asiaticoside on depressive behavior and brain amino acid content in mice [J]. Traditional Chinese Medicine, 2003, 26(12): 870-872.
[16] Chen Yao, Qin Luping, Zheng Hancheng, et al. Effects of asiaticoside on neuroendocrine function in rats with depression [J]. Journal of the Second Military Medical University, 2002, 23(11): 1224-1226.
[17] Zhu Xiaoling, Wang Yongjun, Zhang Yinghua, et al. Effects of asiaticoside and emodin on C3 expression in renal mesangial cells induced by tumor necrosis factor α [J]. Chinese Journal of Clinical Pharmacology and Therapeutics, 2006, 11(4): 414-417.
[18] Ming Zhijun, Sun Meng. Experimental study on the anti-inflammatory effect of asiaticoside [J]. Chinese Medicine Science and Technology, 2002, 9(1): 62.
[19] Zhang Shenghua, Yu Lanxiang, Zhen Ruixian, et al. Antibacterial effect of asiaticoside and its therapeutic effect on experimental urinary tract infection in mice [J]. Chinese Journal of New Drugs, 2006, 15(20): 1746-1749


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






