Study on Centella Asiatica Extract Skin Benefits
Centella asiatica (L 1) U rb1 is a plant in the family Apiaceae, and the whole herb is used in medicine. It is cold in nature, bitter and pungent in taste, and has the effects of clearing away heat and dampness, detoxifying and reducing swelling. It is clinically used for heatstroke in summer, bruises and contusions, infectious hepatitis, epidemic cerebrospinal meningitis, etc.[1]. It was first recorded in Shennong's Classic of Materia Medica, and the whole herb is used in medicine and is classified as medium grade. It is widely distributed in all parts south of the Yangtze River basin.
The whole herb of Centella asiatica was subjected to chemical research, and five compounds were isolated and identified. After chemical and spectral analysis, it was identified as p-sitosterol (β-sitosterol), carotenoid (dauco-sterol), asiatic acid (asiatic acid), vanillic acid (vanillic acid) and asiaticoside (asiaticoside). Asiaticoside is an active ingredient extracted from Centella asiatica. As a traditional Chinese medicine, it is also known by the aliases of Luode Da, Beng Da Wan, and Ma Ti. It is a plant in the Lamiaceae family with sweet, pungent, and cooling properties, and has the effect of clearing away heat and detoxifying [2]. It has been shown that asiaticoside can inhibit fibroblast proliferation, reduce transaminase activity, reduce the amount of acidic mucopolysaccharides and collagen, and inhibit excessive proliferation of the matrix and fibrous components of connective tissue. Experiments have shown that it can promote skin growth, local cell proliferation, proliferation of connective tissue and vascular networks, and increase mucus secretion.

1 Research on the promotion of wound healing by asiaticoside
The topical application of 0.2% asiaticoside to Guinea pig puncture wounds can increase the content of hydroxyproline, increase tensile strength, increase collagen content and improve epithelial formation. In streptozotocin-diabetic mice, wound 12 healing was delayed. Topical application of a 0.4% solution of asiaticoside can increase hydroxyproline content, tensile strength, collagen content and epithelial formation, thereby promoting wound healing. For the Guinea pig puncture model, oral administration of 1 ms/kg of asiaticoside also has wound healing activity. A concentration of 40 ms/disk of asiaticoside can promote angiogenesis in the chick chorioallantoic membrane model. These results show that asiaticoside has a significant wound-healing activity, both in normal and delayed wounds, and is the main active ingredient in Centella asiatica [3].
As a result, the asiaticoside in Centella asiatica extract can promote fibroblast proliferation and extracellular matrix synthesis during wound healing. However, the exact mechanism at the molecular and gene expression levels is still poorly understood. Lu et al. [4-5] used cDNA microarray technology to detect the gene expression profile of human skin fibroblasts in vitro after applying asiaticoside (30 ms/mL). The results showed that 54 known functional genes related to cell proliferation, promotion of the cell cycle, and extracellular matrix synthesis were significantly upregulated at different time points. In addition, the mRNA and protein levels of specific genes responsible for extracellular matrix synthesis (such as those encoding type I and type III collagen) were also significantly altered, and these responses were significantly correlated with the stimulation of cells by asiaticoside. This information can be used to further investigate the target genes that mediate the effects of asiaticoside in fibroblasts.
Antioxidants have been reported to play an important role in wound healing. Topical application of asiaticoside (0.2%) twice a day for 7 days to cutaneous wounds in rats can cause an increase in the levels of enzymatic or non-enzymatic antioxidants in the new tissue, mainly superoxide dismutase, catalase, glutathione peroxidase, vitamin E and ascorbic acid. It can also cause a several-fold decrease in the level of lipid peroxidase. However, after 14 days of continuous administration, there was no significant difference in the levels of antioxidants compared to those in wounds treated with the vehicle. Therefore, the study concluded that asiaticoside can induce an increase in antioxidant levels during the initial stage of wound healing, which may be a key factor in the therapeutic properties of asiaticoside [6].
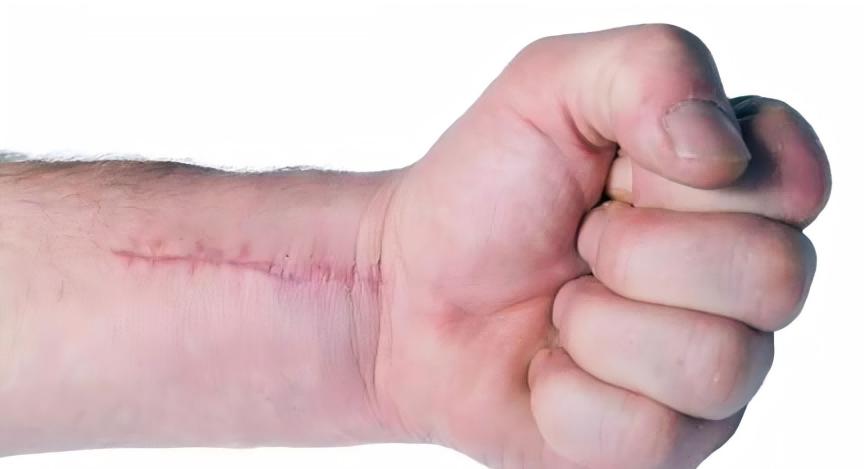
In addition, collagen types I and III are the main components of the dermis. Skin aging is mainly related to a decrease in type I collagen levels, and type I collagen also plays an important role in wound healing. The results of a study by Bonte et al. found that asiaticoside and madecassoside can stimulate collagen secretion, which can increase type I collagen secretion by 2% to 30%, but it was found that only madecassoside can increase type III collagen secretion [7-8].
2 Research on the mechanism of action of asiaticoside in treating scars
By studying the effect of asiaticoside on collagen synthesis in fibroblasts cultured in vitro, as well as its effect on the animal model of proliferative scar in nude mice, it was found that asiaticoside can act on the nucleus of fibroblasts, resulting in a decrease in the nuclear division phase and a decrease or loss of the nucleolus. With increasing drug concentration, intracellular DNA synthesis decreases, and cell growth is inhibited, with an inhibition rate of up to 73%. This suggests that the mechanism of action of asiaticoside is to block the proliferation of fibroblasts, thereby reducing collagen synthesis and preventing scar hyperplasia [9].
On the other hand, there were significant changes in the ultrastructure of the cells in the drug group, which were manifested as loose rough endoplasmic reticulum, reduced content, and the phenomenon of nucleoprotein body exocytosis. In addition, the total collagen synthesis in the drug group was significantly lower than that in the control group, showing a dose-dependent effect. This indicates that asiaticoside may have a direct effect on the rough endoplasmic reticulum, a protein synthesis system. Therefore, asiaticoside prevents the occurrence and development of scars by blocking the growth and proliferation of fibroblasts and inhibiting their ability to synthesize and secrete collagen [10]. In addition to the morphological changes in the animal model of proliferative scar in nude mice, the effect of asiaticoside is similar to that of fibroblasts cultured in vitro. Asiaticoside can promote the apoptosis of scar fibroblasts, reduce the number of immune cells, block blood vessels, reduce the number of blood vessels, and loosen collagen fibers, causing the entire scar tissue to become softer, smaller in size, and degenerative [11].
Transforming growth factor p (transforming growth factor—β, TGF—p) can be divided into three subtypes in mammals: TGF—p, TGF—β2, and TGF—β. It functions by binding to receptors [12]. TGF—β has the effect of promoting scar proliferation, while TGF-β2 has the effect of inhibiting scar proliferation. Among them, TGF-β plays an important role in the formation of keloid scars [13]. Experimental results showed that the expression of TGF-β mRNA in keloid scars treated with asiaticoside was significantly reduced. This is consistent with their previous experiments, which found that asiaticoside can reduce TGF-β expression in proliferative scar fibroblasts cultured in vitro [l4]. Therefore, it has been confirmed from both in vivo and in vitro that asiaticoside can downregulate TGF-mRNA expression in proliferative scars to reduce scar hyperplasia. They also observed that the expression of TGF-1β mRNA in proliferative scars treated with asiaticoside was increased, which was significantly higher than that in the control group. Therefore, they believe that the expression of TGF-1 mRNA in proliferative scars is also one of the mechanisms by which asiaticoside reduces scar hyperplasia.
Currently, TGF-β is considered to be one of the important growth factors that cause the accumulation of extracellular matrix. It regulates the synthesis of type I and III collagen through the Smad3, 7/AP-l signaling pathway and the MMPS/TIMPs pathway, and TGF-p. It mainly upregulates the expression of type I collagen promoter genes, stimulates fibroblasts to produce type I collagen, and causes scar hyperplasia [15]. Zhang Tao et al. [16] confirmed through experiments that the positive expression of type I collagen in proliferative scars treated with asiaticoside was reduced, which was consistent with the results of reduced TGF-β mRNA expression in proliferative scars treated with asiaticoside. At the same time, the expression of tissue inhibitor of metalloproteinase-1 (TIMP1) was reduced in proliferative scars treated with asiaticoside, which further confirmed that asiaticoside reduced the expression of TGF-β, resulting in a significant reduction in the expression of TIMP1, and thus matrix metalloproteinases 1 (MMP1). TIMP1) expression was reduced, which further confirmed that asiaticoside reduced TGF-β expression, and because of its significant reduction in TIMP1 expression, the relative ratio of matrix metalloproteinases 1 (MMP1)/TIMP1 increased, achieving the mechanism of action of promoting type I collagen degradation and reducing scar hyperplasia.
In addition, Pan Mei et al. [17] found through experiments that asiaticoside inhibits scar hyperplasia mainly by increasing the expression of I-Smad transduction signal Smad 7, inhibiting the pathological effects of TGF-16, and blocking the proliferation of fibroblasts. LEE et al. [18] found through further research that it can induce the phosphorylation of Smad2 and Smad3. In addition, they found that asiaticoside can induce the binding of Smad3 and Smad4. Consistent with this result, asiaticoside treatment can induce the nuclear translocation of the Smad3 and Staad4 complex, indicating that asiaticoside is related to the Smad signaling pathway. In addition, SB431542, an inhibitor of TGF-p receptor I kinase, an activator of the Smad pathway, was found to inhibit the phosphorylation of Smad2 induced by asiaticoside and the synthesis of type I collagenase. Therefore, they believe that asiaticoside induces the synthesis of type I collagenase through the TGF-1 kinase-independent Smad pathway.
3 Preliminary studies on the anti-tumor effect of asiaticoside
In terms of anti-tumor effects, it has been found that asiaticoside extract can inhibit the proliferation of tumor cells in vitro, while having no effect on normal lymphocytes. In vivo, it can inhibit the growth of mouse transplanted tumors and prolong the survival of tumor-bearing mice. Asiaticoside extract can also directly inhibit DNA synthesis [19]. Another literature report shows that asiaticoside has an inhibitory effect on the proliferation of L929 and CNE cells cultured in vitro, and also inhibits the growth of S180 transplant tumors, while increasing the survival time of S180-bearing mice [20]. Huang Yunhong et al. [21] found that asiaticoside can inhibit tumor cell proliferation and that multidrug-resistant tumor cells also show similar drug sensitivity to corresponding non-resistant cells. No significant cross-resistance was observed, and asiaticoside can also induce apoptosis of tumor cells.
In addition, they found that asiaticoside and vincristine had a synergistic effect, and asiaticoside could increase the G/M phase block caused by vincristine. They confirmed that the combination of asiaticoside and vincristine significantly increased apoptosis by flow cytometry, agarose gel electrophoresis and morphological testing. The above results were also confirmed by the analysis of the expression of cycle regulation and apoptosis-related proteins Bcl-2, caspase-3, cytochrome B1 and cdc2. The specific mechanism of this synergistic effect is not yet fully understood, but they initially believe that it may be that asiaticoside significantly increases the intracellular calcium concentration and reduces mitochondrial membrane potential early in the induction of apoptosis, thereby affecting mitochondrial function. This may be a key step in the action of asiaticoside on the apoptosis pathway, thereby increasing the sensitivity of cells to apoptosis induced by antitumor drugs, and thus producing a synergistic effect with vincristine. They believe that asiaticoside has the potential to be used as a biochemical regulator in tumor chemotherapy.

4. Preventive and therapeutic effects on breast hyperplasia
Li Ping et al. [22] successfully prepared a rat model of breast hyperplasia by simulating the pathogenesis of human breast hyperplasia. The study found that whether Centella asiatica was administered preventively or therapeutically, it showed an effect on the symptoms of breast hyperplasia in rats caused by an imbalance in the E2, P, and E2/P ratios, such as nipple diameter, breast diameter, and breast height. High doses significantly inhibited estrogen levels, and all dose groups increased progesterone levels (up to 160%), resulting in a significant improvement in the estrogen/progesterone ratio (up to 54% reduction), which significantly reduced the rate of mammary hyperplasia and the degree of hyperplasia of the mammary gland tissue compared to the model control group, and showed a clear dose-effect relationship. Therefore, the researchers believe that the mechanism of Centella asiatica extract in preventing and treating mammary hyperplasia in experimental rats lies in reducing serum E levels, increasing P levels, and regulating and improving the E2/P ratio.
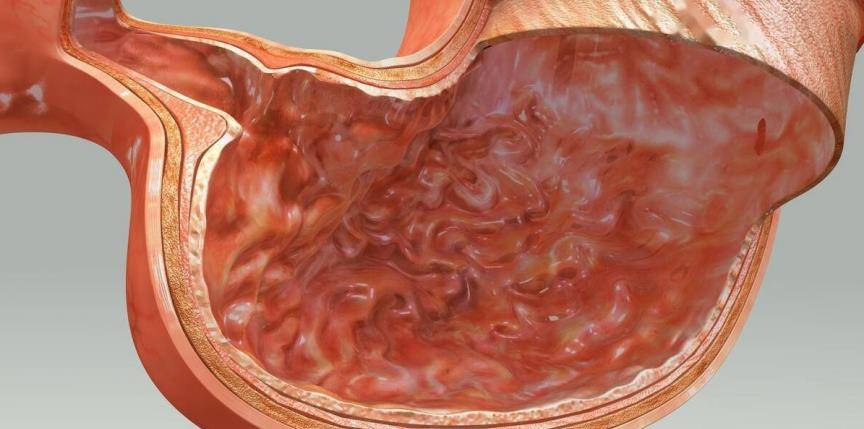
5 Protective effect on gastric mucosa
Hong Kong CHENG et al. [23] found that Centella asiatica extract can resist ethanol-induced gastric mucosal damage in mice by strengthening the mucosal barrier and reducing free radical damage, suggesting that Centella asiatica extract has certain anti-gastric ulcer activity. Le Jinmao et al. [24] found that after 2 weeks of treatment with compound Centella asiatica extract, the blood flow of rats accelerated, the blood flow was linear or mitochondrial, blood cell aggregation decreased, and the healing area of the gastric ulcers reached 99.5%. It was found that Centella asiatica extract has a certain inhibitory effect on histamine-induced gastric juice secretion, gastric juice free acid and total acid, and pepsin.
Guo et al. [25] orally administered different concentrations of Centella asiatica water extract and asiaticoside to rats with acetic acid-induced gastric ulcers. It was found that both could reduce the area of ulcers in a dose-dependent manner 1, 3, and 7 days after ulcer induction. It was also found that during this process, the activity and protein expression level of inducible nitric oxide synthase (iNOS) in ulcer tissue were reduced, and the nitrate, nitrite, and stable end product nitric oxide (NO) in ulcer tissue were also reduced. They concluded that Centella asiatica water extract can reduce gastric ulcer area and inhibit the production of nitric oxide in gastric ulcer. iNOS) activity and protein expression levels were reduced, and nitrate, nitrite and the stable end product nitric oxide (NO) were also reduced in ulcer tissue.
They concluded that Centella asiatica water extract and asiaticoside have anti-inflammatory properties and promote ulcer healing by inhibiting NO synthesis. They also found that the healing of ulcers was accompanied by a decrease in the activity of myeloperoxidase in ulcer tissue, as well as an increase in epithelial cell proliferation and angiogenesis. A key angiogenic factor, basic fibroblast growth factor, was upregulated in ulcer tissue from rats treated with Centella asiatica water extract and asiaticoside. These results further indicate that Centella asiatica and its active ingredients have broad application value as an anti-gastric ulcer drug.
6 Anti-anxiety and antidepressant effects and other pharmacological effects
Chen Yao et al. [26] found that total asiaticoside exerts an antidepressant effect by improving the body's resistance to various non-specific stimuli and avoiding the disorder of the regulatory functions of the hypothalamic-pituitary-adrenal cortex axis and the hypothalamic-pituitary-thyroid axis caused by excessive stress stimuli. WIJEWEERA et al. [27] also found through a rat behavioral model that Centella asiatica, its methanol and acetate liposoluble extracts, and pure asiaticoside showed the same anxiolytic activity. It was also found that asiaticoside did not affect spontaneous activity, indicating that these components have no sedative activity in rodents. GUSEVA et al. [28] also found that total asiaticoside is effective and well tolerated in the treatment of systemic and local scleroderma, and is therefore recommended for oral or topical administration. The indications for oral administration are chronic or subacute systemic scleroderma with limited skin damage, while the combination of tablets and ointment is recommended for progressive or focal scleroderma. In addition, Centella asiatica has anti-cytotoxic, stress-relieving, anti-leprosy, anti-fungal, anti-filariasis, anti-tuberculosis, analgesic, anti-inflammatory and other effects. It is also used in beauty preparations and insect repellents.

7. Summary
The modern application of Centella asiatica is already very extensive, and as one of its main ingredients, asiaticoside, is based on the above-mentioned various special effects, it will definitely play its unique advantages in more and more fields. Although most of the current research on its mechanism is still in its infancy, with the wide application of various cell and molecular biology techniques in the field of drug mechanism research, it is believed that our understanding of asiaticoside will become clearer and clearer, which will provide a basis for the clinical development of new dosage forms and new treatment areas.
References
[1] He Mingfang, Meng Zhengmu, Wo Lianqun. Research on the chemical composition of Centella asiatica [J]. Journal of China Pharmaceutical University, 2000, 31(2): 91-93.
[2] Zhao Yuxin, Li Manling, Feng Weihong, et al. Research progress of Centella asiatica. Chinese Journal of Traditional Chinese Medicine Information, 2002, 9(8): 81-84.
[3] SI-IUKLA A, RASIK A M, JAIN G K, et al. In vitro and in vivo wound healing activity of asiaticoside isolated from centella asiatica [J]. J Ethnopharmacol, 1999, 65(1): 1-11.
[4] LU L, YING K, WEI S, et al. Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts [J]. Int J Dermatol, 2004, (11): 801-807.
[5] Lu L, YINGK, WEI S, et al. Dermal fibroblast-associated gene induction by asiaticoside shown in vitro by DNA microarray analysis [J]. Br J Dermatol, 2004, 151(3): 571-578.
[6] SHUKLA A, RASIK A M, DHAWAN B N. Asiaticoside-induced elevation of antioxidant levels in healing wounds [J]. Phytother Res, 1999, 13(1): 50-54.
[7] LÜ Luo, WEI Shaomin, LIN Huifen, et al. Effects of Centella asiatica extract on the biological characteristics of skin cells [J]. Daily Chemical Industry, 2003, 33(1): 19-2l, 24.
[8] BONTE F, DUMAS M, CHAUDAGNE C, et al. Comparative activity of asiaticoside and madecassoside on type I and 111 coHagen synthesis by cultured human fibroblasts [J]. Ann Pharm Fr, 1995, 53(1): 38-42.
[9] Wang Ruiguo, Wang Jinju, Yu Xiangbin, et al. Effects of asiaticoside on DNA synthesis and collagen synthesis in fibroblasts [J]. Journal of Fujian College of Traditional Chinese Medicine, 2001, 11(2): 41-42.
[10] Xie Julin, Li Tianzeng, Qi Shaohai, et al. The effect of asiaticoside on fibroblasts cultured in vitro [J]. Journal of Zhongshan University of Medical Sciences, 2001, 22(1): 4l-43, 47.
[11] QI S H, XIE J L, LI T Z, et al. Experimental study on the effect of asiaticoside on hypertrophic scars after burns [J]. Chinese Journal of Burns, 2000, 16(1): 53-56.
[12] CHIN D, BOYLE G M, PARSONS P G, et al. What is transforming growth factor—beta (TGF—B)? [J] J Plast Surg, 2004, 57(3): 215—221.
[13] SOC, BEANES S R, HU F Y, et al. Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization [J]. Am J Pathol, 2003, 163(6): 2459-2476.
[14] SUNIYOSHI K, NAKAO A, SETOGUCHI Y, et al. Smads regulate collagen gel contraction by human dermal fibroblasts. Br JDermatol, 2003, 149(3): 464-470.
[15] YAMANE K, SUZUKI H, IHN H, et al. Cell type-specific regulation of the TGF-beta responsive alpha2(I)collagen gene by CpG methylation [J]. J Cell Physiol, 2005, 202(3): 822-830.
[16] Zhang Tao, Rong Xinzhou, Yang Ronghua, et al. Effects of asiaticoside on transforming growth factor-beta 1 mRNA and matrix metalloproteinase expression in proliferative scars [J]. Journal of Southern Medical University, 2006, 26(1): 67-70.
[17] Pan Shu, Li Tianzeng, Li Yeyang, et al. Effects of asiaticoside on proliferation of fibroblasts and Smad signaling pathway in proliferative scars [J]. Chinese Journal of Plastic and Reconstructive Surgery, 2004, 18(4): 291-294.
[18] LEE J, JUNG E, KIM Y, et al. Asiaticoside induces human col·lagen I synthesis through TGF—beta receptor Ikinase (TbetaRIkinase)—independent Smad signaling [J]. Planta Med, 2006, 72(4): 324—328.
[19] SAMPSON J H, RAMAN A, KARLSEN G, et al. In vitro keratinocyte antiproliferant effect of Centella asiatica extract and saponins. Phytomedicine, 2001, 8(3): 230-235.
[2O] Wang Ruiguo, Wang Jinju, Wang Baokui, et al. Preliminary experimental study on the anti-tumor effect of asiaticoside [J]. Fujian Traditional Chinese Medicine, 2001, 32(4): 39-40.
[21] Huang Yunhong, Zhang Shenghua, Zhen Ruixian, et al. Asiaticoside induces apoptosis in tumor cells and enhances the anti-tumor effect of vincristine [J]. Cancer, 2004, 23(12): l 599— 1604 .
[22] Li Ping, Ding Jianhua, Gu Bing, et al. Prevention and treatment of Centella asiatica against breast hyperplasia in experimental rats [J]. Journal of Nanjing Medical University, 2002, 22(6): 470—473 .
[23] CHENG C L, GUO J S, LUK J, et al. The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats [J]. Life Sci, 2004, 74(18): 2237-2249.
[24] Le Jinmao, Pu Peiying, Lei Ying. Effects of compound Centella asiatica extract on chronic experimental ulcers and microcirculation in rats [J]. Jiangsu Traditional Chinese Medicine, 1992, 13(3): 42-44.
[25] GUO J S, CHENG C L, KOO M W. Inhibitory effects of Centella asiatica water extract and asiaticoside on inducible nitric oxide synthase during gastric ulcer healing in rats [J]. Planta Med, 2004, (12): 1150-1154.
[26] Chen Yao, Qin Luping, Zheng Hancheng, et al. Effects of asiaticoside on neuroendocrine function in rats with depression [J]. Journal of the Second Military Medical University, 2002, 23(11): 1224-1226.
[27] WIJEWEERA P, ARNASON J T, KOSZYCKI D. et al. Evaluation of anxiolytie properties of Gotukola- (Centella asiatica) ex-tracts and asiaticoside in rat behavioral models [J]. Phytomedi cine, 26, l3(9-10): 668-676.
[28] GUSEVAN G, STAROVOITOVA M N, MACH E S. Madeeassoltreatment of systemic and localized scleroderma [J]. Ter Arkh, 1998, 70(5): 58-61.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






